Abstract
Exhaled CO2 is an important host-seeking cue for Anopheles mosquitoes, which is detected by a highly conserved heteromeric receptor consisting of three 7-transmembrane proteins Gr22, Gr23, and Gr24. The CO2 receptor neuron has been shown to also respond sensitively to a variety of odorants in Aedes aegypti. The detection of CO2 is important for upwind navigation and for enhancing the attraction to body heat as well as to skin odorants. The orthologs of the CO2 receptor proteins are present in malaria-transmitting mosquitoes like Anopheles coluzzii and Anopheles sinensis. Activators and inhibitors of the CO2-neuron were tested on the maxillary palps in these two species by single-sensillum electrophysiology. The electrophysiological testing of three prolonged-activator odorants identified originally in Aedes aegypti also showed varying ability to reduce the CO2-ellicited increase in spikes. These findings provide a foundation for comparing the functional conservation with the evolutionary conservation of an important class of odorant receptor. The identification of a suite of natural odorants that can be used to modify the CO2-detection pathway may also contribute to odor-blends that can alter the behavior of these disease transmitting mosquitoes.
Similar content being viewed by others
Introduction
Vector-borne diseases cause significant morbidity and mortality throughout the globe. Mosquitoes transmit a variety of pathogenic microorganisms that are responsible for malaria, filariasis, dengue fever, and encephalitis. Anopheline mosquitoes transmit malarial parasites, which infect >216 million people in Africa and Asia, resulting in ~445,000 deaths annually1. Indoor residual insecticide spraying (IRS) and long-lasting insecticide treated bednets (LLINs) provide protection against endophilic/endophagic mosquitoes, but lack of protection from bites for activities outside a bednet or outdoors from exophilic and diurnal mosquitoes require the development of new tools.
Mosquitoes are attracted long-range to carbon dioxide (CO2) exhaled from human breath and short-range to skin odor and body temperature2,3. The heteromeric CO2-receptor is expressed in the capitate peg sensillum A neuron (cpA) on the maxillary palps and consists of three 7-transmembrane proteins of the Gustatory Receptor family (Gr22, Gr23, and Gr24)4,5. This heteromeric receptor also detects skin odorants in Aedes aegypti and Anopheles coluzzii, participating in attraction when tested in A. aegypti6. Several additional classes of odorants have been identified using chemical informatics and electrophysiology that either activate or inhibit the CO2 receptor neuron in A. aegypti6. Given the sequence-level conservation of the orthologous Gr receptors in anopheline species, it would be interesting to study whether or not the physiological responses to known ligands are also conserved. Activators of the CO2 receptor that we identified previously have been shown to lure mosquitoes into a trap when tested in a greenhouse for Ae. aegypti and in semi-field conditions for An. coluzzii6,7. Identification of additional activators of the CO2 receptor in anophelines could therefore assist in development of convenient synthetic odorant lures that can mimic CO2.
Detection of skin odorants also occurs through other receptors belonging to the Or and Ir families8,9,10, as a Gr3 receptor mutant female Ae. aegypti can still seek out a human inside a greenhouse11. While these alternative pathways exist in mosquitoes to sense mammalian hosts, there is still a reduction in host-seeking behavior of Ae. aegypti by interfering with the detection of CO2 using inhibitory odorants6 or genetically when testing mice in a large cage arena12. The identification of odorant ligands of the Anopheles CO2 receptor neuron (cpA) can contribute to the design of attraction masking agents, which could reduce anopheline biting rates.
Using single sensillum recordings, we screened the cpA neuron of two anopheline mosquito species, anthropophilic An. coluzzii and facultative anthropophilic Anopheles sinensis, that transmit malaria with a large set of odorants that were initially identified by chemical-informatics as putative CO2 receptor ligands, and subsequently tested in the Ae. aegypti mosquitoes6. We identify several odorants that show conserved effects as activators, inhibitors, and ultra-prolonged activator of the cpA neuron. Some of these odorants have potential in reducing anopheline-biting rates.
Results
Sequence conservation of CO2 receptor proteins
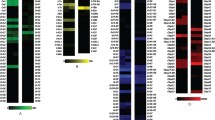
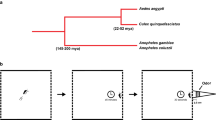
There is a close relationship among CO2 receptors in the Anophelinae (An. coluzzii and An. sinensis) and Culicinae (Ae. aegypti and Culex quinquefasciatus) mosquitoes, and orthologs cluster together in distinct branches (Fig. 1A). The percent amino acid identities between the sequences of these mosquito orthologs ranged as follows: GR22 (81–90%), GR23 (83–97%), and GR24 (74–92%). Similarities ranged for GR22 from 88% to 95%, for GR23 between 94% and 98%, and for GR24 from 85% to 91%.
(A) Maximum likelihood tree depicts the phylogenetic relationship among the CO2 receptors GR22, GR23, and GR24 orthologs in mosquitoes. Mosquito species as labeled as follow: Open circles, An. coluzzii; filled circles, An. sinensis; filled squares, Ae. aegypti, filled triangles, Cx. quinquefasciatus; and filled diamond, D. melanogaster. Bootstrap support for the branches is also shown. (B) An. coluzzii cpA neuron representative traces depicting activation of the cpA neuron upon exposure to 0.5 second pulse (red horizontal bar) of the described odor and (C) mean responses to 68 chemical volatiles (headspace above 1% solution on filter paper). n = 4. error = s.e.m. (D) Dose-responses of the activators of the An. coluzzii cpA neuron by five strong activators and their chemical structures. N = 3. error = s.e.m.
Conservation of response to agonists in An. coluzzii
In order to test conservation of the CO2-receptor neuron responses to different odorants, we tested a structurally diverse set of 67 ligands of the CO2-receptor previously identified in Ae. aegypti and Cx. quinquefasciatus6. The An. coluzzii CO2 neuron responded to several of these odorants with different chemical structures (Fig. 1B). Out of the 67 odors tested 35 (52%) evoked responses ≥30 spikes/sec whereas 14 odors (21%) showed responses lower than the solvent (paraffin oil; Fig. 1C). The odorants that evoked the strongest activation from the An. coluzzii cpA neuron were further evaluated in dose-response assays across a range of five orders of magnitude. All odorants showed a dose-response and four out of the five odors still evoked responses ≥30 spikes/sec with headspace from a 10−3 dilution (Fig. 1D).
Conservation of ultra-prolonged activators in An. coluzzii
Another class of ligands that have been identified in A. aegypti are ultraprolonged activators of the CO2 neuron6,13. In order to test these longer-term responses, recordings were performed as before with three known odorants (Fig. 2A). This analysis revealed that two of three odorants are conserved in their ability to evoke ultraprolonged activation in An. coluzzii. After a 3-s exposure to a (E)-2-methylbut-2-enal stimulus, the cpA neuron continues firing at ~50 spikes/sec for at least 5 min (Fig. 2B,C). Consistent with previous results responses to repeated CO2 stimuli during this 5-min period after the pre-exposure to (E)-2-methylbut-2-enal were significantly reduced (Fig. 2D).
(A) Mean response of An. coluzzii cpA neuron in females to the ultraprolonged activators (headspace above 10% solution on filter paper) and the solvent (n = 5–6). (B) cpA baseline activity exposure to odorant. (C) Representative traces from the cp sensillum to 1 s pulses of 0.15% CO2 prior to and following a 3-s exposure to either solvent (PO-paraffin oil) or (E)-2-methylbut-2-enal (headspace above 10% solution on filter paper). (D) Mean responses of the cpA neuron to 1 s pulses of 0.15% CO2, calculated by subtracting 1-s of baseline activity prior to each stimulus after exposure to paraffin oil (gray) or (E)-2-methylbut-2-enal (headspace above 10% solution on filter paper) (orange). n = 5–6 individuals; t test, ***p < 0.001. Error = s.e.m.
Conservation of inhibitors in An. coluzzii
Among the odorants that induced responses lower than the solvent, six odors actually inhibited the baseline activity of the An. coluzzii cpA neuron when tested with the headspace from 10−2 concentration odor cartridges (Fig. 1A). In order to test whether some of these odorants could constitute potential antagonists of cpA, we tested the ability of 21 odorants at a higher concentration (headspace from 10−1 concentration odor cartridges) to inhibit CO2-mediated (0.15% concentration) activation of the An. coluzzii cpA neuron in overlay assays. Of the 21 odorants tested, 11 were capable of reducing CO2-mediated cpA activation between 20% and 45%, and 5 odorants inhibited CO2 activation by >80% (Fig. 3A). Four top inhibitors were selected for dose-response assays. Four odorants were able to inhibit cpA activation by at least 50% when tested at 10−2 concentration and propanal evoked similar levels of inhibition when tested at 10−3 (Fig. 3B).
Inhibition of CO2 sensing by the An. coluzzii cpA neuron with specific chemical volatiles. (A) Overlays of CO2 and specific inhibitors can fully or partially inhibit the CO2- mediated activation of the cpA neuron. % Inhibition of CO2-evoked responses is measured in relation to exposure of the cpA neuron to CO2 and solvent. n = 4. Vertical bar represents s.e.m. Inset: Representative trace showing an overlay of a three seconds CO2 pulse (green bar) with 0.5 second methyl pyruvate pulse (red bar). (B) Dose-dependent inhibition of CO2-mediated activation of the An. coluzzii CO2 neuron over a range of five orders of magnitude. Chemical structures of the inhibitors are depicted on the right. n = 3. error = s.e.m.
Conservation of inhibitors in An. sinensis
In order to test whether inhibitory odorants of the An. coluzzii cpA neuron could be of utility in An. sinensis, four of the strong inhibitors (propanal, ethyl pyruvate, thiophene-2-thiol, and 4-methyl piperidine) were tested at two concentrations using electrophysiology. All the tested odorants showed some degree of inhibition, but to varying extent. The strongest inhibition was observed for thio-2-thiol and ethyl pyruvate at the higher concentrations (1%) (Fig. 4A). Among the three tested amines, amyl amine (AA) exhibited the highest inhibitory activity followed by butyl amine (BA). Spermidine (SP), on the other hand, acted as a weaker inhibitor in the presence of CO2 (Fig. 4B). Taken together these results indicate that the An. sinensis CO2 receptors respond similarly to Ae. aegypti and An. coluzzi when it comes to CO2 response inhibition.
Inhibition of An. sinensis CO2 receptor neuron (cpA). (A) Representative traces, mean percent inhibition, and chemical structure of cpA background for propanal (3-AL), ethyl pyruvate (EP), thiophene-2-thiol (Thio-2-thiol) and 4- methyl piperidine (4-MP), and (B) amyl amine (AA), butyl amine (BA) and spermidine (SP). n = 6 sensilla. Green and blue bars indicate 0.5 s odor stimulus; black bars indicate the duration of the CO2 stimuli (1 s).
Discussion
Unlike the complex blend of human skin odor, the CO2 in exhaled breath provides a simpler cue to study. Most mosquito species are strongly attracted to CO2 exhaled from human breath2. Carbon-dioxide is detected by the cpA neuron upon binding to a receptor, comprised of three members of the gustatory receptor gene family and named GR22, GR23, and GR24 in An. coluzzii4,5. The detection of CO2 plays several important roles in host-seeking behaviors like long-range navigation towards a live animal2,14. The identification of volatile ligands of the CO2 receptor neuron can contribute to the design of masking agents using inhibitors and trapping-lures using activators, both of which can reduce human contact and prevent disease transmission by mosquitoes6. We previously developed a computational approach15 which we applied to identify novel odorant ligands of the cpA neuron in A. aegypti6. Here we tested the conservation of the ligand responses in two species of anopheline mosquitoes, An. coluzzii and An. sinensis, that transmit malaria in Africa and Asia respectively. An. sinensis is one of the vectors of malaria in Asia and has Gr receptor orthologs that are closely related to ones in An. coluzzi. A high degree of functional conservation was observed amongst the ligands and we identified all three classes of ligands: activators, prolonged activators, and inhibitors.
In general, the responses to activators were weaker in An. coluzzii than in Ae. aegypti6, and only two odorants ((E)-pent-2-enal and methyl acetate) evoked stronger responses in An. coluzzii than in Ae. aegypti6. Conversely, inhibition of CO2-mediated cpA activation is stronger in An. coluzzii, as odorants unable to inhibit the cpA neuron in Ae. aegypti6 were capable of inhibiting the An. coluzzii and An. sinensis CO2 neuron counterparts. Amongst the inhibitors we have identified both high and low volatility compounds that act in both An. coluzzii and An. sinensis.
The detection of CO2 by the cpA neuron also activates attraction to other cues like skin odorants, visual cues, and importantly to body-warmth of 37 °C which is one of the strongest attraction cues at close-quarters12,16,17. The maxillary palp neuron, cpA also plays an important role in detection of human skin odorants6, and inhibitors such ethyl pyruvate and spermidine show reduction in attraction to skin odorants in Aedes6,18. Odorants like these could be useful in reducing host-seeking behavior and transmission of malaria, especially when used alongside others that block receptors detecting skin odorants.
Another approach to modulate behavior is using strong prolonged activators of cpA neurons that make the neuron unresponsive to CO2 as has been shown with 2,3-butanedione and blends on An. coluzzii and Ae. aegypti13. However, at the higher concentration needed for this effect the unpleasant smell of this odorant and health concerns precluded integration into practical solutions. We were able to demonstrate that An. coluzzii showed an ultra-prolonged activation to (E)-2-methylbut-2-enal, which resulted in masking the detection of CO2 significantly for several minutes after by the maxillary palp cpA neurons suggesting that this odor could disrupt detection of CO2 and navigation toward its source as has been shown in Ae. aegypti13 and could be utilized for potential practical applications in preventing mosquito bites and spreading of mosquito-borne diseases19.
Some of the CO2 receptor neuron inhibitors have organoleptic and physicochemical properties that are conducive to development into spatial and short-range masking agents for anopheline mosquitoes. However, ultimately for a masking strategy to work, additional odorants will be needed in a blend to block other human skin receptors that are members of the Odorant receptor (Or) and Ionotropic receptor (Ir) gene families.
Experimental Procedures
Mosquitoes
The M form of Anopheles gambiae (Herein An. coluzzii, Ngousso strain, Cameroon) were maintained in a 12:12 (Light:Dark) photocycle at 27 °C and 70% RH. The An. sinensis mosquitoes were received from MR4 center and then colony was maintained in insectary at the same conditions as An. coluzzii. Adult females were fed on bovine blood through a heated membrane feeding system (Hemostat Laboratories, California, USA).
Electrophysiology
Single-sensillum recording was carried out with 4–12 days old female anopheline mosquitoes as described elsewhere6,13,20. All recording replicates were performed in different specimens. All odorants were obtained from Sigma at >98% purity and were diluted to (as indicated) in paraffin oil or water. A filter paper with 50 μl of the solution was inserted into a Pasteur pipette cartridge and the headspace was injected into a humidified airstream to further dilute it 3-fold as done previously6.
For the ultra-prolonged activators (E)-2-methylbut-2-enal, 3-methylbut-2-enal and 3-methylbutanal were dissolved at 10−1 in paraffin oil or water, from which 50 μl of the solution was added on a filter paper inside a Pasteur pipette and the headspace was used for odor delivery as indicated in the section above. The odor delivery system was modified as shown in6; solvent responses during the same recording session were subtracted. A controlled 3-s stimulus of solvent/stimulus was delivered from a Pasteur pipette into the carrier airstream. Subsequent 1-s of CO2 (0.15%) stimuli was delivered using a MNJ-D microinjector (Tritech Research). Activity was calculated by subtracting baseline activity 1-s prior to each stimulus. Spike counting was done with Clampfit 10.3.
Phylogenetic analysis
The amino acid sequences of the CO2 receptor orthologs of Drosophila melanogaster, An. coluzzii, An. sinensis, Ae. aegypti, and Cx. quinquefasciatus (signal peptide removed) were aligned with the ClustalW software, and the phylogenetic tree was constructed with the MEGA621 software, using the Maximum Likelihood method and LG + G matrix-based model22. Reliability of the branches was inferred by 1,000 bootstrap replicates23.
Sequence access numbers
The GenBank access numbers for the CO2 receptor are as followed: An. coluzzii GR22 (XP_319142), GR23 (XP_312786), and GR24 (ABK97614); An. sinensis GR22 (KFB40998), GR23 (KFB38231), and GR24 (KFB40736); Ae. aegypti GR1 (XP_001655150), GR2 (XP_001654839), and GR3 (XP_001660602); Cx. quinquefasciatus GR1 (XP_001848097), GR2 (XP_001848828), and GR3 (XP_001848689); and Drosophila melanogaster GR21a (ABK97615) and GR63a (ABK97613).
References
WHO. World Malaria Report (2017).
Carde, R. T. G. G. In Ecology of Vector-Borne Diseases (eds Knols, W. & Takken, B. G. J.) 115–141 (Wageningen Academic Publishers, 2010).
Webster, B., Lacey, E. S. & Carde, R. T. Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J Chem Ecol 41, 59–66, https://doi.org/10.1007/s10886-014-0542-x (2015).
Lu, T. et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol 17, 1533–1544 (2007).
Robertson, H. M. & Kent, L. B. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci 9, 19, https://doi.org/10.1673/031.009.1901 (2009).
Tauxe, G. M., MacWilliam, D., Boyle, S. M., Guda, T. & Ray, A. Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell 155, 1365–1379 (2013).
Mburu, M. M. et al. 2-Butanone as a carbon dioxide mimic in attractant blends for the Afrotropical malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar J 16, 351, https://doi.org/10.1186/s12936-017-1998-2 (2017).
Carey, A. F., Wang, G., Su, C. Y., Zwiebel, L. J. & Carlson, J. R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71, https://doi.org/10.1038/nature08834 (2010).
Pitts, R. J., Derryberry, S. L., Zhang, Z. & Zwiebel, L. J. Variant Ionotropic Receptors in the Malaria Vector Mosquito Anopheles gambiae Tuned to Amines and Carboxylic Acids. Sci Rep 7, 40297 (2017).
Wang, G., Carey, A. F., Carlson, J. R. & Zwiebel, L. J. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA 107, 4418–4423 (2010).
DeGennaro, M. et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491 (2013).
McMeniman, C. J., Corfas, R. A., Matthews, B. J., Ritchie, S. A. & Vosshall, L. B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071, https://doi.org/10.1016/j.cell.2013.12.044 (2014).
Turner, S. L. et al. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474, 87–91 (2011).
Ray, A., van Naters, W. G. & Carlson, J. R. Molecular determinants of odorant receptor function in insects. J Biosci 39, 555–563 (2014).
Boyle, S. M., McInally, S. & Ray, A. Expanding the olfactory code by in silico decoding of odor-receptor chemical space. Elife 2, e01120 (2013).
Dekker, T., Geier, M. & Carde, R. T. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol 208, 2963–2972 (2005).
van Breugel, F., Riffell, J., Fairhall, A. & Dickinson, M. H. Mosquitoes Use Vision to Associate Odor Plumes with Thermal Targets. Curr Biol 25, 2123–2129 (2015).
MacWilliam, D., Kowalewski, J., Kumar, A., Pontrello, C. & Ray, A. Signaling Mode of the Broad-Spectrum Conserved CO2 Receptor Is One of the Important Determinants of Odor Valence in Drosophila. Neuron 97, 1153–1167 e1154 (2018).
Ray, A. Reception of odors and repellents in mosquitoes. Curr Opin Neurobiol 34, 158–164 (2015).
Turner, S. L. & Ray, A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 461, 277–281 (2009).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30, 2725–2729 (2013).
Le, S. Q. & Gascuel, O. An improved general amino acid replacement matrix. Mol Biol Evol 25, 1307–1320 (2008).
Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 39, 783–791 (1985).
Acknowledgements
The work was supported by grants from the National Institute of Health RO1AI087785 (NIAID) and RO1DC014092 (NIDCD) to A. Ray and U19 AI089672 (NIAID) to L. Cui and G. Yan. The granting agencies had no role in experimental design or analysis.
Author information
Authors and Affiliations
Contributions
I.V.C.-A. conducted experiments in Figures 1 and 3 and wrote the first draft of the manuscript; K.S. conducted experiments in Figures 2 and 4 and helped edit the manuscript; L.C. and G.Y. secured funding and helped edit the manuscript; A.R. conceived the project, managed the project and wrote the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
A. Ray is founder and equity holder of Sensorygen Llc. A. Ray and I.V. Coutinho-Abreu are listed as inventors in patents submitted by UC Riverside.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coutinho-Abreu, I.V., Sharma, K., Cui, L. et al. Odorant ligands for the CO2 receptor in two Anopheles vectors of malaria. Sci Rep 9, 2549 (2019). https://doi.org/10.1038/s41598-019-39099-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39099-0
- Springer Nature Limited