Abstract
Genome-wide association studies (GWAS) show that many common alleles confer risk for developing Alzheimer’s disease (AD). These risk loci may contribute to MRI alterations in young individuals, preceding the clinical manifestations of AD. Prior evidence identifies vascular dysregulation as the earliest marker of disease progression. However, it remains unclear whether cerebrovascular function (measured via grey-matter cerebral blood flow (gmCBF)) is altered in young individuals with increased AD genetic risk. We establish relationships between gmCBF with APOE and AD polygenic risk score in a young cohort (N = 75; aged: 19–32). Genetic risk was assessed via a) possessing at least one copy of the APOE ɛ4 allele and b) a polygenic risk score (AD-PRS) estimated from AD-GWAS. We observed a reduction in gmCBF in APOE ɛ4 carriers and a negative relationship between AD-PRS and gmCBF. We further found regional reductions in gmCBF in individuals with higher AD-PRS across the frontal cortex (PFWE < 0.05). Our findings suggest that a larger burden of AD common genetic risk alleles is associated with attenuated cerebrovascular function, during young adulthood. These results suggest that cerebral vasculature is a mechanism by which AD risk alleles confer susceptibility.
Similar content being viewed by others
Introduction
Genome-wide association studies (GWAS) have demonstrated that Alzheimer’s disease (AD) has a highly polygenic basis, where potentially thousands of common risk alleles confer susceptibility1. Although the polygenic architecture has shown utility in predicting AD2,3, the neurobiological mechanisms by which these loci confer risk remain poorly understood. Imaging genetics can reveal the neurobiological mechanisms by which genetic loci confer risk for AD. For example, several studies show that common variation in the APOE, CLU, PICALM and BIN1 genes are associated with AD may influence the structure and function of the human brain decades before disease onset4,5,6,7,8,9,10. Although these AD risk alleles are associated with changes in brain volume and blood oxygen level dependent (BOLD)11, little is known about how they are linked to global cerebrovascular function in young asymptomatic individuals.
Cerebrovascular function has been linked to key AD biological pathways such as i) cholesterol metabolism and blood brain barrier permeability12,13 and ii) arteriosclerosis of vessels14. Pivotally, recent evidence suggests that vascular dysregulation is the earliest marker of AD progression, in a cohort of 40–70 year old individuals15. In addition, several initial studies have demonstrated that the presence of AD risk alleles such as APOE ε4 or familial risk of AD are associated with alterations in neurovasculature, where older individuals with risk for developing AD show reduced cerebral blood flow (CBF)16,17,18,19. However, no study has yet explored how the comprehensive polygenetic architecture of AD is linked to cerebrovascular function in young asymptomatic individuals. The observations from such a study could identify links between genetic risk loci that may disrupt cerebrovascular health later in life as a mechanism of increased AD susceptibility.
In the current study, we sought to extend the findings of recent evidence examining vascular dysregulation as one of the earliest markers of disease progression in AD15 by examining vascular effects in young individuals who have increased AD genetic risk. Specifically, we sought to explore the cumulative effects of both APOE and all the other common genetic risk loci (as measured using an AD-PRS) identified by recent GWAS1. We had two key hypotheses. First, given that reductions in grey matter CBF (gmCBF) have been observed in older individuals who possess at least one copy of the ɛ4 allele, we predict this effect would also be observed in young ɛ4 carriers. Second, we anticipated that as cumulative risk for developing AD increased, we would also observe a decrease in gmCBF. Moreover, we suggest that the alterations in gmCBF observed in AD populations15 may be partly influenced by common genetic AD risk factors. We anticipate that the combined effect of these risk alleles can be observed in asymptomatic individuals.
Results
Demographic analysis
Consistent with prior epidemiological reports20, females had higher resting gmCBF than males (Table 1). There were no significant associations between AD-PRS and age (P > 0.09, in all cases) or gender (P > 0.1, in all cases). Grey matter CBF, GMV and AD-PRS were normally distributed (Shapiro test; P > 0.1 in all cases). APOE ɛ4 status was not related to GMV, composite cognition or any of the seven cognitive subdomain measures (P > 0.1, in all cases; see Table 2). Similarly, AD-PRS was also not related to GMV or any cognitive measures (P > 0.1, in all cases).
Genetic analysis of whole brain gmCBF
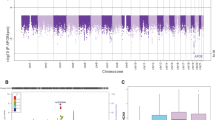
The linear regression revealed a significant association between gmCBF and both APOE (β = −0.213, P = 0.044) and AD-PRS (β = −0.232, P = 0.031) controlling for age, gender and GMV (see Table 1 for full regression model; Fig. 1a,b for effect size estimates). To control for population stratification effects, we repeated the regression, including the first five principle components (PCAs) from the LD-pruned genotype data. Including these additional five covariates did not significantly affect the association between gmCBF and AD-PRS (β = −0.251, P = 0.022). A SNP-wise post-hoc analysis of AD-PRS showed that the majority of alleles over-represented in the AD population (risk alleles) were associated with reduced gmCBF, while alleles over-represented in the healthy population (protective alleles) were mostly associated with increased gmCBF (Fig. 1c). This assumption was confirmed via a sign test (13/17 SNPs with expected direction, P < 0.05). Consistent with an additive polygenic model, no single SNP appeared to drive the AD-PRS effects. Post-hoc analysis of AD-PRS with progressively liberal thresholds demonstrated similar relationships at PT < 5 × 10−6 (β = −0.22, P = 0.045) but not at PT < 5 × 10−4 (β = −0.03, P = 0.74) and PT < 5 × 10−2 (β = 0.01, P = 0.91).
Results for both the APOE status group difference in gmCBF and the association between AD-PRS and gmCBF. (a) Total gmCBF (grey matter cerebral blood flow) stratified by presence of APOE ε4 isoform; (b) association between gmCBF and AD-PRS. R2 and p-values estimated from linear regression, controlling for age, gender, GMV and PRS (in (a)) or APOE (in (b)). (c) Reflects the individual contributions to gmCBF for all SNPs within the AD-PRS (NSNPS = 17), controlling for covariates. CIs = confidence intervals.
Genetic analysis of grey matter density
We found no other evidence of association between APOE status or AD-PRS and grey matter density in a voxel-based search across the brain (PFWE > 0.1, in all cases).
Post-hoc exploratory regional analysis
We conducted a post-hoc exploratory voxel-wide analysis across gmCBF. All voxel-based analyses were estimated accounting for both APOE and AD-PRS (including aforementioned covariates). APOE ε4 status was not regionally associated with CBF in any voxels after correction for multiple comparisons (PFWE > 0.05). However, AD-PRS was significantly associated with gmCBF in several prefrontal regions, which were significant after controlling for multiple comparisons (PFWE < 0.05). All clusters were located in the frontal lobe (including the frontal pole, middle frontal gyrus, inferior frontal gyrus, insular, frontal medial cortex & orbitofrontal cortex; see Fig. 2).
Voxel wide analysis of gmCBF for AD-PRS. Regional association between gmCBF and AD-PRS across all grey matter. All clusters that remain (red-yellow) reflect voxels that survived correction across the whole brain (using threshold free cluster enhancement) with permutation testing (N = 5000) (PFWE < 0.05). Color-bar represents regional t-statistic.
Discussion
We find negative associations between i) APOE ε4 status and ii) AD-PRS with gmCBF, providing evidence that an increasing number common AD genetic risk loci is associated with reduction in resting gmCBF in young asymptomatic individuals. These findings support observations between APOE and reductions in resting gmCBF in older individuals21. We also provide novel evidence that the GWAS significant common AD risk loci identified via GWAS were negatively associated with gmCBF. Our post-hoc observations between AD-PRS in frontal regions conforms with preclinical models demonstrating that reduced frontal gmCBF is linked to genetic risk22, and may aid in the understanding of future cognitive decline23. Together, these analyses provide support for a broader hypothesis that AD genetic risk loci may confer susceptibility via alterations in cerebrovascular health, decades before the onset of clinical symptoms. This is also evidenced by several AD-PRS imaging studies showing associations between an AD-PRS and MRI based measures of brain health, including macrostructure, white matter microstructure and blood oxygen level dependency (BOLD) during memory processing in the medial temporal lobe24,25,26,27,28.
These findings conform to pre-clinical models of AD where functional impairments such as widespread alterations in cerebral blood flow precede grey matter atrophy and/or cognitive impairments15. Our findings can also be assessed in light of other recent evidence showing that reduced CBF is associated with a reduction in cognitive function in older individuals20. In the current study, we find reduced gmCBF in young asymptomatic individuals suggesting that vascular alterations are present prior to the onset of symptoms, supporting recent research conducted in an older sample15. However, putative dynamic relationships between AD-PRS and brain imaging traits linked to AD across the lifespan remain largely untested. Furthermore, it is critical that future research investigates the precise nature of this alteration and assesses the relationship between gmCBF and oxygen dynamics including cerebral metabolic rate of oxygen (CMRO2), oxygen extraction fraction (OEF) and oxygen diffusivity (for methods see29,30,31,32).
Our findings should be taken with the following considerations. We appreciate that while this is a large imaging sample, it is relatively small for a genetic study and further investigations should aim to a) replicate these observations to confirm these associations in younger individuals and b) expand population level imaging protocols to vascular measures. However, considering the moderate impact of both APOE and AD-PRS, we suggest that this study was adequately powered to detect the cumulative effect of these risk factors. Lastly, as the APOE status and AD-PRS effects were in the same direction, we suggest that cumulative genetic risk may be more broadly related to lower gmCBF. Lastly, as we did not see a relationship between AD-PRS and gmCBF at more liberal p-thresholds, suggesting the precise genetic architecture of AD that contributes to imaging measures remains unclear.
Our observations highlight possible therapeutic benefits of exercise (and associated increases in CBF) for neurodegenerative disease33. While evidence suggests that exercise may improve cerebrovascular health, the longitudinal effects of exercise as a therapeutic tool for AD remain unknown. Furthermore, dietary supplements such as nitrates have been shown to increase CBF in healthy adults34. Thus, future research should investigate the beneficial effects of such a supplement for increasing gmCBF in individuals with high genetic risk of developing AD.
In conclusion, we observed an association between reduced CBF and AD genetic risk loci (specifically – evidence for the involvement of APOE and independent top GWAS risk loci). Future studies may be able to investigate biologically informed pathways to understand how AD imaging phenotypes are influenced by genetic risk. We suggest that CBF may be a useful tool in understanding how genetic risk for AD may affect the human brain in young individuals and has implications for functional MRI studies that relate BOLD and models of neurovascular coupling.
Methods
Participants
One hundred healthy, right-handed individuals were recruited via advertisement from Cardiff University. Participants reported no history of psychiatric or neurological illness, and were not taking any psychotropic medication. The study was approved by the ethics committee of the School of Psychology, Cardiff University. Each participant provided written informed consent. Participants were excluded from the study if they were over 35 (n = 2), failed genotyping quality control (n = 12) or failed quality control for the imaging procedure (n = 9). In addition, those who possessed the APOE ε2ε4 isoform (n = 2) were also excluded due to the presence of the protective ε2 allele, as previously described27. Our final sample consisted of 75 individuals, 54 of whom possessed no copy of the ε4 allele (pooled: ε2ε3 & ε3ε3) and 21 individuals who possessed at least one copy of the ε4 allele (pooled: ε3ε4 & ε4ε4). There was no association between APOE status with age, gender, grey matter volume (GMV) or AD-PRS (Table 1). APOE status and AD-PRS were entered into the regression model together (see statistical analysis for further details).
Genotyping and extraction of DNA
Genomic DNA was obtained from saliva using Oragene OG-500 saliva kits (DNA Genotek, Inc., Ontario, Canada). Genotyping was performed using custom Illumina. HumanCoreExome-24 BeadChip genotyping arrays, which contain approximately 500,000 common genetic variants (Illumina, Inc., San Diego, CA). Quality control and imputation were implemented in plink 1.935. Briefly, individuals were excluded for any of the following reasons: 1) ambiguous sex (where samples with undetermined X chromosome heterozygosity were excluded); 2) cryptic relatedness up to third-degree relatives as ascertained using identity by descent; 3) genotyping completeness less than 98%; 4) non-European ethnicity admixture which was determined via population stratification, where samples that clustered outside the CEU HapMap3 population using principal component analysis were excluded); and 5) outliers from an autosomal heterozygosity filter. Single nucleotide polymorphisms (SNPs) were excluded where the minor allele frequency was less than 1%, if the call rate was less than 98%, or if the χ2 test for Hardy-Weinberg equilibrium had a p value less than 1e-6. A total of 233054 genotyped SNPs remained after quality control. Autosomal chromosomes were imputed using the reference panel HRCv1.1 (hrc.r1.1.2016) using a mixed population panel36. Phasing was completed using Eagle v2.337 and imputation was performed using Mimimac338. Imputed data was converted to best-guess genotypes35 with multi-allelic sites removed. SNP filters for HWE (1e-6) and minor allele frequency (1%) were re-applied. SNP ids were updated from chr:bp to rsIDs using dbsnp_138.b37.vcf. A total of 7545595 imputed SNPs for consideration in AD-PRS analysis.
Creation of polygenic scores
Polygenic score calculations were performed according to the procedure described by the International Schizophrenia Consortium39. Training data were from the most recent AD GWAS1. These data are publicly available from http://www.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php. SNPs were removed from all analyses if they had a low minor allele frequency (P < 0.01). Subsequently, the data were pruned for linkage disequilibrium using the clumping function (–clump) in plink 1.935 removing SNPs within 500 kilobase (–clump-kb) and R2 > 0.25 (–clump-r2) with a more significantly associated SNP. We used the –score command in PLINK to calculate polygenic score. For the creation of the AD-PRS, we considered SNPs that were associated with AD that surpassed the GWAS threshold (PT < 5 × 10−8). We chose a conservative P-threshold as prior fMRI neuroimaging studies have demonstrated that conservative P-thresholds have been successful in predicting blood oxygenation level dependent (BOLD) MRI measures linked to AD28. As we aimed to understand the contribution of SNPs outside the APOE locus, we employed a conservative approach, where we removed all SNPs from chromosome 19 before creating an AD-PRS. We aimed to remove any confounding signal from variants in linkage disequilibrium (LD) with the APOE locus. A total of 17 SNPs were considered in the AD-PRS calculation. Removing the entire APOE locus (removing chr19: 45.053–45.73 Mb, rather than the whole of chromosome 19; NSNPS = 19) did not significantly affect our results. We further created 3 additional AD-PRS at progressively liberal P-thresholds to explore optimal model performance (PT < 5 × 10−6; PT < 5 × 10−4; PT < 5 × 10−2). Although our sample predominately included individuals of western European descent, we also aimed to ensure that the population stratification was not accounting for variation linked to AD-PRS. We therefore extracted the first five principle components from the linkage-disequilibrium (LD) pruned genotypes and included these as covariates in the analysis.
Imaging procedure
Imaging data were collected on a 3 Tesla General Electric (GE) scanner. Anatomical T1-weighted images were acquired with a 3D fast spoiled gradient echo sequence (FSPGR) (parameters: 172 contiguous sagittal slices with a slice thickness of 1 mm, TR = 7.9, TE = 3 ms, inversion time of 450 ms, flip angle = 20°, a FOV of 256 × 256 × 176 mm, matrix size 256 × 256 × 192 to yield 1 mm isotropic voxel resolution images).
Resting CBF data was collected using a pseudo-continuous arterial spin labelling (PCASL) sequence40. The study consisted of a single MRI session (which also comprised other functional and structural scans), and the PCASL sequence lasted approximately 6 minutes. Resting-state CBF maps (rCBF) and whole brain CBF (wbCBF) measurements were created with a PCASL sequence identical to the one previously described. Parameters included a 1.5 second tag and a post-labelling delay of 1.5 seconds. The images for the PCASL sequence were acquired using a 3D fast spin echo (FSE) spiral multi-slice readout (parameters: number of excitations = 3, time to echo = 32 ms, echo time train length = 64, TR = 5.5 seconds, matrix size = 48 × 64 × 60, FOV = 18 × 23 × 18 cm).
Grey matter CBF (gmCBF) analysis
Recent evidence suggests vascular dysfunction in AD is uniformly reduced across the brain15, therefore we looked at whether this is also the case in young individuals with increased cumulative risk for developing AD. This was conducted by extracting mean CBF from each grey matter image.
Briefly, grey matter CBF was measured in native space for each of the 75 individuals. First, anatomical T1 weighted FSPGR images were registered to the M0 image acquired as part of the calibration of the CBF image acquisition, generating a transformation matrix. This transformation matrix was then applied to the skull stripped T1w anatomical (with reference/warping to the M0) using FSL’s Brain Extraction Tool41. From here, we used linear registration FSL’s FLIRT42,43 to register the skull stripped anatomical image to the M0 transformation matrix (Montreal Neurological Institute (MNI) space) and the difference was calculated between this and the subjects native space, providing data in the same space as the CBF data. The two transformation matrices for each participant were then concatenated to produce a matrix for the low resolution CBF image. All CBF images were then warped to standard MNI template using FSL’s FLIRT41,42. The priors for the grey matter were then registered to the skull stripped M0 image, creating a mask of each individual’s grey matter where CBF values could be extracted. Grey matter volume (GMV) was approximated as the mean intensity of the segmented grey matter image. See Fig. 3 for a) the mean gmCBF of the cohort and b) for APOE ɛ4- and c) APOE ɛ4+ .
Voxel based morphometry
Regional grey matter density was estimated for each individual. Briefly, structural data was analysed with FSL-VBM44, (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimised VBM protocol45 carried out with FSL tools42. First, structural images were brain-extracted and grey matter-segmented before being registered to the MNI 152 standard space using non-linear registration. The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific grey matter template. Second, all native grey matter images were non-linearly registered to this study-specific template and “modulated” to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated grey matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. These images were entered into same regression models as the standardised gmCBF images.
Power analysis
Current estimates suggest that the combination of APOE locus and GWAS loci (NSNPS = 20) classify AD cases and controls with an accuracy of 66%, assuming a lifetime prevalence of 2%2, equating to a moderate effect size (Cohen’s D = 0.583; r = 0.28)46. Based upon this anticipated effect size, we had approximately 69% power to detect an combined effect of these AD risk loci (N = 75, α = 0.05), calculated with ‘pwr’ in R47.
Cognitive instruments
We assessed cognition via the MATRICS Consensus Cognitive Battery (MCCB), which measures composite cognition across seven sub-domains including working memory, attention, speed of processing, verbal and visual learning and social cognition48.
Statistical analysis
Linear regression models were employed in R (https://www.r-project.org/; version 3.1.3) to assess the impact of both APOE and AD-PRS risk on whole brain grey matter CBF. Whole brain grey matter CBF was introduced as our dependent variable where APOE and AD-PRS were added together as regressors. Age, gender and grey matter volume (GMV) were included as covariates in the analyses. A post-hoc analysis was repeated using the aforementioned linear regression approach (using the same covariates) and was conducted at a voxel-wise level to explore regional effects of APOE and AD-PRS on gmCBF. For whole brain analysis (gmCBF and GMV), the family-wise error rate was controlled with nonparametric permutation testing (5000 permutations) and TFCE (threshold free cluster enhancement) which effectively controls for multiple comparisons, compared to cluster extent thresholding49.
Approval and accordance
The study was approved by the ethics committee of the School of Psychology, Cardiff University. Methods were carried out in accordance with guidelines and regulations.
Informed consent
Informed consent was obtained for all procedures used for this study.
Data Availability
Analysis code and data can be made available upon request to Cardiff University Brain Research Imaging Centre.
References
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45, 1452–1458, https://doi.org/10.1038/ng.2802 (2013).
Escott-Price, V., Shoai, M., Pither, R., Williams, J. & Hardy, J. Polygenic score prediction captures nearly all common genetic risk for Alzheimer’s disease. Neurobiol Aging 49, 214 e217–214 e211, https://doi.org/10.1016/j.neurobiolaging.2016.07.018 (2017).
Escott-Price, V. et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 138, 3673–3684, https://doi.org/10.1093/brain/awv268 (2015).
Zhang, X. et al. Bridging Integrator 1 (BIN1) Genotype Effects on Working Memory, Hippocampal Volume, and Functional Connectivity in Young Healthy Individuals. Neuropsychopharmacology 40, 1794–1803, https://doi.org/10.1038/npp.2015.30 (2015).
Zhang, P. et al. Impacts of PICALM and CLU variants associated with Alzheimer’s disease on the functional connectivity of the hippocampus in healthy young adults. Brain structure & function 220, 1463–1475, https://doi.org/10.1007/s00429-014-0738-4 (2015).
Trachtenberg, A. J. et al. The effects of APOE on the functional architecture of the resting brain. Neuroimage 59, 565–572, https://doi.org/10.1016/j.neuroimage.2011.07.059 (2012).
Filippini, N. et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 106, 7209–7214, https://doi.org/10.1073/pnas.0811879106 (2009).
Lancaster, T. M. et al. Alzheimer’s disease risk variant in CLU is associated with neural inefficiency in healthy individuals. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 11, 1144–1152, https://doi.org/10.1016/j.jalz.2014.10.012 (2015).
Lancaster, T. M. et al. Neural hyperactivation in carriers of the Alzheimer’s risk variant on the clusterin gene. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 21, 880–884, https://doi.org/10.1016/j.euroneuro.2011.02.001 (2011).
Foley, S. F. et al. Multimodal Brain Imaging Reveals Structural Differences in Alzheimer’s Disease Polygenic Risk Carriers: A Study in Healthy Young Adults. Biol Psychiatry 81, 154–161, https://doi.org/10.1016/j.biopsych.2016.02.033 (2017).
Prvulovic, D., Van de Ven, V., Sack, A. T., Maurer, K. & Linden, D. E. Functional activation imaging in aging and dementia. Psychiatry Res 140, 97–113, https://doi.org/10.1016/j.pscychresns.2005.06.006 (2005).
Methia, N. et al. ApoE deficiency compromises the blood brain barrier especially after injury. Molecular medicine 7, 810–815 (2001).
Jones, L. et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One 5, e13950, https://doi.org/10.1371/journal.pone.0013950 (2010).
Yip, A. G. et al. APOE, vascular pathology, and the AD brain. Neurology 65, 259–265, https://doi.org/10.1212/01.wnl.0000168863.49053.4d (2005).
Iturria-Medina, Y. et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature communications 7, 11934, https://doi.org/10.1038/ncomms11934 (2016).
Fleisher, A. S. et al. Cerebral perfusion and oxygenation differences in Alzheimer’s disease risk. Neurobiol Aging 30, 1737–1748, https://doi.org/10.1016/j.neurobiolaging.2008.01.012 (2009).
Wierenga, C. E. et al. Interaction of age and APOE genotype on cerebral blood flow at rest. J Alzheimers Dis 34, 921–935, https://doi.org/10.3233/JAD-121897 (2013).
Wierenga, C. E. & Bondi, M. W. Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychology review 17, 127–143, https://doi.org/10.1007/s11065-007-9025-y (2007).
Suri, S. et al. Reduced cerebrovascular reactivity in young adults carrying the APOE epsilon4 allele. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 11, 648–657 e641, https://doi.org/10.1016/j.jalz.2014.05.1755 (2015).
Wolters, F. J. et al. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.117.027448 (2017).
Filippini, N. et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 54, 602–610, https://doi.org/10.1016/j.neuroimage.2010.08.009 (2011).
Kim, S. M. et al. Regional cerebral perfusion in patients with Alzheimer’s disease and mild cognitive impairment: effect of APOE epsilon4 allele. Neuroradiology 55, 25–34, https://doi.org/10.1007/s00234-012-1077-x (2013).
Chao, L. L. et al. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer disease and associated disorders 24, 19–27, https://doi.org/10.1097/WAD.0b013e3181b4f736 (2010).
Biffi, A. et al. Genetic variation and neuroimaging measures in Alzheimer disease. Arch Neurol 67, 677–685, https://doi.org/10.1001/archneurol.2010.108 (2010).
Harrison, T. M. et al. An Alzheimer’s Disease Genetic Risk Score Predicts Longitudinal Thinning of Hippocampal Complex Subregions in Healthy Older Adults. eNeuro 3, https://doi.org/10.1523/ENEURO.0098-16.2016 (2016).
Mormino, E. C. et al. Polygenic risk of Alzheimer disease is associated with early- and late-life processes. Neurology 87, 481–488, https://doi.org/10.1212/WNL.0000000000002922 (2016).
Lupton, M. K. et al. The effect of increased genetic risk for Alzheimer’s disease on hippocampal and amygdala volume. Neurobiol Aging 40, 68–77, https://doi.org/10.1016/j.neurobiolaging.2015.12.023 (2016).
Xiao, E. et al. Late-Onset Alzheimer’s Disease Polygenic Risk Profile Score Predicts Hippocampal Function. Biological psychiatry. Cognitive neuroscience and neuroimaging 2, 673–679, https://doi.org/10.1016/j.bpsc.2017.08.004 (2017).
Germuska, M. & Bulte, D. P. MRI measurement of oxygen extraction fraction, mean vessel size and cerebral blood volume using serial hyperoxia and hypercapnia. Neuroimage 92, 132–142, https://doi.org/10.1016/j.neuroimage.2014.02.002 (2014).
Jain, V., Langham, M. C. & Wehrli, F. W. MRI estimation of global brain oxygen consumption rate. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 30, 1598–1607, https://doi.org/10.1038/jcbfm.2010.49 (2010).
Wise, R. G., Harris, A. D., Stone, A. J. & Murphy, K. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. Neuroimage 83, 135–147, https://doi.org/10.1016/j.neuroimage.2013.06.008 (2013).
Germuska, M. et al. Dual-calibrated fMRI measurement of absolute cerebral metabolic rate of oxygen consumption and effective oxygen diffusivity. Neuroimage 184, 717–728, https://doi.org/10.1016/j.neuroimage.2018.09.035 (2018).
Lucas, S. J., Cotter, J. D., Brassard, P. & Bailey, D. M. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 35, 902–911, https://doi.org/10.1038/jcbfm.2015.49 (2015).
Wightman, E. L. et al. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiology & behavior 149, 149–158, https://doi.org/10.1016/j.physbeh.2015.05.035 (2015).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7, https://doi.org/10.1186/s13742-015-0047-8 (2015).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 48, 1279–1283, https://doi.org/10.1038/ng.3643 (2016).
Loh, P. R. et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet 48, 1443–1448, https://doi.org/10.1038/ng.3679 (2016).
Das, S. et al. Next-generation genotype imputation service and methods. Nat Genet 48, 1284–1287, https://doi.org/10.1038/ng.3656 (2016).
International Schizophrenia, C. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752, https://doi.org/10.1038/nature08185 (2009).
Dai, W., Garcia, D., de Bazelaire, C. & Alsop, D. C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine 60, 1488–1497, https://doi.org/10.1002/mrm.21790 (2008).
Smith, S. M. Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155, https://doi.org/10.1002/hbm.10062 (2002).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1), S208–219, https://doi.org/10.1016/j.neuroimage.2004.07.051 (2004).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Douaud, G. et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130, 2375–2386, https://doi.org/10.1093/brain/awm184 (2007).
Good, C. D. et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36, https://doi.org/10.1006/nimg.2001.0786 (2001).
Ruscio, J. A probability-based measure of effect size: robustness to base rates and other factors. Psychological methods 13, 19–30, https://doi.org/10.1037/1082-989X.13.1.19 (2008).
Champely, S. Pwr: Basic functions for power analysis. R package version 1.1.1, http://CRAN.R-project.org/package=pwr (2012).
Nuechterlein, K. H. et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165, 203–213, https://doi.org/10.1176/appi.ajp.2007.07010042 (2008).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98, https://doi.org/10.1016/j.neuroimage.2008.03.061 (2009).
Acknowledgements
This study was supported by the National Centre for Mental Health at Cardiff University with funds from the National Institute for Social Care and Health Research (NISCHR) and the Medical Research Council (grant number; MR/K013041/1). We are grateful to all professionals, patients and volunteers involved with the National Centre for Mental Health (NCMH). NCMH is funded by the National Institute for Social Care and Health Research (NISCHR), Welsh Government, Wales (Grant No. BR09). HLC is funded by a Wellcome Strategic Award [104943/Z/14/Z]. KM is funded by the Wellcome Trust [WT200804]. TML is funded by a Wellcome Trust ISSF Fellowship [WT513688] and a Ser Cymru II Fellowship, funded by European Regional Development Funds via the Welsh Government (Case ID: CU149).
Author information
Authors and Affiliations
Contributions
H.L.C. contributed by writing the manuscript, conducting data analysis of MRI data and statistical analysis. T.M.L. edited manuscript, conducted data analysis, and provided access to the dataset. K.E.T. provided genotype quality control and polygenic scores. R.G.W. provided initial version of the PCASL MRI sequence in the original cohort. K.M. edited the manuscript and provided support at early stage of analysis. D.E.J.L. was the principle investigator of the data that was collected.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chandler, H.L., Wise, R.G., Murphy, K. et al. Polygenic impact of common genetic risk loci for Alzheimer’s disease on cerebral blood flow in young individuals. Sci Rep 9, 467 (2019). https://doi.org/10.1038/s41598-018-36820-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36820-3
- Springer Nature Limited
This article is cited by
-
Proof-of-concept recall-by-genotype study of extremely low and high Alzheimer’s polygenic risk reveals autobiographical deficits and cingulate cortex correlates
Alzheimer's Research & Therapy (2023)
-
A review of brain imaging biomarker genomics in Alzheimer’s disease: implementation and perspectives
Translational Neurodegeneration (2022)
-
Polygenic risk for Alzheimer's disease shapes hippocampal scene-selectivity
Neuropsychopharmacology (2020)
-
The multiplex model of the genetics of Alzheimer’s disease
Nature Neuroscience (2020)