Abstract
The regulation of the kallikrein-kinin system is an important mechanism controlling vasodilation and promoting inflammation. We aimed to investigate the role of Toll-like receptor 2 (TLR2) in regulating kinin B1 and B2 receptor expression in human gingival fibroblasts and in mouse gingiva. Both P. gingivalis LPS and the synthetic TLR2 agonist Pam2CSK4 increased kinin receptor transcripts. Silencing of TLR2, but not of TLR4, inhibited the induction of kinin receptor transcripts by both P. gingivalis LPS and Pam2CSK4. Human gingival fibroblasts (HGF) exposed to Pam2CSK4 increased binding sites for bradykinin (BK, B2 receptor agonist) and des-Arg10-Lys-bradykinin (DALBK, B1 receptor agonist). Pre-treatment of HGF for 24 h with Pam2CSK4 resulted in increased PGE2 release in response to BK and DALBK. The increase of B1 and B2 receptor transcripts by P. gingivalis LPS was not blocked by IL-1β neutralizing antibody; TNF-α blocking antibody did not affect B1 receptor up-regulation, but partially blocked increase of B2 receptor mRNA. Injection of P. gingivalis LPS in mouse gingiva induced an increase of B1 and B2 receptor mRNA. These data show that activation of TLR2 in human gingival fibroblasts as well as in mouse gingival tissue leads to increase of B1 and B2 receptor mRNA and protein.
Similar content being viewed by others
Introduction
Kinins are generated by the release from kininogens through the enzymatic action of kallikreins. Since their discovery, these peptides are well known as pro-inflammatory molecules by increasing vasodilation, vascular permeability and cellular migration1. The kinin family is composed of bradykinin (BK) and Lys-bradykinin (Lys-BK), both B2 receptor agonists, and des-Arg9-bradykinin (DABK) and des-Arg10-Lys-bradykinin (DALBK), B1 receptor agonists1. B2 receptors are constitutively expressed in many cell types and are responsible for the classical actions of kinins, while B1 receptors are induced under pathological conditions and are mainly involved in inflammatory events1. Mechanisms controlling the local actions of the kallikrein-kinin system involve release of kinins but also regulation of their receptors2. Thus, pro-inflammatory molecules such as cytokines and lipopolysaccharide (LPS) regulate B1 and B2 receptor expression3,4.
Periodontal disease is a highly prevalent chronic inflammatory disease of the periodontium causing loss of gingival tissue, periodontal ligament and tooth-supporting bone. Colonization of the root surfaces on teeth by complex subgingival biofilms, containing several gram-negative bacteria, including Porphyromonas gingivalis, initiates the cascade of a wide variety of events leading to infiltration of inflammatory cells and the production of molecules that can disturb the remodeling of periodontal tissues, eventually leading to loss of alveolar bone and loosened teeth5. The presence of P. gingivalis impedes or modulates the host protective mechanisms in many different ways and is associated with diseased sites. Therefore, P. gingivalis is potentially a keystone pathogen that modifies the environment supporting the bacterial community to promote periodontal disease6.
We have reported that kinins may play important roles in periodontitis7. Accordingly, B1 and B2 receptors are expressed on osteoblasts and fibroblasts and activation of these receptors causes enhanced bone resorption mediated by increased prostaglandin E2 (PGE2) formation in both cell types and enhanced expression of receptor activator of nuclear factor-κB ligand (RANKL) in osteoblasts3,8,9. Interestingly, P. gingivalis expresses an arginine specific cysteine proteinase (Arg-gingipain-1/RGP-1) that can release kinins from kininogens10, facilitated by components of the kallikrein-kinin system binding to gingipains on the cell surface of P. gingivalis11.
Toll-like receptors are a family of pattern recognition receptors that recognize a plethora of pathogen-associated molecular patterns (PAMPs). To the PAMPs belongs lipopolysaccharide (LPS) from gram-negative bacteria, which is recognized by Toll-like receptors 4 (TLR4)12. The importance of TLR4 for periodontal disease is well studied, but much less is known on the role of TLR2. Interestingly, P. gingivalis has the capacity to activate both TLR2 and TLR413,14. Recently, we reported that P. gingivalis stimulates osteoclast formation in vitro and causes inflammation induced bone loss in vivo through activation of TLR215. This observation and the fact that periodontitis induced by P. gingivalis can not be observed in mice with genetic deletion of TLR2 indicates that TLR2 is also important for the pathogenic properties of P. gingivalis in periodontal disease16,17,18.
Data from human and mouse studies have evidenced an association between periodontal disease and rheumatoid arthritis (RA)19,20,21. The observation that alveolar bone loss in periodontitis patients precede the clinical onset of symptoms of RA21, together with the fact that treatment of periodontitis seems to reduce the severity of RA22,23 indicates a possible cause relationship between the two diseases. Further support for a role of oral infection in RA are studies in mice showing that oral infection with P. gingivalis aggravates arthritic bone erosions in collagen-induced arthritis22,24. The pathogenetic mechanisms involved were, at least in part, dependent on Th17 cells through the activation of TLR2 by P. gingivalis24. Further supporting an association between periodontal disease and RA is the observation that DNA from P. gingivalis has been detected in serum and synovial fluid from RA patients25. The routes used by P. gingvivalis to invade blood vessels in the periodontium and to reach the joints through the circulation are still unknown, but may be attributed to local activation in the periodontal tissues of the kallikrein-kinin system. This hypothesis is supported by the fact that local vascular permeability and bacterial spreading can be enhanced by P. gingivalis through a mechanism that was inhibited by decreasing kinin activity, either by administration of angiotensin converting enzyme (ACE), acting as a kininase enzyme, or by a kinin B2 receptor antagonist. In contrast, increased kinin activity by administration of BK, or the ACE inhibitor captopril, enhanced vascular permeability and bacterial spreading induced by infection with P. gingivalis26. Interestingly, the ability of P. gingivalis to disseminate was strain specific and correlated to generation of kinin activity. Thus, local regulation of kinin receptors in gingival fibroblasts could contribute by increasing the response to BK, leading to the generation of vasoactive mediators, such as prostaglandins, and by promoting bacterial spreading and aggravation of RA in periodontitis patients. In the present study, we have investigated the role of TLR2 for the local regulation of kinin receptors and report the novel finding that activation of TLR2 directly increases the expression of functional B1 and B2 receptors in human gingival fibroblasts as well as in mouse gingival tissue.
Results
Induction of BDKRB1 and BDKRB2 mRNA expression by P. gingivalis LPS and Pam2CSK4 in HGF
Human gingival fibroblasts were isolated from an individual without any clinical signs of gingival inflammation. Exposure of these cells to P. gingivalis LPS (10 μg/ml) for 3–24 hours resulted in time-dependent increased expression of both BDKRB1 (Fig. 1A) and BDKRB2 mRNA (Fig. 1B). The upregulation of BDKRB1 and BDKRB2 mRNA caused by P. gingivalis LPS was concentration dependent, with stimulatory effects seen at and above 100 ng/ml (Fig. 1C,D). Expression of IL6 mRNA has previously been reported to be upregulated by P. gingivalis LPS27; in the present experiments, increased IL6 mRNA was seen at the same concentrations as those stimulating kinin receptor expression (data not shown). BDKRB1 and BDKRB2 mRNA expression was enhanced also by the synthetic TLR2 agonist Pam2CSK4 (50 ng/mL) (Fig. 1E).
P. gingivalis LPS and the TLR2 agonist Pam2CSK4 increase the expression of BDKRB1 and BDKRB2 mRNA in human gingival fibroblasts. Time-course of the expression of BDKRB1 and BDKRB2 in human gingival fibroblasts cultured in the presence or absence of 10 μg/mL of P. gingivalis LPS (A,B). P. gingivalis LPS dose dependently increased mRNA expression of BDKRB1 (C) and BDKRB2 (D) in human gingival fibroblasts after 6 h of treatment with LPS. Pam2CSK4 (50 ng/mL) increased BDKRB1 and BDKRB2 mRNA in human gingival fibroblasts after 6 h of treatment (E). Data were normalized against RPL13A and are expressed as percentage of the means for the controls at 3 h (A) or controls (B–E), which was arbitrarily set to 100%. Values represent means for 3 wells/experimental group and SEM is shown as vertical bar. * and ** indicate significant difference to untreated control cells, P < 0.05 and P < 0.01, respectively. Statistical significance was determined using Student’s t test (A, B and E) or one-way analysis of variance (ANOVA), with Levene’s homogeneity test and Dunnet’s T3 post hoc test (C,D).
In order to evaluate if regulation of kinin receptor expression by P. gingivalis LPS and Pam2CSK4 in gingival fibroblasts was a general phenomenon, we incubated cells isolated from five different individuals with these test substances. In cells from all five individuals, Pam2CSK4 (50 ng/mL) significantly increased both BDKRB1 and BDKRB2 mRNA expression (Fig. 2). P. gingivalis LPS (1 μg/mL) significantly increased both BDKRB1 and BDKRB2 mRNA expression in cells from four of the five patients (Fig. 2).
P. gingivalis LPS and the TLR2 agonist Pam2CSK4 increase the expression of B1 and B2 kinin transcripts in human gingival fibroblasts from different individuals. BDKRB1 (A) and BDKRB2 (B) were up-regulated after 6 h of exposure to P. gingivalis LPS (1 μg/mL) or Pam2CSK4 (50 ng/mL) in cells isolated from five individuals. Each bar represents the average of 3 wells/experimental group and SEM is given as vertical bars. *** and *** indicate significant difference to untreated control cells, P < 0.05, P < 0.01 and P < 0.001 respectively. Statistical significance was determined using one-way analysis of variance (ANOVA), with Levene’s homogeneity test and Dunnet’s T3 post hoc test.
Induction of Bdkrb1 and Bdkrb2 mRNA expression by P. gingivalis LPS in mouse gingiva
In order to assess if the upregulation of BK receptors by P. gingivalis LPS observed in the fibroblasts cultures could be observed also in vivo, we locally exposed gingival tissue in mice to the bacterial LPS. Injection of P. gingivalis LPS (3 μg) every other day for 14 days in mouse gingiva enhanced the mRNA expression of Bdkrb1 and Bdkrb2 (Fig. 3A,B). Bdkrb1 mRNA was increased by 2.3-fold (Fig. 3A), while Bdkrb2 mRNA was increased by 1.6-fold (Fig. 3B).
P. gingivalis LPS increases the expression of Bdkrb1 and Bdkrb2 in mouse gingiva. Injection of LPS from P. gingivalis (3 μg) every other day for 14 days increases the expression of Bdkrb1 (A) and Bdkrb2 (B) in mouse gingiva in comparison with injection of vehicle (Control). The expression was analysed using Taqman assays. Data were normalized against Actb and are expressed as percent of the means for the controls, which was arbitrarily set to 100%. Each symbol represents data from one mouse. The horizontal line represents the mean for each experimental group. ** and *** indicate significant difference to untreated mice, P < 0.01 and P < 0.001, respectively. Statistical analysis was determined using Student’s unpaired t-test.
LPS from P. gingivalis and Pam2CSK4 up-regulate kinin receptor transcripts selectively via TLR2
In order to confirm that up-regulation of kinin receptors by the TLR2 receptor agonists used was a specific effect of TLR2 receptor activation, we knocked down TLR2 by using small interfering RNA designed to silence TLR2 (TLR2-siRNA). To rule out the contribution of TLR4, we also silenced TLR4 using TLR4-siRNA. The mRNA expression levels of TLR2 and TLR4 were decreased by 90%, as compared to cells transfected with a control (scrambled) siRNA (SCR-siRNA; data not shown). Our results showed that knockdown of TLR2 significantly decreased the enhancement of BDKRB1 and BDKRB2 mRNA induced by P. gingivalis LPS, as well as by Pam2CSK4 (Fig. 4A,B). In contrast, knockdown of TLR4 did not significantly affect kinin receptor expression induced by P. gingivalis LPS or by Pam2CSK4 (Fig. 4C,D).
The up-regulation of BDKRB1 and BDKRB2 mRNA by LPS from P. gingivalis is mediated by TLR2. Gingival fibroblasts were transfected with a scrambled siRNA or siRNA targeting TLR2 (A,B) or TLR4 (C,D). Twenty-four hours after transfection, the cells were exposed to LPS form P. gingivalis (1 μg/mL) or Pam2CSK4 (50 ng/mL). After 6 h, the expression of BDKRB1 (A,C) and BDKRB2 (B,D) mRNA was analyzed by qPCR using Taqman Assays. Data were normalized against RPL13A and expressed as percent of control which was arbitrarily set to 100%. Data are expressed as means ± SEM (n = 4 wells/experimental group). *, ** and *** indicate significant difference, P < 0.05, P < 0.01 and P < 0.001, respectively. Statistical analysis was determined using two-way analysis of variance (ANOVA), with Levene’s homogeneity test and Tukey post hoc test. The difference in P. gingivalis-induced response with and without silencing analyzed by two-way ANOVA was statistically significant (interaction P value in (A) and (B) was P < 0.01).
TLR2 agonists up-regulate kinin receptors at protein level
As shown in Fig. 5A,B, gingival fibroblasts pre-treated with Pam2CSK4 for 24 h exhibited enhanced binding to [3H]-BK and [3H]-DALBK, evidencing that the number of correctly folded receptor proteins capable of binding to the kinin receptors was enhanced.
TLR2 activation by Pam2CSK4 enhances the number of B1 and B2 binding sites, and the prostaglandin response induced by kinins. Gingival fibroblasts were pre-treated with Pam2CSK4 (50 ng/ml) or PBS (controls) for 24 h (A–C). The cells were then exposed to radiolabelled ligands for 90 minutes for binding analysis, (A,B) or treated for additional 24 h with kinins in order to assess the amount of PGE2 released (C). The results represent means ± SEM of 4 wells/experimental group. ** and *** indicate significant difference, P < 0.01 and P < 0.001, respectively. Statistical analysis was determined using Student’s unpaired t-test (A,B) or determined using two-way analysis of variance (ANOVA), with Levene’s homogeneity test and Tukey post hoc test. (C) The difference in PGE2 release with and without pretreatment with Pam2CSK4 by two-way ANOVA was statistically significant (interaction P value was P < 0.01).
To analyze the functional relevance of the up-regulation of B1 and B2, we took advantage of the fact that activation of both receptors are linked to increased formation of PGE2 in gingival fibroblasts28,29, as well as in many other cell types. When the fibroblasts were pre-treated with Pam2CSK4 for 6 h (50 ng/mL), the subsequent PGE2 responses to both BK and DALBK were enhanced (Fig. 5C), indicating that activation of TLR2 results in increased number of functional kinin receptors.
Analysis of the participation of IL-1β and TNF-α in P. gingivalis LPS-induced kinin receptor expression
In the human gingival fibroblasts, LPS from P. gingivalis induced the expression of IL-1β and TNF-α mRNAs, which were undetectable in control cells not exposed to LPS (data not shown). We, therefore, evaluated if these cytokines could participate in kinin receptor expression induced P. gingivalis LPS and for these purpose made use of specific neutralizing antibodies tested to verify their effectiveness (Supplemental 2). Neither the antibody neutralizing IL-1β (Fig. 6A), nor the one neutralizing TNF-α (Fig. 6C), affected P. gingivalis LPS (1 μg/mL)-induced increase of the mRNA expression of BDKRB1. At variance, although the treatment with the IL-1β neutralizing antibody caused no effect on BDKRB2 mRNA induced by P. gingivalis LPS (Fig. 6B), the TNF-α neutralizing antibody partially inhibited the up-regulation of BDKRB2 mRNA (Fig. 6D).
The role of IL-1β and TNF-α in P. gingivalis LPS mediated up-regulation of BDKRB1 and BDKRB2 mRNA. Gingival fibroblasts were exposed to 1 μg/mL of LPS from P. gingivalis for 6 h in the presence or absence of anti-IL-1β (0.3 μg/mL) (A,B) or anti-TNF-α (1 μg/mL) (C,D) and the expression of BDKRB1 (A,C) and BDKRB2 (B,D) mRNA was analyzed by qPCR using Taqman Assays. Data were normalized against RPL13A and expressed as percent of control which was arbitrarily set to 100%. Data are expressed as means ± SEM (n = 4 wells/experimental group. *** and *** indicate significant difference, P < 0.05, P < 0.01 and P < 0.001, respectively. Statistical analysis was determined using one-way analysis of variance (ANOVA), with Levene’s homogeneity test and Tukey post hoc test.
Discussion
In the present study, we report that the mRNAs encoding for the B1 and B2 kinin receptors are among those genes regulated by LPS from the periodontopathogenic bacterium P. gingivalis, both in vitro in human gingival fibroblasts and in vivo in mouse gingiva. Interestingly, it has been demonstrated that expression of TLR2 mRNA and protein, one of the receptors activated by P. gingivalis is enhanced by activation of B2 kinin receptor, indicating a bidirectional regulation of kinin receptors and TLR2 by their cognate ligands30.
In order to escape from the host recognition by the innate immune system and promote its adaptive fitness in the mammalian host, P. gingivalis LPS may elicit different responses when bound to TLR2 or TLR413,14. The heterogeneous responses of P. gingivalis LPS observed in vitro and in vivo may be due to the fact that many preparations are contaminated with lipoproteins or other lipid species31,32. Although TLRs are mainly present in inflammatory cells, it has been shown that gingival fibroblasts express a number of proteins belonging to the TLR family, including TLR2 and TLR433. Such data suggest that resident gingival fibroblasts might participate in the first recognition of pathogens. In line with this observation, we report here that P. gingivalis LPS was able to upregulate B1 and B2 receptors, both at the mRNA and at the protein levels. Up-regulation of B1 and B2 receptors in an organ culture model of tracheal segments isolated from mice by Salmonella LPS (TLR2 and TLR4 agonist) and by polyinosinic polycytidylic acid (TLR3 agonist) has also been reported34. Here, we show that the induction of B1 and B2 receptor expression by P. gingivalis LPS is an effect directly mediated by TLR2. One evidence for this is that Pam2CSK4, a specific TLR2 synthetic agonist, also upregulated B1 and B2 receptors in the human gingival fibroblasts. Furthermore, we also demonstrated that the effects elicited by P. gingivalis LPS and Pam2CSK4 were decreased in gingival fibroblasts in which TLR2 expression was robustly decreased by siRNA silencing, but remained unchanged in cells in which TLR4 was likewise siRNA-silenced, although we can not exclude that some remaining TLR4 protein after silencing might have contributed to the response.
It was previously reported that E. coli LPS is capable of regulating the expression of Bdkrb1 in a mouse paw edema model35. In this multicellular system, the purposed sequence of events that leads to the up-regulation of B1 receptor by E. coli involves the release of pro-inflammatory cytokines such as IL-1β and TNF-α, the release of chemoattractant molecules and neutrophil influx. Some years later, it was shown that LPS from P. gingivalis also up-regulates Bdkrb1 in the same model, by a mechanism that also involves neutrophil influx and TNF-α production4. Noteworthy, in the present study we show that human gingival fibroblasts are capable of up-regulating both BDKRB1 and BDKRB2 independently of IL-1β and in the case of BDKRB2, partially dependent of TNF-α.
We have previously reported that IL-1β and TNF-α enhance the expression of BDKRB1 and BDKRB2 in human gingival fibroblasts3. Since induction of pro-inflammatory cytokines is a well-recognized response to TLR2 activation36, we investigated if these cytokines mediated the effects by P. gingivalis LPS and Pam2CSK4 on kinin receptor expression. Using antibodies that specifically neutralize the effects of IL-1β and TNF-α, we show that up-regulation of BDKRB1 occurs independently of the production of both cytokines, whereas BDKRB2 up-regulation is partially dependent on TNF-α production but independent on IL-1β. In the mouse paw model, where inflammatory cells can be recruited to the inflamed site, neutrophil influx and TNF-α production are important events for the regulation of BDKRB1 levels by P. gingivalis LPS4. Although TNF-α expressing neutrophils are present in the inflamed gingiva during periodontitis37, the up-regulation of BDKRB1 in gingival fibroblasts, independently of IL-1β and TNF-α, may be of importance for the actions of kinins in the periodontium in chronic inflammation. As regards the BDKRB2 up-regulation, it has previously been shown that the up-regulation of this receptor by cardiac myocytes challenged with LPS was partially dependent on TNF-α production38, in agreement with our data. Nevertheless, it is interesting to note that the BDKRB2 up-regulation by P. gingivalis LPS was not completely inhibited by TNF-α neutralizing antibody in the gingival fibroblasts, which means that other TNF-α-independent pathways may also be involved in the regulation of the expression of this receptor.
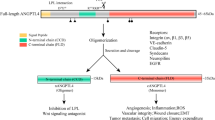
The data presented here may be of clinical relevance, since activation of TLR2 by P. gingivalis is associated with the aggravation of experimental arthritis in mice24. In humans, an association between periodontitis and RA has been demonstrated21,22,24. One possible mechanism has been suggested to be due to the presence of PAMPs derived from oral bacteria in the diseased joints. Supporting this view, DNA from periodontopathogenic bacteria can be detected in the serum and synovial fluid from patients with RA and psoriatic arthritis25,39. The mechanism underlying the invasion of periodontogenic bacteria is still elusive, but one possible route could be local activation of the kallikrein-kinin system from a sequestered infection site to promote vasodilation and facilitate invasion26. Our data can be reconciled with this hypothesis, since activation of TLR2 by P. gingivalis LPS not only increased B1 and B2 receptors mRNA, but also increased the capacity of gingival fibroblasts to produce prostaglandin E2, a potent vasodilator agent40, in response to the kinins. Kinins themselves are also vasodilatory agents, and can be generated at the inflammatory site during periodontal infection by the action of gingipain, a kinin-producing protease expressed by P. gingivalis41. The proposed sequence of events involved in bacterial invasion promoted by P. gingivalis, including a role of TLR2 induced kinin expression in fibroblasts, is outlined in Fig. 7.
Proposed role of kinin receptors in gingival fibroblasts for the invasion of P. gingivalis in gingival blood vessels. LPS from P. gingivalis is released from the biofilm on teeth at the inflammatory site and binds to TLR2, composed either by the heterodimer TLR1/TLR2 or TLR6/TLR2 in the cell membrane of human gingival fibroblasts. At the same time, the kinin-releasing protease gingipain expressed by P. gingivalis promotes the generation of kinins at the inflammatory site. Activation of TLR2 leads to the expression of kinin receptor mRNA and protein by gingival fibroblasts. The binding of BK and DALBK to B2 and B1 receptors, respectively, expressed by the fibroblasts leads to the release of PGE2. Kinins and PGE2 may act as vasodilator agents, facilitating the penetration of bacteria into the blood vessels and their spreading to other tissues.
In conclusion, in this study we report that P. gingivalis LPS is able to up-regulate kinin receptors in human gingival fibroblasts and mouse gingiva by the activation of TLR2. Moreover, our data reveal a new pathway by which these receptors are up-regulated which is independent on the production of IL-1β and TNF-α in the case of B1 receptor, and partially dependent on TNF-α production in the case of B2 receptor. These findings open new horizons for studies investigating mechanisms controlling the expression of B1 and B2 receptors in non-inflammatory cells.
Material and Methods
Materials
Specified in Supplementary Material 1.
Cell culture
Human gingival fibroblasts were isolated from healthy donors with written, informed consent as previously described42. Fibroblasts were from different individuals (males and females between 25–50 years of age (all generally and periodontally healthy) and the cells used in the present study were from passage 5–10. Approval from the Ethical Committee for Human Research at Umeå University was obtained for all the methods described, and all methods were performed in accordance with the relevant guidelines and regulations. The data shown were obtained using cells from one individual but reproduced using cells from another individual with the exception of the experiments used to produce data shown in Fig. 2 which were performed using cells from five different individuals.
In vivo regulation of Bdkrb1 and Bdkrb2 by P. gingivalis LPS
In order to assess the effect of P. gingivalis LPS on the regulation of kinin receptors in vivo, we injected LPS from P. gingivalis (3 μg per injection), or PBS, in the gingiva in the mesial aspect of upper first molar of male 6-weeks old C57Bl/6 mice. The protocol for this experiment was approved by the Ethical Committee on Animal Experimentation at the School of Dentistry in Araraquara – UNESP, Brazil and performed in accordance with the guidelines from the Brazilian College for Animal Experimentation (COBEA). Injections were performed every second day for 14 days, and the animals were sacrificed 6 h after the last injection. The gingival tissue was dissected and the RNA was extracted using RNAqueous-MICRO kit for qPCR analysis.
RNA extraction and cDNA synthesis
After exposure to the test substances for the time indicated in the graphs or figure legends, total RNA was extracted from the cells using RNAqueous-4PCR kit. The cDNA was synthesized with a first-strand cDNA synthesis kit using oligo(dT)15 as primers following the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qPCR)
The mRNA expression of human BDKRB1, BDKRB2, TLR2, TLR4 and the mouse genes Bdkrb1 and Bdkrb2 were assessed using previously described primer sequences3,27. Amplification was performed in an ABI Prism 7900HT sequence detection system using cDNA as template, specific primers and probes and Taqman Universal Mater Mix kit. To control the amount of cDNA input, ribosomal protein L13A (RPL13A) or β-actin (Actb) were used as controls (housekeeping genes) for human and mouse samples, respectively.
Radioligand binding assays
After overnight attachment of the fibroblasts, the media were changed and α-MEM with 1% FCS with or without Pam2CSK4 (50 ng/mL) was added. Twenty-four hours later, binding studies were performed following all the standardizations described previously9. To assess the amount of binding sites, the cells were incubated in MEM/HEPES/0.1% BSA with [3H]-BK 4 nmol/l or [3H]-des-Arg10-Lys-BK 14 nmol/l for 90 min at 4 °C. After extensive washing steps, the cells were detached and the radioactivity analyzed using liquid scintillation counter. The binding of [3H]-BK was competed for by B2, but not B1, ligands and the binding of [3H]-des-Arg10-Lys-BK was competed for by B1, but not B2, ligands (data not shown).
Prostaglandin E2 production
The amount of PGE2 was measured in the supernatant of cells exposed to BK (1 μM) or DALBK (1 μM) for 24 hours by using a commercially available ELISA kit for PGE2. In order to analyze the effect of TLR2 activation on kinin receptors expression, cells were pre-treated with or without Pam2CSK4 (50 ng/mL) for 24 h prior to the addition of kinins.
TLR2 and TLR4 knockdown
TLR2 and TLR4 were knocked down in gingival fibroblasts using siRNA as previously described27. Briefly, the cells were transfected with 30 nM of scrambled (SCR – Ambion, AM4635), TLR2 (Ambion, ID#111285) or TLR4 siRNA (Ambion, ID#112337) using lipofectamin 2000 in α-MEM with 10% FCS without antibiotics. The knockdown was confirmed by qPCR and more than 90% inhibition of TLR2 and TLR4 mRNA was achieved (data not shown). Twenty-four hours after transfection, the media were changed and the cells were exposed to the test substances; 6 h later RNA was extracted for qPCR analysis.
Participation of IL-1β and TNF-α in up-regulation of kinin receptors induced by P. gingivalis LPS
After overnight attachment, human gingival fibroblasts were incubated in α-MEM/1% FCS in the presence or absence of P. gingiavlis LPS, with or without antibodies neutralizing human IL-1β or human TNF-α. The IL-1β neutralizing antibodies blocked IL-1β induced enhancement of BDKRB1 mRNA expression and the TNF-α neutralizing antibodies blocked TNF-α induced increase of BDKRB1 mRNA (Supplementary Material 2).
Statistical analyses
Statistical analysis of multiple treatment groups was performed using analysis of variance (ANOVA), with Levene’s homogeneity test, and Dunnet’s T3 or Tukey post hoc test. For the experiments with two groups, the unpaired Student’s t-test was performed. The data shown in the figures are expressed as means ± standard error of means (SEM) for 3–6 wells per experimental group.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Leeb-Lundberg, L. M., Marceau, F., Muller-Esterl, W., Pettibone, D. J. & Zuraw, B. L. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57, 27–77 (2005).
Costa-Neto, C. M. et al. Non-canonical signalling and roles of the vasoactive peptides angiotensins and kinins. Clinical science 126, 753–774 (2014).
Brechter, A. B., Persson, E., Lundgren, I. & Lerner, U. H. Kinin B1 and B2 receptor expression in osteoblasts and fibroblasts is enhanced by interleukin-1 and tumour necrosis factor-alpha. Effects dependent on activation of NF-kappaB and MAP kinases. Bone 43, 72–83 (2008).
Dornelles, F. N. et al. In vivo up-regulation of kinin B1 receptors after treatment with Porphyromonas gingivalis lipopolysaccharide in rat paw. The Journal of pharmacology and experimental therapeutics 330, 756–763 (2009).
Liu, Y. C., Lerner, U. H. & Teng, Y. T. Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000 52, 163–206 (2010).
Darveau, R. P., Hajishengallis, G. & Curtis, M. A. Porphyromonas gingivalis as a potential community activist for disease. Journal of dental research 91, 816–820 (2012).
Lerner, U. H., Persson, E. M. & Lundberg, P. Kinins and Neuro-osteogenic factors. In Principles of Bone Biology, Vol. 2 (eds Bilezikian, J. P., Raisz, L. G. & Martin, T. J.) 1025–1057 (Academic Press, San Diego, 2008).
Brechter, A. B. & Lerner, U. H. Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum 56, 910–923 (2007).
Brechter, A. B. & Lerner, U. H. Characterization of bradykinin receptors in a human osteoblastic cell line. Regul Pept 103, 39–51 (2002).
Imamura, T., Pike, R. N., Potempa, J. & Travis, J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. The Journal of clinical investigation 94, 361–367 (1994).
Rapala-Kozik, M. et al. Adsorption of components of the plasma kinin-forming system on the surface of Porphyromonas gingivalis involves gingipains as the major docking platforms. Infection and immunity 79, 797–805 (2011).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology 11, 373–384 (2010).
Kocgozlu, L., Elkaim, R., Tenenbaum, H. & Werner, S. Variable cell responses to P. gingivalis lipopolysaccharide. Journal of dental research 88, 741–745 (2009).
Zhang, D., Chen, L., Li, S., Gu, Z. & Yan, J. Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL-1 beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate Immun 14, 99–107 (2008).
Kassem, A. et al. Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF-kappa B Ligand) through Activation of Toll-like Receptor 2 in Osteoblasts. Journal of Biological Chemistry 290, 20147–20158 (2015).
Burns, E., Bachrach, G., Shapira, L. & Nussbaum, G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol 177, 8296–8300 (2006).
Papadopoulos, G. et al. Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol 190, 1148–1157 (2013).
Lin, J. et al. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infection and immunity 82, 4127–4134 (2014).
Mercado, F., Marshall, R. I., Klestov, A. C. & Bartold, P. M. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol 27, 267–272 (2000).
de Pablo, P., Chapple, I. L., Buckley, C. D. & Dietrich, T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol 5, 218–224 (2009).
Kindstedt, E. et al. Marginal jawbone loss is associated with onset of rheumatoid arthritis and is related to plasma level of receptor activator of nuclear factor kappa-B ligand (RANKL). Arthritis & rheumatology (2017).
Al-Katma, M. K., Bissada, N. F., Bordeaux, J. M., Sue, J. & Askari, A. D. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol 13, 134–137 (2007).
Ortiz, P. et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol 80, 535–540 (2009).
de Aquino, S. G. et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol 192, 4103–4111 (2014).
Martinez-Martinez, R. E. et al. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol 36, 1004–1010 (2009).
Hu, S. W., Huang, C. H., Huang, H. C., Lai, Y. Y. & Lin, Y. Y. Transvascular dissemination of Porphyromonas gingivalis from a sequestered site is dependent upon activation of the kallikrein/kinin pathway. J Periodontal Res 41, 200–207 (2006).
Souza, P. P. et al. Stimulation of IL-6 cytokines in fibroblasts by toll-like receptors 2. Journal of dental research 89, 802–807 (2010).
Lerner, U. H. & Modeer, T. Bradykinin B1 and B2 receptor agonists synergistically potentiate interleukin-1-induced prostaglandin biosynthesis in human gingival fibroblasts. Inflammation 15, 427–436 (1991).
Modeer, T., Ljunggren, O. & Lerner, U. H. Bradykinin-2 receptor-mediated release of 3H-arachidonic acid and formation of prostaglandin E2 in human gingival fibroblasts. J Periodontal Res 25, 358–363 (1990).
Gutierrez-Venegas, G. & Arreguin-Cano, J. A. Bradykinin promotes TLR2 expression in human gingival fibroblasts. International immunopharmacology 11, 2079–2085 (2011).
Nichols, F. C., Bajrami, B., Clark, R. B., Housley, W. & Yao, X. Free lipid A isolated from Porphyromonas gingivalis lipopolysaccharide is contaminated with phosphorylated dihydroceramide lipids: recovery in diseased dental samples. Infection and immunity 80, 860–874 (2012).
Hirschfeld, M., Ma, Y., Weis, J. H., Vogel, S. N. & Weis, J. J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 165, 618–622 (2000).
Uehara, A. & Takada, H. Functional TLRs and NODs in human gingival fibroblasts. Journal of dental research 86, 249–254 (2007).
Bachar, O., Adner, M., Uddman, R. & Cardell, L. O. Toll-like receptor stimulation induces airway hyper-responsiveness to bradykinin, an effect mediated by JNK and NF-kappa B signaling pathways. European journal of immunology 34, 1196–1207 (2004).
Passos, G. F. et al. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J Immunol 172, 1839–1847 (2004).
Wang, H. et al. Leptospiral hemolysins induce proinflammatory cytokines through Toll-like receptor 2-and 4-mediated JNK and NF-kappaB signaling pathways. PloS one 7, e42266 (2012).
Nussbaum, G. & Shapira, L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol 38(Suppl 11), 49–59 (2011).
Yayama, K., Hiyoshi, H., Sugiyama, K. & Okamoto, H. The lipopolysaccharide-induced up-regulation of bradykinin B2-receptor in the mouse heart is mediated by tumor necrosis factor-alpha and angiotensin II. Biol Pharm Bull 29, 1143–1147 (2006).
Moen, K. et al. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol 24, 656–663 (2006).
Omori, K., Kida, T., Hori, M., Ozaki, H. & Murata, T. Multiple roles of the PGE2 -EP receptor signal in vascular permeability. Br J Pharmacol 171, 4879–4889 (2014).
Monteiro, A. C. et al. Kinin danger signals proteolytically released by gingipain induce Fimbriae-specific IFN-gamma- and IL-17-producing T cells in mice infected intramucosally with Porphyromonas gingivalis. J Immunol 183, 3700–3711 (2009).
Lerner, U. & Hänstrom, L. Human gingival fibroblasts secrete non-dialyzable, prostanoid-independent products which stimulate bone resorption in vitro. J Periodontal Res 22, 284–289 (1987).
Acknowledgements
This project was supported by grants from the Swedish Science Council, the Swedish Rheumatism Association, the Royal 80 Year Found of King Gustav V, the County Council of Västerbotten, COMBINE, an ALF/LUA research grant from Sahlgrenska University Hospital in Gothenburg, Lundberg Foundation, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Science Without Borders Program (Grant #080/2012) and Fundação de Amparo à Pesquisa do Estado de São Paulo (Grant # 2014/05283-3).
Author information
Authors and Affiliations
Contributions
P.P.C.S. and U.H.L. designed the study; P.P.C.S., I.L. and F.A.C.M. conducted the experiments; P.P.C.S., P.L., C.M.C. and U.H.L. interpreted the data; P.P.C.S. and U.H.L. wrote the first draft of the manuscript which was edited and approved by all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Souza, P.P.C., Lundberg, P., Lundgren, I. et al. Activation of Toll-like receptor 2 induces B1 and B2 kinin receptors in human gingival fibroblasts and in mouse gingiva. Sci Rep 9, 2973 (2019). https://doi.org/10.1038/s41598-018-37777-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37777-z
- Springer Nature Limited
This article is cited by
-
Early reduction of SARS-CoV-2-replication in bronchial epithelium by kinin B2 receptor antagonism
Journal of Molecular Medicine (2022)