Abstract
Mice lacking Sirt2 spontaneously develop tumors in multiple organs, as well as when expressed in combination with oncogenic KrasG12D, leading to pancreatic tumors. Here, we report that after caerulein-induced pancreatitis, Sirt2-deficient mice exhibited an increased inflammatory phenotype and delayed pancreatic tissue recovery. Seven days post injury, the pancreas of Sirt2−/− mice display active inflammation, whereas wild-type mice had mostly recovered. In addition, the pancreas from the Sirt2−/− mice exhibited extensive tissue fibrosis, which was still present at six weeks after exposure. The mice lacking Sirt2 also demonstrated an enhanced whole body pro-inflammatory phenotype that was most obvious with increasing age. Importantly, an accumulation of a cell population with spontaneous cancerous KrasG12D mutations was observed in the Sirt2−/− mice that is enhanced in the recovering pancreas after exposure to caerulein. Finally, transcriptome analysis of the pancreas of the Sirt2−/− mice exhibited a pro-inflammatory genomic signature. These results suggest that loss of Sirt2, as well as increased age, enhanced the immune response to pancreatic injury and induced an inflammatory phenotype permissive for the accumulation of cells carrying oncogenic Kras mutations.
Similar content being viewed by others
Introduction
Sirtuin genes are the human and murine homologs of the S. cerevisiae Sir2 gene that have been shown to direct both metabolism and lifespan1. In addition, sirtuins play a role in longevity by directing critical acetylome signaling networks in response to caloric restriction2. Sirtuin 2 (SIRT2) is a member of sirtuin family of NAD+-dependent deacetylases that are linked to multiple biological and pathological processes, such as the cell cycle, DNA repair, metabolic homeostasis, genomic integrity, and tumorigenesis. Originally, SIRT2 was observed to regulate the cell cycle3 through the deacetylation of H4K16ac4 but later was shown also to function in metabolic processes by inhibiting adipogenesis by deacetylating FOXO1 and facilitating lipolysis by repressing PPARγ activity5. SIRT2 also directs other physiological processes, including gluconeogenesis, insulin sensitivity, and fatty acid oxidation6,7,8, and is critical for insulin-dependent AKT activation, suggesting that the inhibition of SIRT2 may have an impact on cancer growth9.
We have shown that mice lacking Sirt2 develop tumors in multiple organs, including pancreatic ductal adenocarcinomas (PDAC)10, and interestingly, the combination of Sirt2 deletion with an oncogenic KrasG12D mutation induces PDAC at three months11. In this regard, it has also been shown that KRAS is a SIRT2 downstream deacetylation target that directs enzyme activity12. Ninety-five percent of patients diagnosed with PDAC have tumors with a KRAS mutation. However, the percentage of oncogenic mutant KRAS in healthy organs, including the pancreas, are at rates far exceeding the incidence of cancer development. Furthermore, KRAS GTPase activity is higher in cells derived from mutant Kras-driven PDAC as compared to non-transformed pancreatic cells expressing the same mutant Kras13, suggesting that other events are necessary for complete somatic cell dedifferentiation and/or transformation14,15.
Pancreatitis is initiated by the premature activation of digestive enzymes that cause auto-digestion of the gland16,17,18 and commonly is caused by gallstones, excessive alcohol consumption, smoking, medications, and blunt abdomen trauma19. However, severe and recurrent episodes can develop chronic pancreatitis, leading to permanent inflammation of the pancreas and an increased risk of pancreatic cancer. Caerulein-induced pancreatitis is a well-characterized rodent model that mimics clinical pancreatitis16,18,20,21,22. Injury of the pancreas induced by caerulein results in infiltration of inflammatory immune cells, edema, and destruction of a large portion of the pancreatic parenchyma20,21,23. Despite the massive inflammatory response and tissue destruction, the murine pancreas can histologically regenerate and recover after exposure24,25, often within one week20,23,26,27,28.
In this regard, a recent study has revealed the role of SIRT2 in inflammatory responses, in which SIRT2 deacetylates the NF-κB subunit p65, resulting in a decrease of NF-κB-dependent gene expression29. More recently, loss of Sirt2 increases the severity of experimental colitis by modulation of macrophage polarization30 and collagen-induced arthritis with a concomitant increase in NF-κB acetylation31. In addition, agents that inhibit SIRT2 aggravates experimental traumatic brain injury by increasing acetylation and nuclear translocation of NF-κB p6532. Finally, since 2017, there are now over twenty manuscripts that describe, at least in some part, a mechanistic connection between SIRT2, protein acetylation, and a potential link to inflammatory pathways. Thus, it seems reasonable to propose that these studies provide evidence for an anti-inflammatory effect of SIRT2.
As mentioned, we have shown that Sirt2 knockout mice with a KRASG12D mutation background develop pancreatic cancer, suggesting Sirt2 knockout is susceptible for pancreatic cancer11. Also, SIRT2 has been shown to be involved in the inflammatory response29,30,31, and pancreatic inflammation is a key issue in pancreatic cancer incidents in humans. Since Kras mutations are found in more than 95% of pancreatic cancers in human, we hypothesized that sustained inflammation due to the lack of SIRT2 activity accumulates a cell population carrying Kras mutations that originally existed in the pancreas through the disruption-regeneration cycles during pancreatitis. Here, we report that after caerulein-induced pancreatitis, Sirt2-deficient mice exhibited an increased inflammatory phenotype and delayed recovery. In addition, aged Sirt2−/− mice develop a pro-inflammatory permissive phenotype. Interestingly, we found that the oncogenic mutations in Kras accumulation was drastically accelerated in pancreatic cells of Sirt2−/− mice with caerulein-induced acute pancreatitis. Our results indicate that Sirt2 deficiency increases inflammation infiltration during caerulein-induced acute pancreatitis and strongly impairs the recovery from pancreatic tissue injury, as well as leads to the accumulation of oncogenic Kras mutations in the pancreas.
Results
Sirt2 deficiency impairs pancreatic regeneration
It has been previously shown that loss of Sirt2 accelerates the oncogenic properties of oncogenic KrasG12D, and these tumors exhibited an increase in trichrome staining suggesting a potential pro-inflammatory environment in pancreatic cells lacking Sirt211. To address this question, we used a well-established model to induce pancreatitis by 8 hourly intraperitoneal (i.p.) injections of 100 μg/kg caerulein. After two days post injection, wild-type and Sirt2−/− mice exposed to caerulein were sacrificed and both showed similar patterns of moderately severe histological damage (Fig. 1a), and scores of tissue integrity, acinar cell necrosis, as well as inflammatory cell infiltration (Fig. 1b,c) (Supplemental Section, Table S1, for histological scoring criteria). In contrast, wild-type mice pancreas appear to mostly recover at day 7 post injection; however, pancreatic regeneration was severely impaired in Sirt2−/− mice, and the inflammation score remained ~70% of day 2 (Fig. 1a–c). Taken together, these results suggest that loss of Sirt2 led to delayed pancreas tissue recovery after caerulein-induced acute pancreatitis, suggesting SIRT2 may play role in pancreatic tissue regeneration.
Mice lacking Sirt2 exposed to caerulein exhibit impaired pancreatic regeneration. (a) Wild-type (WT) and Sirt2−/− (KO) mice had 8 hourly intraperitoneal (i.p.) injections with PBS or caerulein (100 μg/ml), and the pancreas was harvested on day 2 (D2) or day 7 (D7) post-injection. H&E staining was done for all mice, and representative images are shown. n = 5–10 mice per group. Bars indicate 100 µm. (b) Individual scoring of pancreatic tissue integrity, acinar necrosis, and inflammation parameters from wild-type and Sirt2−/− mice used in Fig. 1a. (c) Combined scoring of pancreatic tissue integrity for the mice treated without or with caerulein and harvested at D2 and D7. The scoring system used is shown in the Supplemental Section, Table 1. (n = 5–10, *p < 0.05, **p < 0.01).
Loss of Sirt2 deficiency leads to inflammatory cell infiltration following caerulein exposure
To determine whether loss of Sirt2 affects the inflammatory cell infiltration in pancreatic tissue during a course of acute caerulein exposure, we assessed the IHC staining of a macrophage maker F4/80. Wild-type and Sirt2−/− mice displayed a similar F4/80+ IHC staining in macrophages infiltrating into the pancreatic tissue at day 2 (Fig. 2a, left and middle panels), as compared to PBS-injected controls. In contrast, F4/80+ staining macrophage infiltration was still observed in Sirt2−/− mice at day 7 (Fig. 2a, right panels).
Deletion of Sirt2 increases the macrophage-predominant inflammatory response. (a) Wild-type (WT) and Sirt2−/− (KO) mice were i.p. injected with PBS or caerulein, and the pancreas was harvested on day 2 (D2) or day 7 (D7) post-injection. Panels showed representative immuno-histochemical staining of a macrophage maker F4/80. Bars indicate 100 µm. (b,c) Pancreas from wild-type and Sirt2−/− mice treated with PBS or caerulein at D7 were harvested, digested, and single cells were isolated and stained with anti-CD45, anti-CD11b, and anti-Gr-1 antibodies. (d) FACS plots characterized the expression of CD45+ cells in wild-type, and Sirt2−/− mice at D7. Wild-type and Sirt2−/− mice were i.p. injected with PBS or caerulein, and the spleen was removed at D7. Splenic cells were isolated and stained with anti-CD45, anti-CD11b, and anti-Gr-1 antibodies. Scatterplots showed the population of CD45+CD11b+Gr1+ myeloid cells in wild-type and Sirt2−/− mice at D7 (circled). (e) Graph showing the percentage of CD45+CD11b+Gr1+ myeloid cells from wild-type and Sirt2−/− at D7. (n = 5, *p < 0.05, **p < 0.01). Bar graphs indicated mean ± standard error of the mean (s.e.m).
The local pancreatic inflammatory response was assessed in isolated pancreas in Sirt2−/− mice and wild-type mice at day 7 post injection (Fig. 2b,c). CD45+ macrophages were increased by caerulein-induced acute pancreatitis in the pancreas from both wild type and Sirt2−/− mice, and the induced levels for both were maintained at day 7 to a similar extent. However, the myeloid-derived suppressor cell (MDSC) population (CD45+CD11b+Gr-1+) was significantly higher in pancreas from Sirt2−/− mice as observed in spleen confirming sustained pancreatitis at day 7. Next, we examined whether the whole body CD45+CD11b+Gr-1+ have been changed in the spleen following caerulein injection. The increase of the CD45+CD11b+Gr-1+ population positively correlates with inflammation and being cancer bearing. A high proportion of CD45+CD11b+Gr-1+ cells were observed in the spleen of Sirt2−/− mice compared to wild-type mice at D7 after the last injection (Fig. 2d,e). IFN-γ producing CD4+ T cells and serum level of IFN-γ also remained high in Sirt2−/− mice at day 7 (Supplemental Section, Fig. S1). Pancreatic tissue damage, as well as macrophage infiltration, was also abnormal, as compared to control mice, up to at least 21 days after the induction of pancreatitis (Supplemental Section, Figs S2 and S3). Since SIRT2 regulates NF-κB activities29, we assessed the NF- κB activity in pancreas from wild-type and Sirt2−/− mice at day 7. The expression levels of NF-κB target genes, Pdgfra (Platelet Derived Growth Factor Receptor Alpha), Fcer1g (Fc Fragment Of IgE Receptor Ig), and Ccl2 (C-C Motif Chemokine Ligand 2) were determined by RT-qPCR and showed sustained NF-κB activity at day 7 in Sirt2−/− mice (Supplemental Section, Fig. S4), indicating the sustained active immunological response in pancreas from Sirt2−/− mice at day 7. Finally, when a chronic pancreatitis model was used, via injection of a lower concentration of caerulein into mice (50 μg/kg, 6 hourly injections once a week for 6 weeks), the pancreas from the Sirt2−/− mice also exhibited more intense tissue damage (Fig. 3a,b) and macrophage infiltration (Fig. 3c,d) although there is no difference in body weight (Fig. 3e). The results of these in vivo experiments suggest that loss of Sirt2 prolonged the whole body inflammatory response by caerulein-induced acute and chronic pancreatitis.
Characterization of wild-type and Sirt2 knockout mice pancreas at 6 weeks after caerulein-induced chronic pancreatitis. (a) Wild-type (WT) and Sirt2−/− (KO) mice had 6 hourly intraperitoneal (i.p.) injections with caerulein (50 μg/ml) once a week for 6 weeks. H&E staining was done for all mice, and representative images are shown. n = 5 mice per group. Bars indicate 100 µm. (b) Wild-type (WT) and Sirt2−/− (KO) mice pancreas with caerulein-induced chronic pancreatitis at 6 weeks were analyzed by Masson’s Trichrome staining. Representative images are shown. Bars indicate 100 µm. (c) Wild-type (WT) and Sirt2−/− (KO) mice were i.p. injected with caerulein, and the pancreas was harvested at 6 weeks post-injection. Panels showed representative immuno-histochemical staining of a macrophage maker F4/80. Bars indicate 100 µm. (d) Measurement of pancreas infiltrated macrophages in wild type and Sirt2−/− mice at 6 weeks after caerulein-induced chronic pancreatitis. (e) Body weight of mice at 6 weeks after caerulein-induced chronic pancreatitis.
Effect of Sirt2 deficiency on immune response by age
Sirt2-deficient mice showed prolonged inflammation in caerulein-induced acute pancreatitis, suggesting that these mice may have intrinsic problems with their immune response. Our previous finding that Sirt2-deficient mice developed pancreatic cancer with an oncogenic mutant Kras background11 also supports our notion since tumor development is closely linked with the increasing age partly due to the impaired immune system integrity33.
To study the effects of Sirt2 deficiency on the systemic immune response, age-matched mice were analyzed by flow cytometry to determine the age-related changes in immune cell proportions, such as CD4+ T cells, CD8+ T cells, active T cells (CD4+CD69+), Tregs (CD4+CD25+Foxp3+), and MDSCs (CD45+CD11b+Gr-1+) in the spleen. Compared to the wild-type mice, the Sirt2−/− mice exhibited an increase in the percentage of the total CD4+ T cells and a decrease in the percentage of total CD8+ T cells in the spleen of 12-month old mice (Fig. 4a,b). In addition, the Sirt2−/− mice also exhibited an increased proportion of activated T cells (CD4+CD69+, Fig. 4c) and Tregs (CD4+CD25+Foxp3+, Fig. 4d) in the spleen of 12-month old mice, but not in 4-month old mice. Notably, Sirt2 deletion leads to a higher proportion of inflammatory CD45+CD11b+Gr-1+ cells and CD45+CD11b+Gr-1− cells, such as red pulp macrophages in the spleen of 12-month old, but not 4-month old mice (Fig. 4e,f). These results suggest that loss of Sirt2 shows enhanced inflammatory responses during aging, even without exposing an agent that induces an inflammation response.
Mice lacking Sirt2 exhibit age-dependent difference in T cell activity, as well as macrophage infiltration. (a) The spleens from 12-month old wild-type (WT) and Sirt2−/− (KO) mice were harvested, and splenocytes were stained with anti-CD3, anti-CD8, and anti-CD4 antibodies. FACS plots showed the populations of CD3+CD8+ T cells and CD3+CD4+ T cells from wild-type or Sirt2−/− mice. (b) Quantitative analysis of the cytotoxic (CD3+CD8+) and helper (CD3+CD4+) T cells from wild-type and Sirt2−/− mice from Fig. 3a. (c) The spleens from age-matched (4 and 12 month) wild-type (WT) and Sirt2−/− (KO) mice were harvested, and splenocytes were stained with anti-CD3, anti-CD4, and anti-CD69 antibodies. The graph shows the percentage of CD3+CD4+CD69+ active T cells from wild-type or Sirt2−/− mice at 4 and 12 months of age. (d) The spleens from age-matched wild-type (WT) and Sirt2−/− (KO) mice were harvested, and splenocytes were stained with anti-CD4, anti-CD25, and anti-Foxp3 antibodies. The graph indicates the percentage of CD4+CD25+Foxp3+ regulatory T cells from wild-type or Sirt2−/− mice at 4 and 12 months of age. (e) The spleens from 12-month old wild-type (WT) and Sirt2−/− (KO) mice were harvested, and splenocytes were stained with anti-CD45, anti-CD11b, and anti-Gr-1 antibodies. Scatterplots showed the population of CD45+CD11b+Gr-1+ cells. (f) Quantitative analysis of the CD45+CD11b+Gr-1+ cells from wild-type and Sirt2−/− mice at 12-month old from Fig. 3e (n = 4, *p < 0.05, **p < 0.01).
Loss of Sirt2 leads to elevated serum amylase and prolonged fibrosis
To monitor tissue damage and regeneration of the pancreas in a caerulein-induced model for acute pancreatitis, the wild-type and Sirt2−/− mice pancreas were stained for CK19, amylase, and DAPI. Wild-type mice, 7 days after exposure, showed a predominate amylase staining pattern, an acinar marker consistent with tissue recovery (Fig. 5a) while in contrast, the Sirt2−/− mice exhibited significant CK19 staining, a ductal marker indicating the presence of tissue damage (Fig. 5a). In addition, the levels of serum amylase in the Sirt2−/− mice were also significantly higher, as compared to wild-type mice, at day 2 and 7 post caerulein injection, indicating sustained tissue and prolonged damage has occurred in mice lacking Sirt2 (Fig. 5b).
Sirt2-deficiency leads to the progression of acute pancreatitis to pre-invasive neoplasms. (a) Wild-type and Sirt2−/− mice were i.p. injected with caerulein, and pancreatic tissue was harvested at D7. Immuno-fluorescent staining was performed with anti-CK19 (green), and pancreatic anti-amylase (red). Colocalization of CK19 and pancreatic amylase is shown. Bars indicate 100 µm. (b) Wild-type and Sirt2−/− mice were i.p. injected with PBS or caerulein, and serum was collected prior to injection and on days 1, 2, and 7 post injection. Serum amylase was measured with an amylase assay kit purchased from Abcam. (n = 5–10, p < 0.05, **p < 0.01). (c) Wild-type and Sirt2−/− mice pancreas with caerulein-induced pancreatitis on day 2 (D2) and day 7 (D7) were analyzed by Masson’s Trichrome staining. Blue staining indicates collagen fibers. Note that the pancreas from the Sirt2−/− mouse still shows histological damage at day 7, whereas the wild type mouse pancreas shows remarkable recovery. Representative images are shown. (d) Quantitative analysis of stromal fibrosis in wild-type and Sirt2−/− mice pancreas from Fig. 4a was performed by scoring the intensity of stromal fibrosis, as determined by the percentage of blue stained areas. (n = 5, *p < 0.05, **p < 0.01).
Pancreatic fibrosis is a characteristic histopathological feature of chronic pancreatitis due to tissue injury. Pancreatic fibrosis, as measured by Masson’s Trichrome staining, was similar in both the wild-type and Sirt2−/− mice on day 2 post injection, as seen by the blue stained areas (Fig. 5c, middle panel). The expression level for α-smooth muscle actin (α-SMA) as a fibrosis marker was also similar between wild-type and Sirt2−/− mice (Supplemental Section, Fig. S5). At day 7 post injection, the pancreas from wild-type mice were mostly recovered, and only sporadic blue sections were found (Fig. 5c, right-upper panel, Fig. 5d). In contrast, pancreas from Sirt2−/− mice still exhibited significant areas of blue staining (Fig. 5c, right-bottom panel, Fig. 5d), suggesting the continued presence of pancreatic fibrosis. Recovery from caerulein-induced pancreatitis is evident at day 7 from pancreas/body weight ratio in wild-type mice (Supplemental Section, Fig. S6a), as well as continuous regeneration/proliferation by Ki67 stain in Sirt2−/− mice (Supplemental Section, Fig. S6b). To assess the elimination mechanism of cells at day 7, we determined p53 expression by RT-qPCR (Supplemental Section, Fig. S7a) and immunohistochemistry (Supplemental Section, Fig. S7b) and found that there was no difference in p53 expression level between wild-type and Sirt2−/− mice at day 7, suggesting a minor contribution of p53-dependent apoptotic pathways.
Enhanced accumulation of spontaneous Kras mutations in Sirt2 knockout mice at 7 days after caerulein-induced pancreatitis
Pancreatic cell KRAS mutations, which commonly occur at amino acid position 12, glycine (G), to valine (V) or aspartic acid (D), are a very early precancerous event34,35,36 and are also found in normal cell populations15,37. In addition, pancreatic fibrosis is also a high-risk factor for PDAC38. Taken together, we hypothesized that the pro-inflammatory/pro-fibrotic permissive phenotype observed in mice lacking Sirt2 may select for cell populations that exhibit growth advantages, such as carrying an oncogenic Kras mutation. Indeed, a cross relationship between inflammation and KRAS mutations has been suggested previously2,39,40,41,42.
To address this question, we have analyzed pancreatic genomic DNA obtained from wild-type and Sirt2−/− mice 7 days after caerulein-induced pancreatitis by competitive allele-specific TaqMan Mutation Detection Assays (using castPCR technology) for KRAS amino acid codon mutations at position 12 for glycine to valine (KrasG12V) or aspartic acid (KrasG12D) (Fig. 6). The detection cut-off value was set to 0.1%, as recommended by the manufacturer’s protocol, and pancreas from 46 wild-type (28 treated with caerulein and 18 non-treated) and 56 Sirt2−/− (28 treated with caerulein and 28 non-treated) mice were subjected to analysis. A KrasG12D mutation was detected in 3.5% of the wild-type mouse pancreas at day 7 after caerulein injection, with no KrasG12V mutations (Fig. 6a, upper column). In contrast, 17.8% of the mice lacking Sirt2 exhibited a Kras mutation (KrasG12D = 7.1%, KrasG12V = 10.7%) at day 7 after caerulein exposure (lower column). Kras mutations were also observed in untreated Sirt2−/− mice (12 to 25-month-old) with 14.3% of KrasG12D mutation in Sirt2−/− mice versus none observed in wild-type mice (Fig. 6a–c). We confirmed Kras mutation detection results by TaqMan PCR detection with a completely different design of primer set from Integrated DNA Technologies (IDT) (data not shown). These results indicate that Sirt2−/− mice accumulated Kras mutations in the pancreas at a frequency of more than 5 times higher than that of wild-type mice through a caerulein-induced pancreatitis.
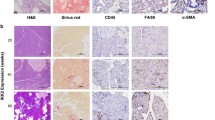
Accumulation of spontaneous tumorigenic Kras mutations in Sirt2-deficient mice. (a) Accumulation of tumorigenic Kras mutations during inflammation-coupled neoplasm. Mouse pancreas genomic DNA from wild-type and Sirt2 KO, with and without caerulein-treatment, were analyzed for KrasG12D or KrasG12V mutations by competitive allele-specific TaqMan PCR. The table shows the summary of analysis for percentage of mice positive for mutations (46 wild-type mice, 56 Sirt2 KO mice) after pancreatitis. (b) Detail description of wild-type and Sirt2 KO mice with KrasG12D and KrasG12V mutations. (c) Bar graph representation of (b). (d) Immunostaining detection of KRAS-G12D mutant protein in mouse pancreas. Mice pancreas tissues were fixed, embedded in paraffin, and immuno-histochemical staining with anti- KRAS-G12D antibody was performed. Representative images are shown. Bars indicate 100 µm.
To confirm the presence of KRAS-G12D mutant protein, we performed immunohistochemistry with an antibody specific for G12D mutation in mouse KRAS (Fig. 6d). We detected multiple KRAS-G12D mutant protein islands in Sirt2−/− but not in wild-type pancreas 7 days after caerulein-induced pancreatitis. These results strongly support our hypothesis that the absence of Sirt2 provides a growth advantage for cells originally carrying oncogenic Kras mutations.
Identification of differentially expressed genes in Sirt2 −/− mice pancreas on day 2 post caerulein
Histopathological analyses show that pancreas from mice lacking Sirt2 exhibited delayed tissue recovery following caerulein exposure. To extend these results at the molecular level and gain insight of a potential mechanism of delayed pancreatic recovery, RNA-seq gene expression profiling was performed. A heat map, from pancreatic RNA in wild-type and Sirt2−/− mice, with and without caerulein exposure, on day 2 showed clear differences in expressed genes (Fig. 7a). Overall, we have identified 529 differentially expressed genes (322 up-regulated genes and 207 down-regulated genes, fold-change >1.5, p < 0.05) as part of the top up- and down-regulated genes (Fig. 7b, Supplemental Section, Fig. S9).
Gene expression profiling of caerulein-induced pancreatitis in wild-type and Sirt2-deficient mice. (a) The heat map illustrates the expression level of statistically significant 529 differentially expressed genes in pancreas between wild-type and Sirt2−/− mice. Each column is a sample, and each row is a gene. The red color represents gene expression greater than the overall mean, and the blue color represents gene expression less than the overall mean. Hierarchical clustering of genes and samples are represented by the dendrograms on the left and across the top of the heat map. (b) The list of top 20 genes for the up- and down-regulated differentially expressed genes in Sirt2−/− mice pancreas compared with wild-type mice on day 2 post caerulein-induced acute pancreatitis. There are 322 up-regulated and 207 down-regulated statistically significant expressed genes detected. (c) KEGG pathway enrichment analysis identified biological pathways associated with the differentially expressed genes detected between wild-type and Sirt2−/− mice at 2 days post caerulein-induced pancreatitis. The top 6 enriched up-regulated and down-regulated pathways are listed based on corrected hypergeometric p-value.
The KEGG pathway analysis revealed 17 up-regulated and 9 down-regulated enriched pathways in Sirt2−/− mice on day 2 post caerulein-induced pancreatitis (Fig. 7c). Notably, up-regulated pathways included those related to extracellular matrix (ECM) reorganization, cell growth and death, and nutrition regulation, suggesting an active reorganization of ECM due to the continuous cell death and cell growth requiring high nutrition in the Sirt2−/− pancreas.
Glycosaminoglycan, including heparin sulfate proteoglycans (HSPGs), biosynthesis is also an enriched pathway in the pancreas of Sirt2−/− mice exposed to caerulein. HSPGs are expressed in most cell surfaces and essential for embryogenesis. HSPGs also play important roles in modulating secreted signaling molecules, including growth factors and cytokines43.
Aggressive “scrap and build” processes are enriched in “pathways in cancer”. Within this category, there are multiple changes in growth factor receptors’ expression including insulin-like growth factor 1 receptor (Igf1r), fibroblast growth factor receptor 3 (Fgfr3), and positive regulators of the Ras pathway, such as nuclear receptor coactivator 4 (Ncoa4), and segment polarity Wnt pathway proteins dishevelled homolog Dvl2 and Dvl3. Other positive and/or negative cell growth regulatory genes identified include the glucose metabolism regulatory gene Ncoa4, peroxisome proliferator-activated receptor gamma (PPARγ), zinc finger, BTB domain-containing protein 16 (Zbtb16), and laminin subunit alpha-2 (Lama2), an extracellular matrix (EMC) reorganization gene actively involved in cancer development.
The “Lysine degradation” pathway is also enriched in the Sirt2−/− mouse, and these genes are involved in pathways that convert lysine to acetyl-coenzyme A (CoA), which is a critical intermediate molecule for lipid metabolism and ATP synthesis, all of which are essential to cell growth and survival. The Ingenuity Pathway Analysis (IPA) also revealed that ECM regeneration was one of the major pathways significantly upregulated in the pancreas from Sirt2−/− mice (Supplemental Section, Fig. 9). Importantly, the IPA independently showed that the pancreas from Sirt2−/− mice 2 days after exposure exhibit tumorigenic “cell death and survival” genomic profile. Finally, the characterization of these differentially expressed genes is shown. Overall, the transcriptome pathway enrichment analysis supports the notion, at the molecular level, that the regeneration of pancreatic tissue appears to still be occurring at 2 days post pancreatitis induction in Sirt2−/− mice.
Validation of differentially expressed genes at day 2 post pancreatitis induction
To confirm the RNA-seq results, the mRNA levels of Ncoa4, Igf1r, Fgfr3, and Zbtb16, which belong to the “Pathways in cancer”, were validated by RT-qPCR, and collectively, the expression of these genes is increased in the Sirt2−/− mice (Fig. 8a). In addition, the expression of (Glycosaminoglycan biosynthesis – heparin sulfate) (Fig. 8b), as well as Ncor2 in “Notch signaling pathway” (Fig. 8c), were also higher in Sirt2−/− mice exposed to caerulein, as compared to control mice. In contrast, the expression of Cldn18 (Cell adhesion molecules, CAMs), is decreased in Sirt2−/− mice exposed to caerulein. These results confirm the RNA-seq data, and thus, support the results from the pathway enrichment analysis. We further confirmed differential expression in protein level by immunohistochemical stain for IGF1R and CLDN18 (Supplemental Section, Fig. S8a) and by western blotting for FGFR3 (Supplemental Section, Fig. S8b).
Validation of differentially expressed genes from RNA-seq analysis by RT-qPCR. (a) Relative expression levels of Ncoa4, Zbtb16, Igf1r, and Fgfr3 by RT-qPCR. These genes belong to “Pathways in Cancer”. (b) Expression level of Extl1 that belongs to a pathway “Glycosaminoglycan biosynthesis – heparin sulfate”. (c) Expression of Ncor2, which belongs to “Notch signaling pathway”. (d) Expression of Cldn18, which belongs to a pathway of “Cell adhesion molecules (CAMs), Tight junction, Leukocyte transendothelial migration”. Error bars represent ± SEM. Overall, the trend of gene expression by RT-qPCR mirrors that of the RNA-seq analysis. *p < 0.05, **p < 0.01.
Discussion
We have reported previously that mice lacking Sirt2, which also express a KrasG12D mutation, develop pancreatic cancer11. Acute or chronic inflammation in the pancreas has been demonstrated to be susceptible to oncogenic Kras-mediated transformation38, and loss of Sirt2 leads to a pro-inflammatory phenotype, at least in some part, due to the dysregulation of the acetylation status of the NF-κB subunit29. Thus, we proposed that mice lacking Sirt2 might exhibit a pancreatitis permissive phenotype, as well as Kras mutations. In this regard, we have shown that pathologically obvious pancreatic tissue damage was observed at 7 days after the last injection of caerulein, which continued up to at least 6 weeks, suggesting that mice lacking Sirt2 exhibit a pancreatitis permissive phenotype. In addition, transcriptome analysis revealed differential gene expression patterns, at early time points, indicating that pancreatitis in Sirt2−/− mice display enrichment of pathways related to tissue injury and inflammation.
Kras mutations are present in the pancreas of healthy individuals37, and it seems reasonable to assume that an enhanced inflammatory response or increasing age may play a role, at least in some part, in this process. Caerulein is considered an analogue to cholecystokinin (CCK), a natural hormone synthesized and secreted by enteroendocrine cells in the duodenum, which induces pancreatic enzyme secretion and an inflammatory response44,45. Since sirtuins, including SIRT2, play a role both in aging and the immune response, we hypothesized that caerulein exposure might create a mutagenic environment. Our results clearly show that oncogenic KrasGly12 mutations accumulate after caerulein-induced pancreatitis especially in Sirt2−/− mice possibly due to a growth advantage of oncogenic Kras mutation. This result is an important evidence to postulate a mechanism of how inflammation contributes to the oncogenic Kras mutation initiated pancreatic cancer and show another scientific murine model example of multidimensional interactions between the inflammatory microenvironment and oncogenic Kras mutations40,41,42. Recently, it has been reported that inflammation induced Kras-independent pancreatic cancers in p53−/− mice46. Their results imply that Kras mutation is not preferred during tumorigenesis when DNA repair checkpoint is disrupted (p53-null). This could mean that Kras mutations have a potential to escape from p53-dependent genomic integrity severance mechanism or to overcome the elimination pressure by the tumor suppressor function of p53.
Recently, Boggs et al. reported transcriptome profiling results by RNA-seq in the recovering pancreas from caerulein-induced pancreatitis47. They have acquired transcriptome data at 7 days and 14 days post caerulein-induced pancreatitis, and since the experimental system they used was similar to our study, we have compared their 7 days post pancreatitis expression profiling data with our 2 days post pancreatitis expression profiling data (Supplemental Section, Figs S10 and 11). The heat map depicts a total of 4,102 statistically significant (fold-change >1.5, p-value < 0.05) differentially expressed genes (up-regulated 2066 genes, down-regulated 2036 genes) from our data, which is larger than that of Boggs et al. (total 319 genes), possibly due to the date of the analysis. We have focused on the initial stage of pancreatic tissue regeneration to observe the initial difference in recovery pathway between Sirt2−/− mice and wild type mice, and Boggs et al. analyzed 7 days after pancreatitis in wild type mice when most of the inflammatory response is gone. Indeed, Venn diagrams (Supplemental Section, Fig. S11) revealed that nearly half of the differentially expressed genes identified by Boggs et al. overlapped with our data, and a large number of genes are unique for our analysis. Pathway analysis in each category in the Venn diagram (Supplemental Section, Figs S11–13) indicated that the common enriched pathways for both groups related to EMC regeneration, whereas the pathways specifically enriched in ours (Quan et al.) are related to cell growth, cell death, and inflammation, supporting the pathological differences in 2 days and 7 days post caerulein-induced pancreatitis that we observed. These results confirmed at the molecular level that 2 days post caerulein-induced pancreatitis is an early time point of pancreatic tissue regeneration.
In closing, our results demonstrate that mice lacking Sirt2 exhibit a pancreatic inflammation permissive phenotype, as well as accumulate oncogenic Kras mutations that is accelerated during recovery from caerulein-induced pancreatitis. Since our previous results have shown that Sirt2−/− mice carrying the KrasG12D mutant progress to pancreatic cancer11, there is a reasonable possibility to assume that spontaneous KrasGly12 mutations accumulated during pancreatitis that could increase the risk of PDAC later in life. Indeed, it has been shown that the level of NAD+, an essential substrate for sirtuin activity, declines in the pancreas with age in mice48. If the decrease in SIRT2 activity in the pancreas accelerates the accumulation of oncogenic Kras mutations, it seems reasonable to propose that caerulein-induced pancreatitis in Sirt2−/− mice would be a useful model system for spontaneous Kras mutation-initiated pancreatic cancer in humans.
Methods
Animals
Acute pancreatitis (AP) was induced into 4-5-month-old female and male Sirt2 wild-type and knockout mice by 8 hourly intraperitoneal injection of caerulein (100 μg/kg) (Sigma, St. Louis, MO) for one day. Chronic pancreatitis was induced in mice by 6 hourly intraperitoneal injection of caerulein (50 μg/kg) (Sigma, St. Louis, MO) once a week for 6 weeks. Age-matched control mice were administered comparable injections of PBS. For all experiments, age- and sex-matched mice were used, and the same background wild type mice served as controls. Injection procedures were performed as outlined in our murine protocols that have been approved by Northwestern University’s Institutional Animal Care and Use Committee (IACUC, institutional protocol number: 2012–2989). For all experiments, age- and sex-matched mice were used, and the same background wild type mice served as controls. The mice were sacrificed at various time points (day 1, day 2, and day 7) after the last injection, and the tissues were taken for subsequent analyses. Mice were housed and bred in pathogen-free conditions at Northwestern University’s barrier facility with a 12-hour light-dark cycle and could obtain food and water ad libitum. All animal housing, handling, and experimental procedures were conducted in strict accordance to the National Institutes of Health and IACUC guidelines. The Northwestern animal resources program is under the direction of full-time veterinarians and is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Northwestern complies with the NIH policy on animal welfare, the Animal Welfare Act, and all other applicable federal, state and local laws.
Histological scoring
Pancreatic tissues were fixed in 10% formalin for 24 hrs at room temperature with slow shaking and embedded in paraffin blocks. Paraffin-embedded tissue samples were cut into 4-μm-thick sections, deparaffinized in xylene, and rehydrated through a graded series of ethanol solutions. To evaluate the pancreatic tissue damage, Hematoxylin-eosin staining was performed. The severity of acute pancreatitis was quantified by assessment of tissue integrity, acinar cell necrosis, and inflammatory cell infiltration. The individual scores and combined scores for these three categories were performed by 3 random images from 5 slides and shown in Supplemental Table 1. To detect tissue fibrosis, the sections were also subjected to Masson’s Trichrome staining. The intensity of stromal fibrosis was scored as: 0: Absent; 1: <5%; 2: 5–10%; 3: 10–20%; 4: >20% fibrosis.
Immunohistochemistry and immunofluorescence
Pancreatic tissues were fixed in 10% formalin, embedded in paraffin, cut into 4-μm-thick sections. Paraffin sections were deparaffinized in xylene, rehydrated by ethanol gradient, and then heated in a pressure cooker in a citrate-based antigen retrieval buffer. The sections were blocked and incubated with primary antibody α-F4/80 (1:200; Cat. No. ab100790, Abcam, Cambridge, MA) at 4 °C overnight. Subsequently, secondary antibody was added for 30 min, followed by DAB substrate buffer (Abcam, Cambridge, CA) for 5 min. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted under a coverslip. Immunofluorescence staining was performed according to conventional methods using the following antibodies: α-Amylase (1:200; Cat. No. ab21156, Abcam, Cambridge, MA), α-CK19 (1:200; Cat. No. TROMA-III, University of Iowa Hybridoma Bank), α-PCNA (1:50; Cat. No. sc-56, Santa Cruz Biotech, Santa Cruz, CA) and α-KRASG12D (1:1000; Cat. No. ab221163, Abcam, Cambridge, MA). AlexaFluro 488 (Cat. No. ab150157, Abcam, Cambridge, MA) and 594 conjugated secondary antibodies (Cat. No. ab150084, Abcam, Cambridge, MA) were used at a 1:200 dilution. The sections were mounted in DAPI containing media (Santa Cruz Biotech, Santa Cruz, CA), exposed to DAPI, FITC, and Texas Red filters, and images were superimposed.
Flow cytometry and intracellular cytokine staining
Single-cell suspensions from the spleen and pancreas were stained at 4 °C using predetermined optimal concentrations of antibodies for 30 min. Cells with the forward and side scatter properties of lymphocytes were analyzed using Fortessa flow cytometer (BD Bioscience, San Jose, CA). Background staining was assessed using isotype matched control antibodies. The following antibodies were used: FITC-conjugated anti-mouse CD45 (Cat. No. 553079, BD Biosciences, San Jose, CA), PE-conjugated anti-mouse CD11b (Cat. No, 557397, BD Biosciences), APC-conjugated anti-mouse Gr-1 (Ly-6G) (Cat. No. 560599, BD Biosciences), PE-conjugated anti-mouse CD8α (Cat. No. 553032, BD Biosciences), APC-conjugated anti-mouse CD69 (Cat. No. 560689, BD Biosciences), Pacific Blue-conjugated anti-mouse CD4 (Cat. No. 558107, BD Biosciences), FITC-conjugated anti-mouse CD3 (Cat. No. 100203, BD Biosciences, San Jose, CA), APC-conjugated anti-mouse CD25 (Cat. No. 102012, Biolegend, San Diego, CA). For the detection of Tregs, splenocytes were stained with Pacific Blue-conjugated anti-mouse CD4 and APC-conjugated anti-mouse CD25 antibodies, fixed, permeabilized, and subsequently stained with PE-conjugated anti-mouse Foxp3 Ab (Cat. No. 12-5773-82, eBioscience, San Diego, CA).
For intracellular cytokine staining, splenocytes (1 × 106/well in 96 plates) were stimulated at 37 °C in a CO2 incubator for 4 hr with Leukocyte Activation Cocktail containing phorbol 12-myristate 13-acetate (PMA), ionomycin, brefeldin A and BD GolgiplugTM. This was followed by staining for cell surface CD4 and intracellular interferon-γ (IFN-γ) (Cat. No. 562020, BD Pharmingen, San Diego, CA) or tumor necrosis factor-α (TNF-α) (Cat. No. 561063, BD Pharmingen) using the Intracellular Cytokine Staining Starter Kit (BD Pharmingen). The percentage of IFN-γ and TNF-α producing CD4+ T cells was analyzed by Fortessa flow cytometer and FlowJo software.
Serum amylase activity assay
Fresh blood was collected from experimental mice at various time points (day 1, day 2, and day 7) using a BD Microtainer blood collection tube (BD Biosciences, San Jose, CA) after the last injection. Following centrifugation, serum was kept frozen at −80 °C until assayed. Serum amylase activity was detected with an Amylase Assay Kit (Abcam, Cambridge, MA).
Identification of Kras mutations
Mutations in Kras gene for G12D or G12V were detected by the competitive allele-specific TaqMan PCR in 46 wild-type and 56 Sirt2−/− mice. Genomic DNA isolated from mouse pancreas were subjected to TaqMan PCR with custom TaqMan probes specific for mouse Kras mutation G12D or G12V (Applied Biosystems). CastPCR Mutation Detection custom made TaqMan probe by Thermo Fisher Scientific (castPCR assay design for Ensemble: ENSMUSG00000030265 Northwestern for mouse KRAS G12V mutation and mouse KRAS G12D mutation custom part number #4476206. Detection cutoff value was set as 0.1%. The CastPCR method was verified using Integrated DNA Technologies (IDT) designed primer set for KRAS G12D GGT/GAT:
KRAS_G12_For: TTTATTGTAAGGCCTGCTGAAA
KRAS_G12_Rev: CTGAATTAGCTGTATCGTCAAGG
KRAS_G12_WT_Allele: TACGCCACCAGC
KRAS_G12D_Allele: TACGCCATCAG
Next-generation RNA sequencing analysis and bioinformatics
Total RNAs were extracted from pancreatic tissue from PBS or caerulein treated Sirt2 wild-type and knockout mice using PureLink RNA Mini Kit. The stranded total RNA-seq was conducted in the Northwestern University NUSeq Core Facility. Briefly, total RNA examples were checked for quality on Agilent Bioanalyzer 2100, and quantity with the Qubit fluorometer. The Illumina TruSeq Stranded Total RNA Library Preparation Kit was used to prepare sequencing libraries from 500 ng of total RNA samples. The Kit was performed without modifications. This procedure includes rRNA depletion, remaining RNA purification and fragmentation, cDNA synthesis, 3′ end adenylation, Illumina adapter ligation, library PCR amplification and validation. The lllumina NextSeq. 500 Sequencer was used to sequence the libraries with the production of single-end, 75 bp reads.
Real-time RT-PCR
Total RNAs were extracted from the pancreatic tissue using PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA). Reverse transcription was performed from 1 μg total RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA), and complementary DNA (cDNA) was used for SYBR green real-time PCR. Amplification was performed with forward and reverse primers using PowerUpTM SYBR Green Master Mix on a QuantStudioTM 7 system (Applied Biosystems, Foster City, CA). The expression of each gene was normalized by corresponding amount of GAPDH mRNA for each condition. Expression of each product were calculated by the comparative ΔΔCt method. Primers are listed in Supplemental Table 2.
Statistical analysis of expression profiles
The quality of DNA reads, in fastq format, was evaluated using FastQC. Adapters were trimmed, and reads of poor quality or aligning to rRNA sequences were filtered. The cleaned reads were aligned to the Mus musculus genome (mm10) using STAR. Read counts for each gene were calculated using htseq-count in conjunction with a gene annotation file for mm10 obtained from UCSC (University of California Santa Cruz; http://genome.ucsc.edu). Normalization and differential expression were determined using DESeq249. The cutoff for determining significantly differentially expressed genes was an FDR-adjusted p-value less than 0.05. Heat map analysis was performed by Morpheus (https://software.broadinstitute.org/morpheus), and Venn diagrams were drawn with BioVenn.
Pathway enrichment analysis
The enrichment of differentially expressed genes in KEGG pathways were analyzed using GeneCoDis3. Ingenuity Pathway Analysis (IPA) was performed on the differentially expressed genes to identify enriched canonical pathways and gene networks50.
Accession number
GSE115758. The following secure token has been created to allow review of record GSE115758 while it remains in private status: olwlyuqgzxoflql.
Statistical analysis
Ordinary one-way ANOVAs and two-tailed unpaired t-tests were conducted using GraphPad Prism 6. The statistical significance was reported when p < 0.05.
Data Availability
The datasets generated during the current study are available in the GEO repository. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE115758.
References
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230, https://doi.org/10.1038/35065638 (2001).
Guerra, C. et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302, https://doi.org/10.1016/j.ccr.2007.01.012 (2007).
Dryden, S. C., Nahhas, F. A., Nowak, J. E., Goustin, A. S. & Tainsky, M. A. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol 23, 3173–3185 (2003).
Serrano, L. et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev 27, 639–653, https://doi.org/10.1101/gad.211342.112 (2013).
Wang, F. & Tong, Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1′s repressive interaction with PPARgamma. Mol Biol Cell 20, 801–808, https://doi.org/10.1091/mbc.E08-06-0647 (2009).
Zhang, M. et al. Sirtinol promotes PEPCK1 degradation and inhibits gluconeogenesis by inhibiting deacetylase SIRT2. Sci Rep 7, 7, https://doi.org/10.1038/s41598-017-00035-9 (2017).
Arora, A. & Dey, C. S. SIRT2 regulates insulin sensitivity in insulin resistant neuronal cells. Biochem Biophys Res Commun 474, 747–752, https://doi.org/10.1016/j.bbrc.2016.05.029 (2016).
Krishnan, J. et al. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev 26, 259–270, https://doi.org/10.1101/gad.180406.111 (2012).
Ramakrishnan, G. et al. Sirt2 deacetylase is a novel AKT binding partner critical for AKT activation by insulin. J Biol Chem 289, 6054–6066, https://doi.org/10.1074/jbc.M113.537266 (2014).
Kim, H. S. et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 20, 487–499, https://doi.org/10.1016/j.ccr.2011.09.004 (2011).
Song, H. Y. et al. SIRT2 deletion enhances KRAS-induced tumorigenesis in vivo by regulating K147 acetylation status. Oncotarget 7, 80336–80349, https://doi.org/10.18632/oncotarget.12015 (2016).
Yang, M. H. et al. HDAC6 and SIRT2 regulate the acetylation state and oncogenic activity of mutant K-RAS. Mol Cancer Res 11, 1072–1077, https://doi.org/10.1158/1541-7786.Mcr-13-0040-t (2013).
Ji, B. et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology 137, 1072–1082, 1082 e1071-1076, https://doi.org/10.1053/j.gastro.2009.05.052 (2009).
Yakubovskaya, M. S. et al. High frequency of K-ras mutations in normal appearing lung tissues and sputum of patients with lung cancer. Int J Cancer 63, 810–814 (1995).
Yan, L. et al. Molecular analysis to detect pancreatic ductal adenocarcinoma in high-risk groups. Gastroenterology 128, 2124–2130 (2005).
Kloppel, G., Dreyer, T., Willemer, S., Kern, H. F. & Adler, G. Human acute pancreatitis: its pathogenesis in the light of immunocytochemical and ultrastructural findings in acinar cells. Virchows Arch A Pathol Anat Histopathol 409, 791–803 (1986).
Willemer, S. & Adler, G. Histochemical and ultrastructural characteristics of tubular complexes in human acute pancreatitis. Dig Dis Sci 34, 46–55 (1989).
Willemer, S., Kloppel, G., Kern, H. F. & Adler, G. Immunocytochemical and morphometric analysis of acinar zymogen granules in human acute pancreatitis. Virchows Arch A Pathol Anat Histopathol 415, 115–123 (1989).
Yadav, D. & Lowenfels, A. B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144, 1252–1261, https://doi.org/10.1053/j.gastro.2013.01.068 (2013).
Lampel, M. & Kern, H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol 373, 97–117 (1977).
Lerch, M. M. & Gorelick, F. S. Models of acute and chronic pancreatitis. Gastroenterology 144, 1180–1193, https://doi.org/10.1053/j.gastro.2012.12.043 (2013).
Saluja, A. et al. In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physiol 249, G702–710, https://doi.org/10.1152/ajpgi.1985.249.6.G702 (1985).
Eisses, J. F. et al. A computer-based automated algorithm for assessing acinar cell loss after experimental pancreatitis. PLoS One 9, e110220, https://doi.org/10.1371/journal.pone.0110220 (2014).
Murtaugh, L. C. & Keefe, M. D. Regeneration and repair of the exocrine pancreas. Annu Rev Physiol 77, 229–249, https://doi.org/10.1146/annurev-physiol-021014-071727 (2015).
Stanger, B. Z. & Hebrok, M. Control of cell identity in pancreas development and regeneration. Gastroenterology 144, 1170–1179, https://doi.org/10.1053/j.gastro.2013.01.074 (2013).
Adler, G., Hupp, T. & Kern, H. F. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch A Pathol Anat Histol 382, 31–47 (1979).
Jensen, J. N. et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128, 728–741 (2005).
Niederau, C., Ferrell, L. D. & Grendell, J. H. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 88, 1192–1204 (1985).
Rothgiesser, K. M., Erener, S., Waibel, S., Luscher, B. & Hottiger, M. O. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123, 4251–4258, https://doi.org/10.1242/jcs.073783 (2010).
Lo Sasso, G. et al. SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS One 9, e103573, https://doi.org/10.1371/journal.pone.0103573 (2014).
Lin, J., Sun, B., Jiang, C., Hong, H. & Zheng, Y. Sirt2 suppresses inflammatory responses in collagen-induced arthritis. Biochem Biophys Res Commun 441, 897–903, https://doi.org/10.1016/j.bbrc.2013.10.153 (2013).
Yuan, F. et al. SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-kappaB p65 acetylation and activation. J Neurochem 136, 581–593, https://doi.org/10.1111/jnc.13423 (2016).
Foster, A. D., Sivarapatna, A. & Gress, R. E. The aging immune system and its relationship with cancer. Aging health 7, 707–718, https://doi.org/10.2217/ahe.11.56 (2011).
Aguirre, A. J. et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 17, 3112–3126, https://doi.org/10.1101/gad.1158703 (2003).
Hingorani, S. R. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003).
Klimstra, D. S. & Longnecker, D. S. K-ras mutations in pancreatic ductal proliferative lesions. Am J Pathol 145, 1547–1550 (1994).
Eshleman, J. R. et al. KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol 13, 963–969 e964, https://doi.org/10.1016/j.cgh.2014.11.028 (2015).
Spector, I. et al. Involvement of host stroma cells and tissue fibrosis in pancreatic tumor development in transgenic mice. PLoS One 7, e41833, https://doi.org/10.1371/journal.pone.0041833 (2012).
Carriere, C., Young, A. L., Gunn, J. R., Longnecker, D. S. & Korc, M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun 382, 561–565, https://doi.org/10.1016/j.bbrc.2009.03.068 (2009).
Fendrich, V. et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology 135, 621–631, https://doi.org/10.1053/j.gastro.2008.04.011 (2008).
Maniati, E. et al. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Ppargamma expression and promotes pancreatic cancer progression in mice. J Clin Invest 121, 4685–4699, https://doi.org/10.1172/JCI45797 (2011).
Siveke, J. T. et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology 134, 544–555, https://doi.org/10.1053/j.gastro.2007.11.003 (2008).
Kreuger, J. & Kjellen, L. Heparan sulfate biosynthesis: regulation and variability. J Histochem Cytochem 60, 898–907, https://doi.org/10.1369/0022155412464972 (2012).
Hyun, J. J. & Lee, H. S. Experimental models of pancreatitis. Clin Endosc 47, 212–216, https://doi.org/10.5946/ce.2014.47.3.212 (2014).
Kim, H. Caerulein pancreatitis: oxidative stress, inflammation, and apoptosis. Gut Liver 2, 74–80, https://doi.org/10.5009/gnl.2008.2.2.74 (2008).
Swidnicka-Siergiejko, A. K. et al. Chronic inflammation initiates multiple forms of K-Ras-independent mouse pancreatic cancer in the absence of TP53. Oncogene 36, 3149–3158, https://doi.org/10.1038/onc.2016.461 (2017).
Boggs, K. et al. Pancreatic gene expression during recovery after pancreatitis reveals unique transcriptome profiles. Sci Rep 8, 1406, https://doi.org/10.1038/s41598-018-19392-0 (2018).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14, 528–536, https://doi.org/10.1016/j.cmet.2011.08.014 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550, https://doi.org/10.1186/s13059-014-0550-8 (2014).
Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530, https://doi.org/10.1093/bioinformatics/btt703 (2014).
Acknowledgements
D.G. is supported by 2R01CA152601-A1, 1R01CA152799-01A1, 1R01CA168292-01A1, 1R01CA214025-01, the Avon Foundation for Breast Cancer Research, the Lynn Sage Cancer Research Foundation, the Zell Family Foundation, Hirshberg Foundation for Pancreatic Cancer Research, and the Chicago Biomedical Consortium, as well as support from the Searle Funds at The Chicago Community Trust. Imaging work was performed at the Northwestern University Center for Advanced Microscopy generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. Melissa Stauffer, PhD, of Scientific Editing Solutions, provided editorial assistance. The authors acknowledged Drs Kristy Boggs and Sohail Z. Husain for providing a whole differentially expressed genes list described in their manuscript. The authors thanks Dr. Hidayatullah G. Munshi for his helpful comments on the manuscript. The authors also thank to Drs. Matthew J. Schipma and Brian Wray for the initial analysis of differentially expressed genes.
Author information
Authors and Affiliations
Contributions
S.Q., D.R.P., P.J.G., D.R.G. and N.H. designed the research. S.Q., D.R.P., S.-H.P. performed the research. S.Q., D.R.P., A.E.D., D.R.G. and N.H. analyzed the data. S.Q., D.R.P., A.E.D., S.-H.P., P.J.G., D.R.G. and N.H. participated in intellectual discussions. S.Q., D.R.P., A.E.D., D.R.G. and N.H. wrote the paper. All authors approved the final edited version.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quan, S., Principe, D.R., Dean, A.E. et al. Loss of Sirt2 increases and prolongs a caerulein-induced pancreatitis permissive phenotype and induces spontaneous oncogenic Kras mutations in mice. Sci Rep 8, 16501 (2018). https://doi.org/10.1038/s41598-018-34792-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34792-y
- Springer Nature Limited
Keywords
This article is cited by
-
SIRT2 alleviated renal fibrosis by deacetylating SMAD2 and SMAD3 in renal tubular epithelial cells
Cell Death & Disease (2023)
-
SIRT2-mediated deacetylation and deubiquitination of C/EBPβ prevents ethanol-induced liver injury
Cell Discovery (2021)