Abstract
Oxidative stress plays a major role in development of cardiovascular disease in patients with chronic kidney disease (CKD). Human mercaptalbumin (HMA), a reduced form of serum albumin, and non-mercaptalbumin (HNA), an oxidized form of serum albumin, are known as indicators for evaluating oxidative stress in systemic circulation, including end-stage renal disease cases. We investigated factors associated with fraction of HNA [f(HNA)] in 112 pre-dialysis CKD patients (63.6 ± 14.0 years old; 59 males, 53 females) using a newly established anion-exchange column packed with hydrophilic polyvinyl alcohol gel as well as high performance liquid chromatography. Mean f(HNA) in our CKD patients was 30.0 ± 6.1%, higher than that previously reported for healthy subjects. In multiple regression analysis, age (β = 0.200, p = 0.014), eGFR (β = −0.238, p = 0.009), hemoglobin (β = −0.346, p < 0.001), and ferritin (β = 0.200, p = 0.019) were significantly and independently associated with f(HNA) (R2 = 0.356, p < 0.001). In addition, factors related to CKD-mineral and bone disorder (CKD-MBD), including intact-PTH (β = 0.218, p = 0.049) and 1,25-dihydroxyvitamin D (1,25(OH)2D) (β = −0.178, p = 0.040), were significantly and independently associated with serum f(HNA) (R2 = 0.339, p < 0.001), whereas fibroblast growth factor-23 was not. These findings indicate the importance of management of hemoglobin and ferritin levels, as well as appropriate control of CKD-MBD factors for a better redox state of serum albumin in CKD patients.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) and end-stage renal disease (ESRD) are strongly associated with cardiovascular disease (CVD)1. Oxidative stress, which is involved with production of excessive levels of reactive oxygen species (ROS), is closely related to the progression of CKD2, while it has also been proposed to play a major role in development of CVD in CKD patients3.
Human serum albumin (HSA) is a simple protein comprised of 585 amino acids with a molecular weight 66 kD and produced in the liver4. Among the 35 HSA cysteine residues, only N-terminal cysteine 34 (Cys34) remains free. HSA is capable of scavenging hydroxyl radicals with its reduced (-SH) cysteine residue Cys34. Based on the state of Cys34, HSA exists in 2 main forms; the oxidized form of human non-mercaptalbumin (HNA) and reduced form of human mercaptalbumin (HMA)5,6.
HNA and HMA have been shown to be good indicators for evaluating oxidative stress in systemic circulation of various types of patients, including those with chronic liver failure7,8 and ESRD9,10. A higher HNA level has been reported to be a considerable risk factor for CVD in patients undergoing hemodialysis and peritoneal dialysis10,11. Thus, it is clinically important to measure HNA and HMA in patients with an elevated risk of CVD, i.e., those with CKD and ESRD. However, few investigations have examined the relationship between serum fraction of HNA [f(HNA)], a serum marker of oxidative stress determined using the formula HNA/(HNA + HMA)*1009,12, and clinical parameters in pre-dialysis CKD patients13.
Conventional methods for measurements of HNA and HMA have been developed, including high performance liquid chromatography (HPLC)14, HPLC-post column bromocresol green15, and anion exchanging chromatography6. However, the protocols used for analysis with those are complicated and/or extremely time consuming, and a more rapid and sensitive clinical laboratory method for assessment of HNA and HMA has been anticipated. Therefore, we recently developed a novel method to measure serum HNA and HMA that utilizes an anion-exchange column packed with hydrophilic polyvinyl alcohol gel, along with HPLC12. This assay technique features a shorter analytical time, and provides highly accurate and reproducible measurements of HNA and HMA12. In healthy adults examined with this system, approximately 20–25% of serum albumin has been shown to be HNA9,12, with the remaining 75–80% found to be HMA16.
In the present study, we conducted a cross-sectional single center investigation of 112 CKD patients, in whom we measured serum HNA and HMA using our newly developed method. With these results, the relationship between f(HNA) and clinical CKD-related parameters was examined.
Results
Clinical characteristics of patients with pre-dialysis CKD
The clinical characteristics of the CKD patients are presented in Table 1. Average (±SD) age, serum creatinine level, and eGFR were 63.6 ± 14.0 years, 2.2 ± 1.4 mg/dL, and 30.7 ± 15.6 mL/min/1.73 m2, respectively. Percent f(HNA) was calculated using the formula HNA/(HNA + HMA)*1009,12. The mean value in the present patients was 30.0 ± 6.1%, which was higher than that previously reported for healthy subjects [f(HNA) 25.1 ± 3.0%; age 63.1 ± 9.9 years, serum creatinine 0.80 ± 0.19 mg/dL]9. Sixty-eight of 112 patients were given renin-angiotensin-aldosterone system (RASS) inhibitors. However, f(HNA) was not significantly different between CKD patients with and without RAAS inhibitor administration (30.4 ± 7.6% vs. 30.2 ± 5.9%, p = 0.809).
Correlations between f(HNA) and clinical parameters in CKD patients
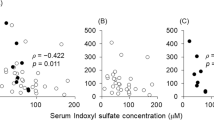
The correlations between patient clinical parameters and f(HNA) were examined using simple regression analysis (Table 2). Age and levels of creatinine, blood urea nitrogen, ferritin, chloride, phosphate, intact-parathyroid hormone (PTH), and FGF-23 were significantly and positively correlated with f(HNA) in our CKD patients (ρ = 0.302, p < 0.001; ρ = 0.410, p < 0.001; ρ = 0.457, p < 0.001; ρ = 0.286, p = 0.002; ρ = 0.230, p = 0.015; ρ = 0.265, p = 0.006; ρ = 0.357, p < 0.001; ρ = 0.420, p < 0.001, respectively) (Table 2, Fig. 1). Furthermore, eGFR, and levels of hemoglobin and sodium-chloride, the latter a marker of metabolic acidosis17, were also significantly and negatively correlated with f(HNA) (ρ = −0.439, p < 0.001; ρ = −0.382, p < 0.001; ρ = −0.294, p = 0.002, respectively) (Table 2, Fig. 1).
Correlations between serum fraction of human non-mercaptalbumin [f(HNA)] and clinical parameters in CKD patients. Age (A), blood urea nitrogen (C), ferritin (E), phosphate (G), intact-PTH (H) and FGF-23 (I) were significantly and positively correlated with f(HNA). Estimated glomerular filtration rate (eGFR) (B), hemoglobin (D), and sodium-chloride (F) were significantly and negatively correlated with f(HNA).
Multivariate analysis of clinical parameters associated with f(HNA)
Next, we investigated clinical parameters associated with f(HNA) using multivariate analysis, with age, gender, body mass index (BMI), aspartate transaminase (AST), hemoglobin (marker of renal anemia), uric acid, sodium-chloride (marker of metabolic acidosis), intact-PTH [marker of CKD mineral and bone disorder (CKD-MBD)], eGFR, and urinary protein included as explanatory variables. The results showed that age (β = 0.255, p = 0.005), hemoglobin (β = −0.341, p = 0.001), intact PTH (β = 0.221, p = 0.039), and eGFR (β = −0.262, p = 0.003) were significantly and independently associated with serum f(HNA) in our CKD patients (R2 = 0.365, p < 0.001) (Table 3).
Multivariate analyses to elucidate renal anemia parameters associated with serum f(HNA) in CKD patients
Renal anemia-related parameters, such as hemoglobin and ferritin, were significantly correlated with serum f(HNA) in the present patients (Table 2). To further identify renal anemia-related parameters independently associated with serum f(HNA), we performed additional multivariate analyses using the parameters ferritin, transferrin saturation (TSAT), use of erythropoiesis-stimulating agent (ESA), and iron supplementation including iron-based phosphate binders as explanatory variables. In addition to age, eGFR, and hemoglobin, the level of serum ferritin was significantly and independently associated with serum f(HNA) in all 4 models, whereas TSAT, use of ESA, and iron supplementation were not (Table 4). When CKD patients were subdivided into 4 groups according to the median values for hemoglobin (12.4 g/dL) and ferritin (100 ng/mL), f(HNA) in patients with higher hemoglobin and lower ferritin levels was significantly lower as compared to those with lower hemoglobin and higher ferritin levels (Supplemental Fig. 1).
Multivariate analyses to elucidate CKD-MBD parameters associated with serum f(HNA) in CKD patients
CKD-MBD related markers, such as phosphate, intact-PTH, and FGF-23, were significantly correlated with serum f(HNA) in the present CKD patients (Table 2). Therefore, to further identify CKD-MBD-related parameters independently associated with serum f(HNA), we performed additional multivariate analyses with the parameters corrected calcium, phosphate, intact-PTH, FGF-23, and 1,25-dihydroxyvitamin D (1,25(OH)2D) included as explanatory variables. Our results showed that intact-PTH was significantly and independently associated with serum f(HNA) (Model 1) (β = 0.218, p = 0.049) (R2 = 0.339, p < 0.001), whereasFGF-23 was not (Model 2) (β = 0.084, p = 0.476) (R2 = 0.317, p < 0.001). Notably, 1,25(OH)2D was significantly and inversely associated with f(HNA) in our CKD patients in analyses with both Model 1 and 2 (Table 5).
Discussion
In the present study, we investigated factors associated with serum levels of HNA (oxidized form of serum albumin) and HMA (reduced form of serum albumin) in pre-dialysis CKD patients using a newly established, highly sensitive assay based on an anion-exchange column packed with a hydrophilic polyvinyl alcohol gel, along with HPLC. Our findings clearly demonstrated that decreased renal function, represented by lower eGFR, was strongly associated with f(HNA) in pre-dialysis CKD patients. We also found that the serum level of f(HNA) was significantly higher in CKD patients with anemia as compared to those without. Multivariate analysis revealed that age, renal function, hemoglobin, and ferritin levels were significantly and independently associated with serum f(HNA) after adjustment for other confounders. These findings suggest the importance of determining renal function, hemoglobin and ferritin levels for assessment of the redox state of serum albumin in CKD patients. In addition, the present results demonstrate that intact-PTH and 1,25(OH)2D are significantly and independently associated with f(HNA), for the first time.
In a previous study, the proportion of HNA, measured with a method different from ours, was demonstrated to be significantly increased in pre-dialysis CKD patients, and serum creatinine levels were significantly and positively correlated with f(HNA)13. Our result of significant association of renal function (eGFR) and f(HNA) was consistent with that of the previous study. Differences between the previous study13 and the present study were summarized in Supplemental Table 1. Firstly, in the present study, data for several CKD-related parameters not included in the previous study13 have been added, including eGFR, urinary protein, sodium-chloride, TSAT, use of ESA or iron supplementation, intact-PTH, FGF-23, 1,25(OH)2D, plasma glucose, and hemoglobin A1c. Those parameters as well as others were evaluated in the present study in relation to the level of f(HNA). We consider that measurement of those markers in the present study is one of the advantages. Secondary, the results of multivariate analyses were different. We found that in addition to eGFR, hemoglobin, ferritin, intact-PTH, and 1,25(OH)2D were also significantly associated with f(HNA) in our CKD patients. In the previous study, multivariate analysis showed that hemoglobin was not significantly associated with f(HNA), while creatinine and uric acid each had a significant association13. In general, oxidative stress is increased with age. In our study, age was found to be a significant factor associated with f(HNA), in agreement with several other reports18,19,20, whereas the previous study did not find such a significant association13. This difference might have been due to the fact that the number of CKD patients in their study (n = 55) was fewer as compared to ours (n = 112). Another explanation for these different findings may be due to differences in the analytical methods used for f(HNA). Novel findings in our study include the results of multivariate analysis showing that hemoglobin, ferritin, intact-PTH, and 1,25(OH)2D were significantly associated with f(HNA) in CKD patients. Finally, the measurement method of f(HNA) was different. With our method, the measurement time is far shorter, and the accuracy is very high with the CV values of 0.3%12.
In our previous study, f(HNA) in healthy subjects was 25.1 ± 3.0%9, while that in the present pre-dialysis CKD patients was 30.0 ± 6.1%, higher than in those healthy subjects. In another study, though the method used for measurement was different, f(HNA) in patients undergoing hemodialysis was 41.5%21, higher than the value for pre-dialysis CKD patients in the present study. In addition, eGFR was significantly and inversely associated with f(HNA) in our study. These findings suggest that decreased renal function is associated with oxidization of serum albumin, shown by f(HNA) level.
In previous studies that used conventional measurement methods, serum f(HNA) was found to be increased with aging in healthy subjects18,19,20. With our novel assay method for HNA and HMA, age was also significantly and positively correlated with f(HNA) in healthy subjects9,12. Although the age of the healthy subjects (63.1 ± 9.9 years) in those previous studies was similar to that of the present patients (63.6 ± 14.0 years), f(HNA) in the present study was 30.0 ± 6.1%, higher as compared to our previous healthy subjects. Furthermore, strong and significant correlations were observed between f(HNA) and eGFR, and between f(HNA) and age in this study, suggesting a close correlation of f(HNA) with oxidative stress, renal function, and age. Also, our multiple regression analysis results demonstrated for the first time that age and renal function are significantly and independently associated with f(HNA).
In patients with ESRD undergoing hemodialysis, f(HNA) has been reported to be decreased from approximately 40% before to 30% after a dialysis session22. That study also noted that such a rapid alteration of redox state of serum albumin before and after dialysis resembled the alteration of serum urea nitrogen levels seen with hemodialysis treatment. Furthermore, a significant reduction in oxidized albumin ratio was reportedly observed after administration of AST-120, a carbonaceous adsorbent drug, in 5/6 nephrectomized CKD rats23. In the present study, blood urea nitrogen was significantly and highly correlated with serum f(HNA). Among uremic toxins, indoxyl sulfate is known to induce oxidative stress via promotion of free radical production24. In a previous study of CKD rats, exogenous administration of indoxyl sulfate increased the ratio of oxidized albumin23. Furthermore, AST-120 was found to decrease serum indoxyl sulfate levels in CKD patients receiving dialysis25 as well as pre-dialysis patients with CKD26. Together, these findings suggest that administration of AST-120 may reduce f(HNA) in CKD patients. Nevertheless, additional longitudinal studies to confirm the effects of AST-120 on the redox state of serum album are considered to be clinically necessary.
In pre-dialysis CKD patients, the present results showed that f(HNA) is significantly and independently associated with hemoglobin and ferritin. That positive and significant association between serum ferritin, a marker of iron status of CKD27, and f(HNA) suggests that increased iron storage impairs the redox state of serum albumin. Previously, free iron was shown to be a powerful source of hydroxyl radicals through the Fenton reaction28, while iron loading alters anti-oxidant systems29. Furthermore, emerging evidence supporting the role of iron overload in cardiovascular complications has been presented30,31, which is thought to be due to endothelial dysfunction induced by ROS increase32. Indeed, serum f(HNA) in hemodialysis patients receiving intravenous iron administration was significantly higher (41.7 ± 6.3%) as compared to those without that administration (36.0 ± 6.0%)21. Nevertheless, we did not find a significant association of oral iron supplementation with f(HNA) in our study. A recent investigation conducted to compare the effects of oral and parenteral iron in a CKD population was terminated early, because the group receiving parenteral iron supplementation experienced a 2.51-fold higher incidence of CVD events and a 2-fold greater incidence of hospitalization for heart failure as compared with the oral iron-treated group33. Those findings indicate that oral iron supplementation for maintaining an adequate ferritin level may not be so harmful, at least as compared to parenteral iron supplementation. In contrast, TSAT is used as an index for responsiveness to ESA rather than absolute iron deficiency, because TSAT results show a large circadian variation and are easily affected by factors other than iron status, such as inflammation and nutritional status34. Thus, we consider that TSAT is not associated with f(HNA), even though ferritin showed a significant association with f(HNA) in the present study. Additional studies that examine a larger number of CKD patients are needed in order to investigate the effects of ESA and oral or parenteral iron supplementation on serum albumin redox status.
As for factors related to CKD-MBD, both PTH34,35 and FGF-2336,37 have been shown to mediate oxidative stress. Thus, investigations of the association between CKD-MBD related markers and f(HNA) are important for treatment of CKD patients. In the present CKD patients, both simple and multiple regression analyses revealed that intact-PTH was associated significantly and positively with serum f(HNA). In contrast, multiple regression analysis findings showed that FGF-23 did not have a significant association. Intact-PTH was shown to be significantly correlated with inflammatory biomarkers, which are known to induce oxidative stress35. In addition, administration of paricalcitol, a selective vitamin D receptor activator, for 12 weeks reduced the levels of oxidative stress markers, including malondialdehyde, nitrites, and carbonyl groups, as well as intact-PTH levels in patients receiving hemodialysis38. Notably, serum 1,25(OH)2D, an active form of vitamin D, had a significantly negative association with f(HNA) in our multiple regression analysis results. Our new findings suggest that among CKD-MBD related factors, PTH and 1,25(OH)2D have effects on the redox state of serum albumin. Based on these results, treatment of CKD-MBD using vitamin D receptor activators, which reduce PTH and increase 1,25(OH)2D, may have an effect on amelioration of the redox state of serum albumin in affected patients. Nevertheless, a longitudinal study is necessary.
The present study has some limitations. First, the number of patients examined was relatively small, mainly because the subjects were enrolled from a single institution. Also, patients with liver dysfunction, reported to have effects on the redox state of serum albumin8,19,39, were excluded, because we intended to examine f(HNA) in CKD patients. Furthermore, this study had a cross-sectional design and the findings cannot be used demonstrate causality of the factors, i.e., we could not conclude that control of uremic toxins, such as using AST-120, control of renal anemia, such as with use of ESA, or decreased ferritin leads to decreased serum f(HNA). Additional studies that explore whether serum f(HNA) can be reduced through strict control of hemoglobin levels in CKD patients are necessary, particularly regarding the link between improvement of anemia and decrease in serum f(HNA), which could be confirmed in experimental animal models. Other in vivo studies may also be necessary to determine the potential effects of some uremic toxins and ferritin on f(HNA). Finally, ankle brachial pressure index (ABI) and (PWV) pulse wave velocity have been shown to be strong predictors of cardiovascular events, not only in the general population40, but also in CKD patients41. An additional longitudinal study is needed to investigate the association between f(HNA) and ABI/PWV.
In conclusion, this is the first study to show that serum f(HNA) is significantly associated with decreased renal function and renal anemia levels in CKD patients, independent of age. Our results also demonstrated that intact-PTH and 1,25(OH)2D are significant factors associated with serum albumin redox state. Together, the present findings suggest the importance of management of hemoglobin and ferritin levels, as well as appropriate control of CKD-MBD factors to provide a better redox state of serum albumin in CKD patients.
Methods
Ethics statement
The study protocol was approved by the Ethics Committee of Osaka City University Graduate School of Medicine (approval #603366). All study participants provided written informed consent for both collection of blood and urine samples, and examination of clinical records relevant to the present study. The research was conducted in accordance with the Declaration of Helsinki.
Patients
CKD was defined according to criteria proposed by the Kidney Disease: Improving Global Outcomes Organization42. A total of 112 CKD patients with eGFR < 60 mL/min/1.73 m2 and regularly followed by nephrologists at Osaka City University Hospital were enrolled between March and April 2016. The etiologies of these patients included hypertensive nephrosclerosis (n = 21), IgA nephropathy (n = 16), membranous nephropathy (n = 16), diabetic nephropathy (n = 15), anti-neutrophil cytoplasmic antibody (MPO-ANCA)-associated glomerulonephritis (n = 7), autosomal dominant polycystic kidney disease (n = 7), tubulointerstitial nephritis (n = 5), minimal change nephrotic syndrome (n = 3), membranoproliferative glomerulonephritis (n = 3), purpura nephritis (n = 3), focal and segmental glomerulosclerosis (n = 2), lupus nephritis (n = 2), and unknown cause (n = 12). Since liver dysfunction has been reported to have an effect on the redox state of serum albumin8,19,39, patients with hepatitis C (n = 6), hepatitis B (n = 2), alcoholic liver cirrhosis (n = 2), fatty liver (n = 8), drug-induced liver injury (n = 4), and liver cancer (n = 4) were excluded from the present analysis (Supplemental Fig. 2).
Blood sampling
Spot blood and urine samples were collected from all subjects in the morning after overnight fasting. The urine samples were kept on ice for 1 hour and then centrifuged at 630 × g for 10 minutes, as previously described43. All laboratory measurements were performed using routine assays with automated methods43. Estimated glomerular filtration rate (eGFR) was calculated using the new Japanese coefficient for the abbreviated Modification of Diet in Renal Disease Study equation, including a correction factor of 0.739 for females44. Serum calcium was corrected based on serum albumin and determined as corrected calcium, as previously described45. Serum intact- PTH was measured using a second-generation Elecsys PTH IRMA assay (Roche Diagnostics, Mannheim, Germany), as previously reported45. Serum full-length fibroblast growth factor-23 (FGF-23) was measured using a CL-JACK System (Kyowa Medex Co. Ltd., Tokyo, Japan), a fully automated random access chemiluminescence immuno-analyzer, as previously reported45. Serum 1,25(OH)2D was measured with a 1,25(OH)2D RIA kit (Immunodiagnostic Systems Limited, Boldon, England), as previously described45.
Measurements of human non-mercaptalbumin (HNA) and mercaptalbumin (HMA)
Measurements of HNA were performed using freshly frozen samples kept at −80 °C until the time of the assay utilizing the recently established assay that employs anion-exchange HPLC9,12. A LabSolutions system (Shimazu Co., Ltd., Kyoto, Japan), consisting of a degasser (DGU20A3R), 2 pumps (LC-20AT), an auto-sampler (SIL30AC), a thermostatic oven (CTO-20AC), a fluorescence detector (RF-20Axs), and a system controller (CBM-20A), was used for the present study9,12.
The gel and column for analysis of HNA and HMA were produced as follows. A polyvinyl alcohol cross-linked gel (9 µm in diameter) (Asahipak GS-520, Asahi Kasei Co., Ltd., Tokyo, Japan) was dried in a vacuum for more than 16 hours, then suspended in 10 ml of dimethyl sulfoxide (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) per 1 g of dried gel. Next, 20 mmol of epichlorohydrin for each 1 g of dried gel was added to suspend the gel and the reaction was allowed to proceed for 20 hours at 30 °C. The activated gel was subsequently filtrated and reacted with a 10% aqueous solution of diethyl amine (Wako Pure Chemical Ind., Co., Ltd, Osaka, Japan) for 20 hours. The synthesized anion-exchange gel was then packed into a stainless column (50 × 7.6 mm I.D.).
HPLC analysis conditions were as follows. The solution of 25 mM phosphoric acid buffer containing 60 mM sodium sulfate (pH 6.0) was employed for the first eluent (eluent A) to absorb and separate the albumin fraction. A high concentration magnesium chloride solution was used for eluting HMA and HNA from the column as an eluate (eluent B). The flow rate was set at 1 mL/min after equilibrating the column for 4.5 min with eluent A. The linear gradient time program from eluent A (100%) to eluent B (100%) lasted for 7.5 min and started at the time of sample injection. The total measurement time was 12 min per sample, including both column equilibration and the analysis time. 3 mL of serum was used for analysis. Analysis temperature was 40 °C. The influence of substances interfering with this measurement method was examined using the actual HPLC chromatograms and no abnormal chromatograms with effects on the measurement results were found.
Statistical analyses
Statistical analyses were performed using Graphpad Prism version 6.0 for Windows (Graphpad Software, San Diego, CA) and JMP software version 10 (SAS Institute Inc., Cary, NC). Values are expressed as the mean ± SD. Comparisons of f(HNA) between CKD patients with and without renal anemia were made using an unpaired Student’s t-test or Mann-Whitney U test for continuous variables, or a chi-square test for categorical variables. One-way ANOVA was used to assess difference in mean values between groups. Correlations between human f(HNA) and clinical data were examined by Spearman’s analysis. Independent associations between clinical variables and f(HNA) in the present CKD patients were assessed by multiple regression analyses, with dummy variables of 0 and 1 included for users and non-users of ESA, respectively, and iron supplementation (yes, no), respectively. BMI, renal function, AST and uric acid levels were found to be significantly correlated with f(HNA) in healthy Japanese subjects9, while hemoglobin and serum uric acid were shown to be significantly correlated with f(HNA) in CKD patients13. Furthermore, chronic metabolic acidosis has been shown to decrease albumin synthesis46. Since intact-PTH was found to be significantly correlated with inflammatory biomarkers, which induce oxidative stress35, that may also have an effect on the redox state of serum albumin. Additionally BMI, renal function, AST, uric acid, sodium-chloride, and intact-PTH may also have effects on the redox state of serum albumin, thus those parameters were added as explanatory variables for multivariate analysis (Table 3). P values < 0.05 were considered to indicate statistical significance. The datasets generated and analyzed for the current study are available from the corresponding author upon reasonable request.
References
Wannamethee, S. G., Shaper, A. G. & Perry, I. J. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke 28, 557–563 (1997).
Himmelfarb, J. Linking oxidative stress and inflammation in kidney disease: which is the chicken and which is the egg? Semin Dial 17, 449–454, https://doi.org/10.1111/j.0894-0959.2004.17605.x (2004).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62, 1524–1538, https://doi.org/10.1046/j.1523-1755.2002.00600.x (2002).
Meloun, B., Moravek, L. & Kostka, V. Complete amino acid sequence of human serum albumin. FEBS Lett 58, 134–137 (1975).
Artigas, A., Wernerman, J., Arroyo, V., Vincent, J. L. & Levy, M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care 33, 62–70, https://doi.org/10.1016/j.jcrc.2015.12.019. (2016).
Era, S. et al. Further studies on the resolution of human mercapt- and nonmercaptalbumin and on human serum albumin in the elderly by high-performance liquid chromatography. Int J Pept Protein Res 31, 435–442 (1988).
Nakao, T. et al. Best practice for diabetic patients on hemodialysis 2012. Ther Apher Dial 19(Suppl 1), 40–66, https://doi.org/10.1111/1744-9987.12299 (2015).
Oettl, K. et al. Oxidative albumin damage in chronic liver failure: relation to albumin binding capacity, liver dysfunction and survival. J Hepatol 59, 978–983, https://doi.org/10.1016/j.jhep.2013.06.013 (2013).
Masudo, R. et al. Evaluation of human nonmercaptalbumin as a marker for oxidative stress and its association with various parameters in blood. J Clin Biochem Nutr 61, 79–84, https://doi.org/10.3164/jcbn.17-5 (2017).
Terawaki, H. et al. The redox state of albumin and serious cardiovascular incidence in hemodialysis patients. Ther Apher Dial 14, 465–471, https://doi.org/10.1111/j.1744-9987.2010.00841.x (2010).
Terawaki, H. et al. A lower level of reduced albumin induces serious cardiovascular incidence among peritoneal dialysis patients. Clin Exp Nephrol 16, 629–635, https://doi.org/10.1007/s10157-012-0610-x (2012).
Yasukawa, K., Shimosawa, T., Okubo, S. & Yatomi, Y. A simple, rapid and validated high-performance liquid chromatography method suitable for clinical measurements of human mercaptalbumin and non-mercaptalbumin. Ann Clin Biochem 55, 121–127, https://doi.org/10.1177/0004563217693257 (2018).
Terawaki, H. et al. Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int 66, 1988–1993, https://doi.org/10.1111/j.1523-1755.2004.00969.x (2004).
Sogami, M., Nagoka, S., Era, S., Honda, M. & Noguchi, K. Resolution of human mercapt- and nonmercaptalbumin by high-performance liquid chromatography. Int J Pept Protein Res 24, 96–103 (1984).
Ueyama, J. et al. A revised method for determination of serum mercaptalbumin and non-mercaptalbumin by high-performance liquid chromatography coupled with postcolumn bromocresol green reaction. Ann Clin Biochem 52, 144–150, https://doi.org/10.1177/0004563214531930 (2015).
Roche, M., Rondeau, P., Singh, N. R. & Tarnus, E. & Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett 582, 1783–1787, https://doi.org/10.1016/j.febslet.2008.04.057 (2008).
Mallat, J. et al. Use of sodium-chloride difference and corrected anion gap as surrogates of Stewart variables in critically ill patients. PLoS One 8, e56635, https://doi.org/10.1371/journal.pone.0056635 (2013).
Anraku, M., Chuang, V. T., Maruyama, T. & Otagiri, M. Redox properties of serum albumin. Biochim Biophys Acta 1830, 5465–5472, https://doi.org/10.1016/j.bbagen.2013.04.036 (2013).
Nagumo, K. et al. Cys34-cysteinylated human serum albumin is a sensitive plasma marker in oxidative stress-related chronic diseases. PLoS One 9, e85216, https://doi.org/10.1371/journal.pone.0085216 (2014).
Oettl, K. & Marsche, G. Redox state of human serum albumin in terms of cysteine-34 in health and disease. Methods Enzymol 474, 181–195, https://doi.org/10.1016/S0076-6879(10)74011-8 (2010).
Anraku, M. et al. Intravenous iron administration induces oxidation of serum albumin in hemodialysis patients. Kidney Int 66, 841–848, https://doi.org/10.1111/j.1523-1755.2004.00813.x (2004).
Terawaki, H., Era, S., Nakayama, M. & Hosoya, T. Decrease in reduced-form albumin among chronic kidney disease patients: new insights in cardiovascular complications. Ther Apher Dial 15, 156–160, https://doi.org/10.1111/j.1744-9987.2010.00889.x (2011).
Shimoishi, K. et al. An oral adsorbent, AST-120 protects against the progression of oxidative stress by reducing the accumulation of indoxyl sulfate in the systemic circulation in renal failure. Pharm Res 24, 1283–1289, https://doi.org/10.1007/s11095-007-9248-x (2007).
Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr 20, S2–6, https://doi.org/10.1053/j.jrn.2010.05.002 (2010).
Niwa, T. et al. Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant 6, 105–109 (1991).
Niwa, T. et al. Indoxyl sulfate and progression of renal failure: effects of a low-protein diet and oral sorbent on indoxyl sulfate production in uremic rats and undialyzed uremic patients. Miner Electrolyte Metab 23, 179–184 (1997).
Beerenhout, C., Bekers, O., Kooman, J. P., van der Sande, F. M. & Leunissen, K. M. A comparison between the soluble transferrin receptor, transferrin saturation and serum ferritin as markers of iron state in hemodialysis patients. Nephron 92, 32–35, https://doi.org/10.1159/000064468 (2002).
Gammella, E., Buratti, P., Cairo, G. & Recalcati, S. Macrophages: central regulators of iron balance. Metallomics 6, 1336–1345, https://doi.org/10.1039/c4mt00104d (2014).
Bartfay, W. J. et al. A biochemical, histochemical, and electron microscopic study on the effects of iron-loading on the hearts of mice. Cardiovasc Pathol 8, 305–314 (1999).
Becker, B. N., Himmelfarb, J., Henrich, W. L. & Hakim, R. M. Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol 8, 475–486 (1997).
Kletzmayr, J. & Horl, W. H. Iron overload and cardiovascular complications in dialysis patients. Nephrol Dial Transplant 17(Suppl 2), 25–29 (2002).
Tawfik, H. E., Cena, J., Schulz, R. & Kaufman, S. Role of oxidative stress in multiparity-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol 295, H1736–1742, https://doi.org/10.1152/ajpheart.87.2008 (2008).
Agarwal, R., Kusek, J. W. & Pappas, M. K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 88, 905–914, https://doi.org/10.1038/ki.2015.163 (2015).
Bross, R. et al. Association of serum total iron-binding capacity and its changes over time with nutritional and clinical outcomes in hemodialysis patients. Am J Nephrol 29, 571–581, https://doi.org/10.1159/000191470 (2009).
Emam, A. A., Mousa, S. G., Ahmed, K. Y. & Al-Azab, A. A. Inflammatory biomarkers in patients with asymptomatic primary hyperparathyroidism. Med Princ Pract 21, 249–253, https://doi.org/10.1159/000334588 (2012).
Singh, S. et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90, 985–996, https://doi.org/10.1016/j.kint.2016.05.019 (2016).
Richter, B., Haller, J., Haffner, D. & Leifheit-Nestler, M. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflugers Arch 468, 1621–1635, https://doi.org/10.1007/s00424-016-1858-x (2016).
Izquierdo, M. J. et al. Paricalcitol reduces oxidative stress and inflammation in hemodialysis patients. BMC Nephrol 13, 159, https://doi.org/10.1186/1471-2369-13-159 (2012).
Stauber, R. E. et al. Human nonmercaptalbumin-2: a novel prognostic marker in chronic liver failure. Ther Apher Dial 18, 74–78, https://doi.org/10.1111/1744-9987.12024 (2014).
Mattace-Raso, F. U. et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113, 657–663, https://doi.org/10.1161/CIRCULATIONAHA.105.555235 (2006).
Tanaka, M. et al. Vitamin D receptor activator reduces oxidative stress in hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial 15, 161–168, https://doi.org/10.1111/j.1744-9987.2010.00890.x (2011).
Inker, L. A. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63, 713–735, https://doi.org/10.1053/j.ajkd.2014.01.416 (2014).
Nakatani, S. et al. Urinary Iron Excretion is Associated with Urinary Full-Length Megalin and Renal Oxidative Stress in Chronic Kidney Disease. Kidney Blood Press Res 43, 458–470, https://doi.org/10.1159/000488470 (2018).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53, 982–992, https://doi.org/10.1053/j.ajkd.2008.12.034 (2009).
Nakatani, S. et al. Fibroblast Growth Factor-23 and Vitamin D Metabolism in Subjects with eGFR >/=60 ml/min/1.73 m(2). Nephron 130, 119–126, https://doi.org/10.1159/000430870 (2015).
Ballmer, P. E. et al. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J Clin Invest 95, 39–45, https://doi.org/10.1172/JCI117668 (1995).
Acknowledgements
K.Y., Y.Y. and H.I. disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by JSPS KAKENHI (25253040) and The Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering.
Author information
Authors and Affiliations
Contributions
S.N., E.I., K.Y., and M.I. contributed to the concept and design of the study, data analysis and interpretation, and writing the manuscript. K.Y., Y.Y. and H.I. contributed to measurements of non-mercaptalbumin and mercaptalbumin. A.N., N.T., S.Y. and E.I. contributed to data acquisition and interpretation. H.U., A.T., K.M. and M.E. contributed to the study concept and data interpretation. All authors have read and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakatani, S., Yasukawa, K., Ishimura, E. et al. Non-mercaptalbumin, Oxidized Form of Serum Albumin, Significantly Associated with Renal Function and Anemia in Chronic Kidney Disease Patients. Sci Rep 8, 16796 (2018). https://doi.org/10.1038/s41598-018-35177-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35177-x
- Springer Nature Limited
Keywords
This article is cited by
-
Assessing the effects of tempol on renal fibrosis, inflammation, and oxidative stress in a high-salt diet combined with 5/6 nephrectomy rat model: utilizing oxidized albumin as a biomarker
BMC Nephrology (2024)
-
Non-mercaptalbumin is significantly associated with the coronary plaque burden and the severity of coronary artery disease
Scientific Reports (2021)
-
A rapid method for measuring serum oxidized albumin in a rat model of proteinuria and hypertension
Scientific Reports (2019)