Abstract
Metal/mineral dyshomeostasis has been implicated in the development of Alzheimer’s disease (AD). The aim of the study was to investigate the difference in absolute and percentage levels of plasma phosphorus, calcium, iron, zinc, copper, selenium in cognitively normal (CN) and AD subjects. Total reflection X-ray fluorescence (TXRF) spectroscopy was used to detect plasma metals/minerals in CN and AD subjects (n = 44 per group). TXRF detected significantly increased plasma levels of phosphorus (p = 1.33 × 10−12) and calcium (p = 0.025) in AD compared to CN subjects, with higher phosphorus/calcium (p = 2.55 × 10−14) ratio in the former. Percentage concentrations calculated for phosphorus, calcium, iron, zinc, copper, selenium by dividing the concentration of each element by the total concentration of these elements and multiplying by 100%, demonstrated phosphorus was higher in AD compared to CN subjects, while calcium, iron, zinc, copper and selenium were lower in AD subjects, with area under the curves as high as 0.937 (p = 6 × 10−5) computed from receiver operating curves. With exclusion of high levels of phosphorus and calcium from percentage calculations, iron levels remained low in AD whereas zinc was higher in AD, and copper and selenium levels were similar. We demonstrate altered distribution of elements in the plasma of AD subjects with high interdependencies between elemental levels and propose the potential of TXRF measurements for disease monitoring.
Similar content being viewed by others
Introduction
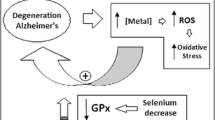
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly population with a predicted incidence of 115.4 million cases by 2050. It is characterised by pathological inclusions of extracellular ß-amyloid (Aß) plaques and intracellular neurofibrillary tangles1. With the lack of a cure, and only mildly effective symptomatic treatments at best, there is a great impetus to understand this incurable dementia. Metal dyshomeostasis (particularly iron, zinc and copper) has recently been implicated in the development of AD2. These metals can accentuate plaque formation and affect tau hyperphosphorylation albeit to a lesser extent. Aberrant homeostasis of these transition elements and enhancement of protein aggregation and detrimental interactions with protein aggregates can generate oxidative stress contributing to neurodegeneration in AD2. Also, there has been considerable debate regarding the contribution of aluminium to AD. A common feature of neurodegenerative diseases such as AD are changes in ratios of various metals3.
Dyshomeostasis of other micronutrients such as calcium is also evident in the AD brain4. Circulating calcium and phosphorus are regulated by parathyroid hormone (PTH). In secondary hyperthyroidism, PTH levels are high, alongside normal to low calcium and a concurrent decrease in vitamin D levels5. High serum phosphate has been associated with increased incidence of dementia6 as well as changes in phosphorus/calcium ratios7.
AD has a well-known prodromal stage1 which provides a therapeutic window when pathological processes are still responsive to treatment. However, determining this prodromal stage is difficult and drives much of the current biomarker research. While cerebrospinal fluid (CSF) Aß, phosphorylated and total-tau are used as biomarkers of AD1, their use to monitor the prodromal phase is debatable and impractical. CSF collection is by lumbar puncture, an uncomfortable sampling method for the individual, especially the elderly and associated with morbidity, and while used to assist diagnosis, it is inappropriate for screening or repeat sampling for disease monitoring. Thus, there is the impetus towards peripheral-based biomarkers such as those in plasma which can be readily collected and repeatedly, if necessary.
Investigators have tested whether alterations of brain metals and other elements are peripherally reflected to potentially aid in diagnosis and disease monitoring5,6,8. No consensus has been obtained with regards to whether the peripheral concentrations of various elements are modulated in AD owing to discrepant findings. This may be attributable to plasma levels, being not only affected by AD pathogenesis, but also affected by diet, nutritional status, sex, age, etc. Additionally, the analytical method used may impact the measurements, some methods requiring sample preparation, the complexity varying between analysis methods; whether free or bound elements or their isotopes are measured, etc. A few studies do report analysis of a panel of metals by inductively-coupled plasma mass spectroscopy (ICP-MS) showing differences between cognitively normal (CN), mild cognitive impairment (MCI) and AD2,3.
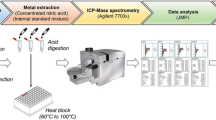
In this study, we quantify the plasma elemental concentrations of phosphorus, calcium, iron, copper, selenium and zinc using total reflection X-ray fluorescence (TXRF) spectrometry in age- and sex-matched AD and cognitively normal (CN) individuals. TXRF measures all the isotopes of an element and detection limits can be comparable to atomic absorption spectroscopy (AAS), inductively coupled plasma optical emission spectroscopy (ICP-OES) or ICP-MS9. Matrix effects are minimised enabling higher sensitivity and low detection limits in the order of parts per billion. A key advantage of TXRF is the relatively simple sample preparation required compared to other techniques, contributing to its robust nature, with errors relating to pipetting small volumes rather than extraction efficiency of metals from biofluids or matrix effects. Other advantages include smaller analytical volumes needed (as low as 10 µl)10; simple quantification using an internal standard; and simultaneous multi-element trace analysis without bias towards expected elemental compositions11,12. We aim to determine whether plasma phosphorus, calcium, iron, copper, selenium and zinc; and phosphorus/calcium ratio, differentiates between CN and AD individuals and may be putative peripheral biomarkers of AD. This is the first study that we are aware of, applying TXRF to the analysis of blood for biomarker discovery in AD, and proposes a possible role of TXRF instrument in the clinical chemistry laboratory.
Materials and Methods
Subjects
Plasma samples from AD and CN controls were selected from the AddNeuroMed cohort13. Informed consent was obtained from all participants according to the Declaration of Helsinki (1991), and protocols and procedures were approved by The Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee. The diagnosis of probable AD was made according to the Diagnostic and Statistical Manual for Mental Diagnosis, fourth edition and National Institute of Neurological, Communicative Disorders and Stroke–AD and Related Disorders Association criteria, with Mini-Mental State Examination (MMSE) score of <24 for cognition. The APOE single nucleotide polymorphisms (SNPs) rs429358 and rs7412 were genotyped using Taqman SNP genotyping assays (determined by allelic discrimination assays based on fluorogenic 50 nuclease activity) and the allele inferred. In total, we examined 88 subjects: n = 44, AD; n = 44, CN; age- and sex-matched.
Blood Collection
Blood collection was performed in the morning after an overnight fast. Venous blood was collected into lithium heparin tubes (Becton and Dickinson) and centrifuged. Plasma was separated, frozen and stored at −80 °C prior to TXRF analysis.
TXRF of Plasma
Plasma were thawed and 20 μl of a gallium standard solution (2 mg/l, Kraft GmbH, Germany) was added to each sample volume of 20 µl. After thorough mixing, 10 μl of each solution was put onto TXRF quartz glass carriers and air dried: duplicates of each sample were performed. TXRF data was collected over 1000 s on a TXRF spectrometer (S2 PICOFOX, Bruker Nano GmbH, Germany) with a molybdenum tube excitation source operating at 50 kV/600 μA. TXRF spectra were inspected, and all elements identified, prior to deconvolution of the spectra using the PICOFOXTM software. Elemental concentrations of phosphorus, calcium, iron, copper, zinc and selenium were calculated by reference to the gallium standard in each sample.
Accuracy of the TXRF measurements was validated by analysing two certified reference samples (SeronormTM serum levels 1 and 2, Sero, Norway). The lower limit of detection (with three-sigma detection) for an element e (LLDe) was calculated according to the following equation:
where Ce is the elemental concentration, and Ae and Abg are the areas of the fluorescence peak for element e and the background subjacent to the fluorescence peak for e, respectively. The measurement time for the calculation of LLDe was also 1000 s, the same as for the plasma samples. Each Seronorm™ standard was measured ten times.
The method detection limit (MDL) was also calculated. The MDL is defined as ‘the minimum concentration of a substance that can be measured and reported with 99% confidence that the analyte concentration is greater than zero and is determined from analysis of a sample in a given matrix containing the analyte’. The MDLe for an element e was calculated (according to the U.S. Environmental Protection Agency, Title 40 Code of Federal Regulations Part 136, Appendix B, revision 1.11) using the following equation:
where t(n=1,99%) is the students’ t-value for a 99% confidence level and a standard deviation (SD) with n − 1 degrees of freedom.
Statistical Analysis
Data analysis was performed using SPSS IBM version 24.0. Normal distribution was checked graphically using box plots, histogram, Q-Q plots, stem and leaf diagrams and numerically using Shapiro-Wilk’s test. The following variables were square-root transformed to normality: phosphorus, copper, selenium; and calcium, iron and zinc were log transformed. Analysis of Co-Variance (ANCOVA) was used to determine elemental differences between the CN and AD groups with co-variates: diagnosis, sex, APOE status, diagnosis x sex, diagnosis x APOE, diagnosis x sex x APOE and sex x APOE. A p-value of ≤ 0.05 was considered significant. The phosphorus/calcium ratio was also calculated as in previous studies and underwent ANCOVA testing.
Absolute concentrations of metals/elements in serum have been shown to be highly variable14 and possibly interdependent. Thus, partial correlation analysis adjusted for age, sex, APOEε4 and diagnosis status was performed to determine interdependencies between the elements: phosphorus, calcium, iron, copper, zinc and selenium. A p-value of ≤ 0.05 was considered significant. Percentage concentrations were also calculated for phosphorus, calcium, iron, zinc, copper, selenium by dividing the concentration of each element by the total concentration of these elements and multiplying by 100%. As percentages of the elements were not normally distributed, the Mann-Whitney U test was performed to determine differences between the CN and AD, with p-value ≤ 0.05 being considered significant. As phosphorus and calcium levels were comparatively much higher than that for iron, zinc, copper and selenium, percentages for the latter elements were re-calculated with the exclusion of phosphorus and calcium and again tested for significance.
Binary logistic regression model adjusted for age, sex and APOEε4 status was used to calculate predictive probabilities of elemental percentages. These standardized predictive probabilities were then used to calculate the receiver-operator curves (ROC) and areas under the curve (AUC). For regression models, we ensured normal distribution of residuals and absence of multi-collinearity. A p-value of ≤ 0.05 was considered significant.
Results
Demographics, clinical data, and plasma elemental concentrations for CN and AD groups are shown in Table 1 and a dot plot of the elemental concentrations shown in Fig. 1. TXRF measurements of elemental concentrations were validated using Seronorm™ Levels 1 and 2 (Table 2). Of the elements studied, selenium had the lowest abundance in the plasma and levels as low as 0.012 mg/l could be measured.
Dot plot of Total Reflectance X-Ray Fluorescence (TXRF) measurements of elements in plasma from cognitively normal (CN) and Alzheimer’s Disease (AD) subjects. P-values for each element and the phosphorus/calcium ratio are shown following normalisation of data and ANCOVA testing (see Table 1).
Absolute Plasma Elemental Concentrations and Interdependencies
Plasma phosphorus (p = 1.33 × 10−12) and calcium (p = 0.025) were significantly higher in AD compared to CN (Tables 1 and 3). Further, a higher phosphorus/calcium (p = 2.55 × 10−14) ratio was observed in AD patients (Tables 1 and 3). Absolute plasma concentrations of iron, zinc, copper and selenium were similar between the groups (Table 1). By partial correlation analysis, plasma levels of elemental phosphorus, calcium, iron, zinc, copper and selenium were shown to be strongly interdependent (Fig. 2). Hence, further statistical analysis between the CN and AD groups was performed on percentages of these elements.
Heat map of partial correlations between the phosphorus, calcium, iron, copper, selenium and zinc (of the transformed data), controlling for age, sex, APOE status and diagnosis. (The correlation coefficient, r, is the top value in the matrix cell with the p-value below.) Phosphorus, copper and selenium have been square-root transformed, while calcium, iron and zinc were log-transformed.
Percentages of Plasma Elemental Concentrations
Of the six elements quantified, the percentages of phosphorus and calcium were much higher in the plasma than that for iron, zinc, copper and selenium (Table 4). Further, the percentage of phosphorus in the plasma was higher in AD than CN (p = 7.55 × 10−11, Table 4), whereas the percentage of calcium (p = 6.04 × 10−11), iron (9.85 × 10−9), zinc (p = 1.20 × 10−5), copper (p = 1.63 × 10−10) and selenium (p = 9.22 × 10−12) were lower in AD (Table 4). As phosphorus and calcium were at much higher levels than the other elements, their inclusion for the calculation of elemental percentages could potentially bias the analysis. Thus, the analysis was repeated but percentages of iron, zinc, copper and selenium were re-calculated with the exclusion of phosphorus and calcium. Again, plasma iron was significantly lower in the AD patients compared to CN subjects (Table 4; p = 0.028). However, zinc was higher in AD than CN (Table 4; p = 1.74 × 10−4), whereas plasma copper and selenium levels were comparable between CN and AD.
Logistic Regression Analysis
ROC based on the binary logistic regression model were used to determine the sensitivity of percentages of the elements in the plasma to predict CN or AD. The AUC of the base model (age, sex and APOEε4 genotype) was 0.643 (Fig. 3A), which was considerably improved by the addition of phosphorus (AUC = 0.930, p = 1.2 × 10−5; Fig. 3B) or calcium (AUC = 0.927, p = 1.6 × 10−5, Fig. 3C). The AUC also improved with addition of iron, copper or selenium (AUCs: 0.863, 0.912 and 0.937; p = 1.96 × 10−3, 6.00 × 10−5 and 6.00 × 10−5, respectively; Figs 2E,F and 3D). Zinc had no effect on the base model AUC (p = 0.120, Fig. 3G), while the phosphorus/calcium ratio significantly improved the AUC (0.926, p = 2 × 10−5, Fig. 3H).
Receiver operating curves (ROC) of binary logistic regression modelling of cognitively normal (CN) and Alzheimer’s Disease (AD) subjects. Base model includes age, sex, APOEε4 genotype (A) and addition of percentage phosphorus (B); calcium (C); iron (D), copper (E), selenium (F) and zinc (G), and phosphorus/calcium (H). AUC – Area under the curve.
Discussion
We report a pattern of increased percentage of phosphorus but decreased calcium, iron, copper, zinc and selenium in the plasma from AD subjects compared to CN. High absolute levels of phosphorus and calcium were best able to discriminate between demented and non-demented cases, especially phosphorus. We demonstrate strong interdependencies between plasma levels of phosphorus, calcium, iron, copper, selenium and zinc. These findings extend and support previous studies that suggest the involvement of metal dyshomeostasis in AD but that there appears an even greater contribution from those systems regulating phosphorus and calcium metabolism.
The higher plasma phosphorus (both absolute and percentage levels) observed in AD patients in our study is consistent with a recent study showing high serum phosphate was associated with increased risk of AD, vascular dementia and Lewy Body dementia6. Binary logistic regression modelling demonstrated the importance of phosphorus to distinguishing AD from CN subjects. Dementia is a recognised comorbidity with chronic kidney disease in which serum phosphate is often raised15. Accompanying elevated plasma phosphate is an increase in fibroblast growth factor-23 (FGF-23) which itself is associated with cerebral small vessel disease that strongly drives cognitive decline and dementia16. Serum phosphate and FGF-23 can be increased by increasing dietary intake17; food, especially processed foods, commonly contain phosphate additives18. High phosphorus intake itself is also associated with increased cardiovascular risk and mortality19. Interestingly, high serum phosphate has been shown to be independently associated with the pro-inflammatory cytokine, IL-620, with high phosphate intake inducing inflammation and increased serum TNFα in uremic rats21. Increased peripheral pro-inflammatory cytokines is characteristic of AD22 and high circulating phosphate may contribute to the increased inflammatory milieu in AD. Whether reducing phosphorus intake and lowering blood phosphate levels attenuates dementia remains to be investigated. Note that we have assumed that the elemental phosphorus measured by TXRF is mainly from phosphate, which is the usual form of phosphorus assessed by conventional clinical chemistry methods.
Increased plasma calcium in AD is concomitant with the increased phosphorus in our study, although decreased if expressed as a percentage of the panel of six elements which illustrates the difficulties in interpreting elemental concentrations. Alternatively, expressing the ratio of phosphorus/calcium may aid interpretation, with the ratio being higher in AD than CN. Calcium dyshomeostasis has been consistently implicated in AD23. Serum calcium levels are modulated by APOE, appearing to increase neuronal calcium influx to increase Aβ and associated with worse cognitive function in APOEε4 carriers, but this interaction is lost in APOEε2 carriers24. However, calcium changes can be mediated by non-APOE mechanisms. Plasma calcium levels are regulated by vitamin D and PTH. Hyperparathyroidism, characterised by elevated PTH, with high or elevated calcium, usually with low vitamin D levels, has been associated with impaired cognitive function and dementia25. Elevated blood phosphate and calcitriol (synthesized from vitamin D) deficiency may increase PTH secretion26 and explain the higher plasma calcium in AD observed in this study.
Lower percentage of plasma selenium in AD in this study is, consistent with previous studies. However, absolute levels were comparable between AD and CN or when comparing percentages calculated with exclusion of phosphorus and calcium, and explains the lack of consistency in alterations in blood selenium levels in AD27. Inclusion of selenium as a percentage of all elements studied gives the highest sensitivity and specificity to AD in this study. Selenium is often a cofactor in antioxidant molecules/pathways and the low peripheral levels in AD observed may contribute to increased susceptibility to oxidative stress, a major aspect of AD pathology. Indeed, a selenium and/or vitamin E supplementation trial had been undertaken to determine if supplementation can attenuate AD28. AD was not attenuated/prevented by selenium supplementation, but the study was underpowered, only involved men, and supplementation was of relatively short duration. However, selenium status has been shown to be determined by local geology and if sufficient, selenium has little impact on AD29.
Zinc has been reported to be decreased or unchanged in the serum and in CSF of AD patients30 but decreased serum zinc has been proposed to be due to ageing rather than AD31. Additionally, a recent ICP-MS study suggested serum zinc is increased with subjective memory complaint but decreased in MCI and AD14. Absolute zinc levels were comparable between CN and AD in this study but was significantly lower in the latter when normalised to the total concentrations of the panel of six elements. Conversely, zinc was higher in AD than CN if the percentage was calculated with the exclusion of phosphorus and calcium. The strong interdependences observed here and in other studies3,32 may contribute to the disparate findings in studies which usually only measure absolute levels of one or two metals. By considering panels of elements/metals rather than one or two of them, variations in exposure and nutrition may be accounted for somewhat (see below).
Anemia and attenuated hemoglobin levels are associated with an approximately two-fold increased risk for developing AD, establishing anemia as a risk factor for cognitive loss8,33. Significantly lower percentage plasma iron observed in the present study could arise due to deficient iron-loading into transferrin8,34 by dysfunctional ceruloplasmin, a ferroxidase that facilitates iron loading into transferrin35, reported in serum and CSF of AD (and PD) subjects36. Low peripheral iron levels may compromise hemoglobin production, leading to a significant decline in hemoglobin levels in AD8. Previous evidence has implicated transferrin and its receptor alleles as risk factors for AD37.
Copper dyshomeostasis has been implicated in AD, with reduced plasma copper and ceruloplasmin levels correlated to impaired cognitive performance in AD subjects38. Overexpression of APP in Tg2576 mice lead to copper deficiency, associated with impaired superoxide dismutase and ceruloplasmin prior to the appearance of amyloid neuropathology39. Copper supplementation has been proposed to be beneficial in AD by normalizing reduction of plasma copper38. In this study, we show that the percentage of copper is lower in AD than CN. However, absolute copper levels and copper percentages with the exclusion of phosphorus and calcium, were similar in CN and AD and consistent with larger published studies40.
Ageing is associated with a chronic inflammatory state, both peripherally and centrally and even more so, in AD21,22. Inflammation induces hepcidin secretion from the liver which promotes ferroportin degradation to increase iron retention in enterocytes and peripheral macrophages41,42. Thus, peripheral inflammation in AD may explain the decreased percentage of circulating iron levels observed in this study. Iron deficiency directly stimulates bone production and cleavage of FGF23 as well as via enhanced renal erythropoietin production17. Elevated peripheral inflammatory cytokines will also stimulate of FGF23 production and cleavage16 and as mentioned above, elevated FGF23 levels are associated with increased risks of cardiovascular disease and cognitive impairment/dementia.
Further research is required to understand the true meaning of plasma values in the context of AD. Considering the variability in exposure to environmental and/or nutritional factors (particularly in the context of malnourishment) which are significant variables, it is difficult to make precise conclusions on the liaison between the elements measured and AD. We and others2,3, have attempted to account for environmental factors and/or nutritional status to some degree by normalising values to a panel of elements. Changes in peripheral circulating levels of elements may reflect decreased or increased body intake/absorption and/or re-distribution between cellular and tissue compartments, e.g., increased brain accumulation. Our dataset does not include information on dietary intake of the various elements analysed in the study or their excretion from the body; any co-morbidities; and/or levels of proteins that integrate the metabolism of the elements measured. However, our cohort was age-matched, and sex balanced. There is also the concern that plasma mineral and metal measurements may reflect weight loss and malnutrition, common in AD, particularly at end stage disease. Thus, our patient cohort comprise only mild-moderate AD (MMSE ~ 20), where only 3% of individual would be expected to be malnourished43. We are unable to establish causality in this observational study but propose pathways worthy of further investigation in AD.
In the present study, we demonstrate altered distribution of minerals and metals in the plasma of AD and considerable interdependency in their circulating levels. Future studies should take the nutritional and environmental elemental exposure status into account to more accurately reflect the association between the elements and AD. We propose the plasma signature of high absolute levels of phosphorus and calcium, a high phosphorus/calcium ratio with a low percentage of iron may be indicative of cognitive impairment and/or AD. Further investigations are needed to determine if this pattern is common to dementia, useful for assessing the prodromal stage of AD, and/or monitoring AD progression.
Change history
16 April 2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Jack, C. R. Jr. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216, https://doi.org/10.1016/S1474-4422 (2013).
Gonzalez-Dominguez, R., Garcia-Barrera, T. & Gomez-Ariza, J. L. Homeostasis of metals in the progression of Alzheimer’s disease. Biometals 27, 539–549, https://doi.org/10.1007/s10534-014-9728-5 (2014).
Paglia, G. et al. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci Rep 6, 22769, https://doi.org/10.1038/srep22769 (2016).
Canzoniero, L. M. & Snider, B. J. Calcium in Alzheimer’s disease pathogenesis: too much, too little or in the wrong place? J Alzheimers Dis 8(147–154), 209–115 (2005).
Gilli, P. & De Bastiani, P. Cognitive function and regular dialysis treatment. Clin Nephrol 19, 188–192 (1983).
Li, T., Xie, Y., Bowe, B., Xian, H. & Al-Aly, Z. Serum phosphorus levels and risk of incident dementia. PLoS One 12, e0171377, https://doi.org/10.1371/journal.pone.0171377 (2017).
Landfield, P. W., Applegate, M. D., Schmitzer-Osborne, S. E. & Naylor, C. E. Phosphate/calcium alterations in the first stages of Alzheimer’s disease: implications for etiology and pathogenesis. J Neurol Sci 106, 221–229 (1991).
Faux, N. G. et al. An anemia of Alzheimer’s disease. Mol Psychiatry 19, 1227–1234, https://doi.org/10.1038/mp.2013.178 (2014).
Stosnach, H. Environmental trace-element analysis using a benchtop total reflection X-ray fluorescence spectrometer. Anal Sci 21, 873–876 (2005).
Liu, Y., Imashuku, S. & Kawai, J. Multi-element analysis by portable total reflection X-ray fluorescence spectrometer. Anal Sci 29, 793–797 (2013).
Szoboszlai, N., Polgari, Z., Mihucz, V. G. & Zaray, G. Recent trends in total reflection X-ray fluorescence spectrometry for biological applications. Anal Chim Acta 633, 1–18, https://doi.org/10.1016/j.aca.2008.11.009 (2009).
Wobrauschek, P. Use of total reflection X-ray fluorescence analysis in the life sciences. Biol Trace Elem Res 43-45, 65–71 (1994).
Lovestone, S. et al. AddNeuroMed–the European collaboration for the discovery of novel biomarkers for Alzheimer’s disease. Ann N Y Acad Sci 1180, 36–46, https://doi.org/10.1111/j.1749-6632.2009.05064.x (2009).
Goulle, J. P. et al. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci Int 153, 39–44, https://doi.org/10.1016/j.forsciint.2005.04.020 (2005).
Bugnicourt, J. M., Godefroy, O., Chillon, J. M., Choukroun, G. & Massy, Z. A. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 24, 353–363, https://doi.org/10.1681/ASN.2012050536 (2013).
Wright, C. B. et al. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS). Neurology 82, 1700–1706, https://doi.org/10.1212/WNL.0000000000000410 (2014).
Ferrari, S. L., Bonjour, J. P. & Rizzoli, R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90, 1519–1524, https://doi.org/10.1210/jc.2004-1039 (2005).
Leon, J. B., Sullivan, C. M. & Sehgal, A. R. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr 23, 265–270 e262, https://doi.org/10.1053/j.jrn.2012.12.003 (2013).
Chang, A. R., Lazo, M., Appel, L. J., Gutierrez, O. M. & Grams, M. E. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am J Clin Nutr 99, 320–327, https://doi.org/10.3945/ajcn.113.073148 (2014).
Navarro-Gonzalez, J. F. & Mora-Fernandez, C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19, 433–442, https://doi.org/10.1681/ASN.2007091048 (2008).
Yamada, S. et al. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am J Physiol Renal Physiol 306, F1418–1428, https://doi.org/10.1152/ajprenal.00633.2013 (2014).
Swardfager, W. et al. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68, 930–941, https://doi.org/10.1016/j.biopsych.2010.06.012 (2010).
Thibault, O., Gant, J. C. & Landfield, P. W. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell 6, 307–317, https://doi.org/10.1111/j.1474-9726.2007.00295.x (2007).
Veinbergs, I., Everson, A., Sagara, Y. & Masliah, E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. J Neurosci Res 67, 379–387, https://doi.org/10.1002/jnr.10138 (2002).
Papageorgiou, S. G. et al. Dementia as presenting symptom of primary hyperparathyroidism: favourable outcome after surgery. Clin Neurol Neurosurg 110, 1038–1040, https://doi.org/10.1016/j.clineuro.2008.06.004 (2008).
Moe, S. M. Disorders involving calcium, phosphorus, and magnesium. Prim Care 35, 215–237, v–vi, https://doi.org/10.1016/j.pop.2008.01.007 (2008).
Loef, M., Schrauzer, G. N. & Walach, H. Selenium and Alzheimer’s disease: a systematic review. J Alzheimers Dis 26, 81–104, https://doi.org/10.3233/JAD-2011-110414 (2011).
Kryscio, R. J. et al. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 74, 567–573, https://doi.org/10.1001/jamaneurol.2016.5778 (2017).
B., R. C. et al. The APOE epsilon4 Allele Is Associated with Lower Selenium Levels in the Brain: Implications for Alzheimer’s Disease. ACS Chem Neurosci 8, 1459–1464, https://doi.org/10.1021/acschemneuro.7b00014 (2017).
Li, D. D., Zhang, W., Wang, Z. Y. & Zhao, P. Serum Copper, Zinc, and Iron Levels in Patients with Alzheimer’s Disease: A Meta-Analysis of Case-Control Studies. Front Aging Neurosci 9, 300, https://doi.org/10.3389/fnagi.2017.00300 (2017).
Rembach, A. et al. Decreased serum zinc is an effect of ageing and not Alzheimer’s disease. Metallomics 6, 1216–1219, https://doi.org/10.1039/c4mt00060a (2014).
Gonzalez, C. et al. Serum zinc, copper, insulin and lipids in Alzheimer’s disease epsilon 4 apolipoprotein E allele carriers. Eur J Clin Invest 29, 637–642 (1999).
Shah, R. C., Buchman, A. S., Wilson, R. S., Leurgans, S. E. & Bennett, D. A. Hemoglobin level in older persons and incident Alzheimer disease: prospective cohort analysis. Neurology 77, 219–226, https://doi.org/10.1212/WNL.0b013e318225aaa9 (2011).
Hare, D. J. et al. Decreased plasma iron in Alzheimer’s disease is due to transferrin desaturation. ACS Chem Neurosci 6, 398–402, https://doi.org/10.1021/cn5003557 (2015).
Osaki, S., Johnson, D. A. & Frieden, E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem 241, 2746–2751 (1966).
Kristinsson, J., Snaedal, J., Torsdottir, G. & Johannesson, T. Ceruloplasmin and iron in Alzheimer’s disease and Parkinson’s disease: a synopsis of recent studies. Neuropsychiatr Dis Treat 8, 515–521, https://doi.org/10.2147/NDT.S34729 (2012).
Lehmann, D. J. et al. Transferrin and HFE genes interact in Alzheimer’s disease risk: the Epistasis Project. Neurobiol Aging 33, 202 e201–213, https://doi.org/10.1016/j.neurobiolaging.2010.07.018 (2012).
Kessler, H. et al. Cerebrospinal fluid diagnostic markers correlate with lower plasma copper and ceruloplasmin in patients with Alzheimer’s disease. J Neural Transm (Vienna) 113, 1763–1769, https://doi.org/10.1007/s00702-006-0485-7 (2006).
Maynard, C. J. et al. Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem 277, 44670–44676, https://doi.org/10.1074/jbc.M204379200 (2002).
Rembach, A. et al. Decreased copper in Alzheimer’s disease brain is predominantly in the soluble extractable fraction. Int J Alzheimers Dis 2013, 623241, https://doi.org/10.1155/2013/623241 (2013).
Ashraf, A., Clark, M. & So, P. W. The Aging of Iron Man. Front Aging Neurosci 10, 65, https://doi.org/10.3389/fnagi.2018.00065 (2018).
Ganz, T. Hepcidin and the global burden of iron deficiency. Clin Chem 61, 577–578, https://doi.org/10.1373/clinchem.2014.229179 (2015).
Guerin, O. et al. Nutritional status assessment during Alzheimer’s disease: results after one year (the REAL French Study Group). J Nutr Health Aging 9, 81–84 (2005).
Acknowledgements
The authors would like to thank Biotechnology and Biological Sciences Research Council (BBSRC), King’s College London and Perspectum Diagnostics Ltd for funding Azhaar Ashraf’s industrial PhD studentship. All data are provided in the supplemental file of this paper in accordance with UK Research Councils’ common principles on Data Policy. Also, we would like to thank the Wellcome Trust for funding the London Metallomics Facility (grant reference 202902/Z/16/Z). This work was supported by the Alzheimer’s Research UK King’s College London Network Centre. Samples were collected by the AddNeuroMed consortium which is supported by InnoMed, an Integrated Project funded by the European Union Sixth Framework programme priority FP6‐2004‐LIFESCIHEALTH‐5, Life Sciences, Genomics and Biotechnology for Health.
Author information
Authors and Affiliations
Author notes
A comprehensive list of consortium members appears at the end of the paper.
Consortia
Contributions
P.-W.S. and J.P. conceived the study and implemented the experimental design. A.H. and S.L. provided samples and clinical information. H.S. acquired the TXRF data. A.A. and H.G.P. performed data analysis. P.-W.S. and A.A. wrote the main manuscript text.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ashraf, A., Stosnach, H., Parkes, H.G. et al. Pattern of Altered Plasma Elemental Phosphorus, Calcium, Zinc, and Iron in Alzheimer’s Disease. Sci Rep 9, 3147 (2019). https://doi.org/10.1038/s41598-018-37431-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37431-8
- Springer Nature Limited
This article is cited by
-
Multi-element Exposure and Cognitive Function in Rural Elderly Chinese
Biological Trace Element Research (2024)
-
Predicting the Cognitive Ability of Young Women Using a New Feature Selection Algorithm
Journal of Molecular Neuroscience (2023)
-
Associations Between Mild Cognitive Impairment and Whole Blood Zinc and Selenium in the Elderly Cohort
Biological Trace Element Research (2023)