Abstract
Shift metabolism profile from mitochondrial oxidative phosphorylation to aerobic glycolysis (Warburg effect) is a key for tumor cell growth and metastasis. Therefore, suppressing the tumor aerobic glycolysis shows a great promise in anti-tumor therapy. In the present study, we study the role of shikonin, a naphthoquinone isolated from the traditional Chinese medicine Lithospermum, in inhibiting tumor aerobic glycolysis and thus tumor growth. We found that shikonin dose-dependently inhibited glucose uptake and lactate production in Lewis lung carcinoma (LLC) and B16 melanoma cells, confirming the inhibitory effect of shikonin on tumor aerobic glycolysis. Treatment of shikonin also decreased tumor cell ATP production. Furthermore, pyruvate kinase M2 (PKM2) inhibitor or activator respectively altered the effect of shikonin on tumor cell aerobic glycolysis, suggesting that suppression of cell aerobic glycolysis by shikonin is through decreasing PKM2 activity. Western blot analysis confirmed that shikonin treatment reduced tumor cell PKM2 phosphorylation though did not reduce total cellular PKM2 level. In vitro assay also showed that shikonin treatment significantly promoted tumor cell apoptosis compared to untreated control cells. Finally, when mice implanted with B16 cells were administered with shikonin or control vehicle, only shikonin treatment significantly decreased B16 tumor cell growth. In conclusion, this study demonstrates that shikonin inhibits tumor growth in mice by suppressing PKM2-mediated aerobic glycolysis.
Similar content being viewed by others
Introduction
Compared to normal non-proliferating cells, tumor cells display a high aerobic glycolysis (Warburg effect). In fact, metabolic switch from oxidative phosphorylation to aerobic glycolysis is a major feature of tumor cell and a key for tumor cell maintaining rapid growth and metastasis1,2,3,4. As the final rate-limiting enzyme of cell glycolysis, pyruvate kinase M2 (PKM2) plays a critical role in tumor cell metabolic switch from oxidative phosphorylation to aerobic glycolysis5,6,7. Therefore, reagents that can suppressive aerobic glycolysis particularly modulating PKM2 activity have shown a great potential in developing anti-tumor drug8.
Shikonin is a natural product isolated from the roots of the Chinese herbs Lithospermum erythrorhizon, Arnebia euchroma and Onosma paniculata9,10,11. Previous studies demonstrate that shikonin has a broad therapeutic effects ranging from anti-inflammatory, anti-oxidant, anti-cancer, wound healing to anti-microbial12,13,14. Recently shikonin has been shown to kill certain cancer cells and inhibit the migration and invasion of cancer cells15 through a number of possible mechanisms, including the inhibition of protein tyrosine kinase (PTK)16, the activities of DNA topoisomerases17, and tumor necrosis factor receptor-associated protein 1 (TRAP1) expression18. Other mechanisms involved in shikonin-induced cancer cell death include upregulation of p5319. However, the exact mechanism by which shikonin inhibits tumor cell proliferation, migration and invasion remains incompletely understood. It is not clear whether shikonin can be used as an effective anti-cancer reagent in vitro and in vivo.
In the present study, we tested the effect of shikonin on the proliferation and apoptosis of various cancer cells in vitro and in vivo. Our results show that shikonin dose-dependently inhibits tumor cell aerobic glycolysis and growth while promotes tumor cell apoptosis. The mechanistic study also suggests that the mechanism underlying the anti-cancer effect of shikonin is to inhibit the phosphorylation of PKM2 and thus suppress PKM2-switched tumor cell aerobic glycolysis.
Results
Shikonin suppresses tumor cell proliferation
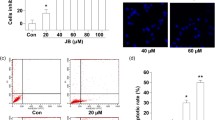
Previous study in our laboratory has demonstrated that shikonin could reduce exocytosis process in tumor cells20, an event closely related to tumor cell aerobic glycolysis. To test the effect of shikonin on tumor cell growth, we assessed the proliferation of LLC and B16 tumor cells in the presence of various concentration of shikonin. Shikonin was dissolved in DMSO and DMSO served as vehicle control. As shown in Fig. 1, after 24 h treatment, shikonin dose-dependently inhibited both LLC and B16 tumor cell growth, suggesting that shikonin may affect a common signaling pathway of tumor proliferation.
Shikonin inhibits tumor cell aerobic glycolysis
Compared to normal cells, rapid proliferating tumor cells switch their metabolism from oxidative phosphorylation to aerobic glycolysis (Warburg effect)21,22,23. Given that aerobic glycolysis is a key for most tumor cells to maintain rapid growth and metastasis, we tested whether shikonin inhibited tumor cell growth via blocking the aerobic glycolysis in tumor cells. In this experiment, we assessed lactate production and glucose uptake in B16 cells with or without shikonin treatment. As shown in Fig. 2, shikonin dose-dependently reduced the glucose uptake and lactate production in B16 cells, suggesting that shikonin suppressed tumor cell aerobic glycolysis. This observation is in agreement with previous findings of shikonin as a Warburg effect inhibitor2,24. In agreement with that shikonin treatment suppressed tumor cell aerobic glycolysis, the ATP level in B16 cells was decreased by shikonin in a dose-dependent manner (Fig. 3).
Shikonin suppresses tumor cell aerobic glycolysis. (A) Shikonin (SK) decreased relative glucose uptake in B16 cells in a dose-dependent manner. (B) Shikonin (SK) reduced relative lactate production in B16 cells in a dose-dependent manner. Data were presented as a means ± SD of 3–4 individual experiments with three samples under each condition. *P < 0.05. **P < 0.01.
Shikonin inhibits cell aerobic glycolysis via decreasing PKM2 phosphorylation
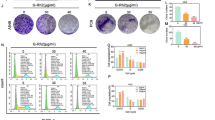
Tumor cell aerobic glycolysis is controlled by various factors, including glycolysis substrates and metabolic rate-limiting enzymes. As the final rate-limiting enzyme, PKM2 has been shown to play a critical role in switching tumor cell metabolism from oxidative phosphorylation to aerobic glycolysis3,4,25. To test whether PKM2 is involved in the inhibitory effect of shikonin on tumor cell aerobic glycolysis, we transfected tumor cells with PKM2 siRNA to knock down PKM2 level and then treated tumor cells with shikonin. As shown in Fig. 4A,B, control cells treated with 10 nM shikonin displayed a significantly reduced glucose uptake and lactate production. In contrast, tumor cells treated with PKM2 siRNA showed no significant difference of glucose uptake and lactate production before and after shikonin treatment. This result implies that shikonin may execute its function through altering PKM2 expression. It has been known that PKM2 activity can be modulated by pTyr, a phosphotyrosine peptide that can specifically phosphorylate PKM2 and promote PKM2 dimeric formation4, as well as fructose (1,6) bisphosphate (FBP) and serine (Ser), which arrest PKM2 in their active tetramer confirmation3. We also treated tumor cells with pTyr, FBP or Ser in the presence or absence of shikonin. The results showed that control cells (treated with PBS solution) displayed a lower glucose uptake and lactate production following 10 μM shikonin treatment, whereas no significant effect of shikonin on tumor cell aerobic glycolysis was detected after modulating PKM2 activity with pTyr, FBP or Ser (Fig. 4C,D). These results are in agreement with the notion that shikonin inhibits tumor cell aerobic glycolysis through affecting PKM2 expression or activity.
Effect of shikonin on suppressing tumor cell aerobic glycolysis is dependent on PKM2. (A) Knockdown of PKM2 in B16 cells via PKM2 siRNA abolished the effect of shikonin on tumor cell glucose uptake. (B) Knockdown of PKM2 in B16 cells abolished the effect of shikonin on tumor cell lactate production. (C) Modulation of PKM2 activity affected the relative glucose uptake in B16 cells. (D) Modulation of PKM2 activity affected the relative lactate production in B16 cells. Data were presented as a means ± SD of three individual experiments with three samples under each condition. *P < 0.05.
To test this, we tested the protein expression level and phosphorylation of PKM2 in B16 cells with or without shikonin treatment by western blot analysis. As shown in Fig. 5A,B, shikonin treatment dose-dependently reduced the PKM2 phosphorylation in B16 cells although it did not affect the tumor cell PKM2 protein level. The result suggests that shikonin treatment may affect the PKM2 activity instead of expression level.
Shikonin (SK) treatment decreases phosphorylation of PKM2 (p-PKM2). (A) Representative western blot images of p-PKM2, PKM2 and GAPDH in B16 cells treated with or without shikonin. The raw WB data of Fig. 5A were shown in Supplementary Material. (B) Analysis results of western blot images in panel A. Data were presented as a means ± SD of three individual experiments. *P < 0.05. **P < 0.01.
Shikonin promotes tumor cell apoptosis
Given that aerobic glycolysis plays a critical role in tumor cell survival22,23, we next examined the potential effect of shikonin on tumor cells apoptosis using flow cytometry. In this experiment, B16 cells and gastric cancer MKN-45 cells were treated with 0, 1 or 10 μM shikonin at different time points, respectively. Cells were then labeled with fluorescently Annexin V–FITC and propidium iodide (PI) for measuring early and late apoptosis of tumor cells24. As shown in Fig. 6, shikonin treatment increased apoptosis of two different tumor cells in both dose-dependent and time-dependent manner.
Shikonin treatment increases tumor cell apoptosis in a dose-dependent and time-dependent manner. (A) Left panel: representative flow cytometry image of B16 cell apoptosis labeled with FITC-Annexin V/PI. Right panel: analysis results of flow cytometry images. (B) Left panel: representative flow cytometry image of gastric cancer cell apoptosis labeled with FITC-Annexin V/PI. Right panel: analysis results of flow cytometry images. Data were presented as a means ± SD of three individual experiments in triplicate. *P < 0.05. **P < 0.01. ***P < 0.001.
Shikonin suppresses tumor cell growth in mouse model
To test the effect of shikonin on tumor growth in vivo, we performed experiments using 6-week-old male SCID mice. In the experiment, B16 melanoma cells were injected subcutaneously into SCID mice (1 × 106 cells per mouse, 6 mice per group). After the xenografts were established, the tumor-bearing mice were administered with shikonin (0, 0.1, 1 and 10 mg/kg, respectively) via intraperitoneal injection. As shown in Fig. 7, shikonin treatment inhibited B16 cell growth in SCID mice in a dose-dependent manner compared to PBS (control) and DMSO treatment. A significant reduction of tumor size (Fig. 7B) and weight (Fig. 7C) was observed when shikonin was injected at concentration of 1 or 10 mg/kg.
Shikonin treatment inhibits the growth of implanted tumor in SCID mice. (A) Representative tumor images in SCID mice implanted with B16 melanoma. (B) B16 tumor size in mice on various days following injection with various doses of shikonin. (C) B16 tumor weight in mice on day 9 after treatment with various doses of shikonin. Data were presented as a means ± SD (n = 6). **P < 0.01.
Discussion
In the present study, we demonstrate that shikonin can inhibit tumor proliferation in vitro and in vivo through decreasing PKM2-mediated aerobic glycolysis switch in tumor cells. This study provides shikonin as an effective anti-cancer drug candidate.
In recent years, accumulating evidences demonstrate that metabolic switch from oxidative phosphorylation to aerobic glycolysis (Warburg effect) is critical for tumor cells maintaining high proliferation and metastasis21,22,23. Blockade of tumor cell aerobic glycolysis particularly the PKM2-mediated aerobic glycolysis switch thus shows a great potential in anti-cancer therapy. Employing cell and mouse model, we have characterized the inhibitory effect of shikonin on tumor cell proliferation, as well as the possible mechanism under such event. Several pieces of evidence support that shikonin inhibits tumor proliferation through decreasing PKM2-mediated aerobic glycolysis switch. Firstly, shikonin reduced the proliferation of LLC and B16 tumor cells and this effect was correlated with its inhibitory effect on tumor cell aerobic glycolysis, Secondly, the effect of shikonin on suppressing tumor cell aerobic glycolysis could be offset by modulating PKM2 level and activity. As shown in Fig. 4, PKM2 knockdown in tumor cells via PKM2 siRNA or modulation of PKM2 activity by pTyr, FBP or serine largely abolished the inhibition of tumor cell aerobic glycolysis by shikonin. Finally, western blot analysis directly showed that shikonin treatment decreased the phosphorylation of PKM2 in B16 cells though did not affect the total cellular PKM2 level.
Although our results demonstrate that shikonin suppresses tumor cell aerobic glycolysis via inhibiting PKM2 phosphorylation, the molecular basis of reduction of PKM2 phosphorylation by shikonin remains unknown at this stage. Through studying the activity of PKM2 after treating PKM2 with different small molecules, previous studies have shown that PKM2 Activator II (DASA), as well as glycolytic intermediates FBP and serine, can modulate PKM2 activity through arresting PKM2 in tetramer structural form26,27. It may be true that shikonin affects PKM2 activity in a similar manner. In addition, as protein kinase Akt2 has been reported to be able to promote PKM2 phosphorylation in tumor cells28,29, shikonin may inhibit PKM2 phosphorylation through suppressing the expression and activity of such protein kinase. Given that PKM2 phosphorylation may switch the conformation of PKM2 from tetramer to dimer, inhibition of PKM2 phosphorylation by shikonin may have a similar role in preventing PKM2 tetramer-to-dimer conformation switch.
Materials and Methods
Animal model
6-week-old male severe combined immune deficiency (SCID) mice (nu/nu) were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China) and maintained under specific pathogen-free conditions at Nanjing University. The experiments on mice were approved by Institutional Animal Care and Use Committee, Nanjing University, and all experiments were performed in accordance with relevant guidelines and regulations. B16 melanoma cells were injected subcutaneously into SCID mice (106 cells per mouse, 6 mice per group). After the xenografts were established, the tumor-bearing mice were administered with shikonin (0, 0.1, 1, 10 mg/kg) via intraperitoneal injection. Shikonin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in DMSO (Sigma-Aldrich). The length, width and height of the tumors were measured with digital calipers every day and tumor volume was calculated accordingly30. On the ninth day, the mice were sacrificed and the tumors were weighed.
Cell culture
B16 cells and gastric cancer MKN-45 cells were obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China) and maintained in RPMI 1640 medium (Gibco, NY) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin within a humidified atmosphere containing 5% CO2 at 37 °C. Cells using for functional and mechanism studies in this study were tested and authenticated using short tandem repeat (STR) method by Shanghai Institute of Cell Biology. For evaluating the effect of shikonin on the viability and metabolic status of B16 cells, different concentration of shikonin were added into B16 cell culture medium and cells were incubated for 24 h.
Cell proliferation
Cell proliferation was assayed by WST (water-soluble tetrazolium salt) assay using Cell Counting Kit-8 (Sigma-Aldrich) according to the manufacturer’s instructions31. Briefly, LLC and B16 cells were seeded into 96-well plates (Corning) at a density of 104 cells per well in DMEM and incubated for 24 h (37 °C and 5% CO2). The medium was then replaced with either serum-free DMEM or serum-free DMEM containing various concentrations (0, 0.01, 0.1, 1 or 10 µM) of shikonin (the total volume in each well was 200 µl). After incubation for another 24 h, the number of viable cells was determined by measurement of the absorbance (OD450 nm).
Apoptosis assay
Apoptosis of cells was detected using an Annexin V–FITC/propidium iodide (PI) staining assay. Flow cytometric analysis of apoptotic cells was carried out using an Annexin V–FITC/PI staining kit (Invitrogen). After washes with cold PBS, the cells were re-suspended in binding buffer (100 mM HEPES, 100 mM NaCl, and 25 mM CaCl2, pH 7.4) and stained with Annexin V-FITC/PI at room temperature in darkness for 15 min. Apoptotic cells were then evaluated by gating PI and Annexin V–positive cells on an FACSCalibur (BD Biosciences). All experiments were performed in triplicate.
Western blot
Cellular proteins were extracted as described previously20,24. Antibodies against PKM2, p-PKM2 purchased from Abcam (Shanghai, China) were used for western blotting. GAPDH (Cell Signaling Technology, CA) served as an internal control.
Measurement of lactate production, glucose uptake and ATP production
The lactate level in the cell culture medium was measured with lactate assay kit (#K607-100, BioVision, Milpitas, CA, USA) according to the method described previously32. Glucose in cell lysates was measured with glucose assay kit (BioVision, #K606-100). For detecting glucose uptake and lactate production, the culture supernatants of tumor cells with different treatments were collected and the fresh culture media was used as control. Equal amounts (2–10 µl) of samples were added to a 96-well plate and the volume of each well was then adjusted to 50 µl with Glucose or Lactate Assay Buffer. Meantime, a standard curve was prepared with the same protocol. After 30 min reaction at 37 °C in dark, the absorbance (OD570 nm) or fluorescence intensity (Ex/Em = 535/590 nm) were measured. The uptake of glucose was determined by subtracting the glucose level in tested samples from the initial glucose level in fresh media. The production of lactate was determined referring standard curve. Considering the cell number of individual sample may be different, all the levels of glucose or production of lactate were finally normalized to the protein level. ATP levels were measured using an ATP assay kit (Celltiter-Glo Luminescent Cell Viability Assay, Promega).
Statistical analysis
Each experiment was representative of at least three independent experiments. The data were presented as the means ± SD of at least three independent experiments. Differences between groups were analyzed using Student’s t-test and the differences were considered to be statistically significant at P < 0.05.
References
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27, 441–464 (2011).
Yang, L. et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 5, 4436 (2014).
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008).
Christofk, H. R., Vander Heiden, M. G., Wu, N., Asara, J. M. & Cantley, L. C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452, 181–186 (2008).
Tamada, M., Suematsu, M. & Saya, H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res 18, 5554–5561 (2012).
Palsson-McDermott, E. M. & O’Neill, L. A. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays 35, 965–973 (2013).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013).
Ganapathy-Kanniappan, S. & Geschwind, J. F. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12, 152 (2013).
Huang, W. R., Zhang, Y. & Tang, X. Shikonin inhibits the proliferation of human lens epithelial cells by inducing apoptosis through ROS and caspase-dependent pathway. Molecules 19, 7785–7797 (2014).
Liu, T. et al. Optimization of shikonin homogenate extraction from Arnebia euchroma using response surface methodology. Molecules 18, 466–481 (2013).
Damianakos, H. et al. Antimicrobial and cytotoxic isohexenylnaphthazarins from Arnebia euchroma (Royle) Jonst. (Boraginaceae) callus and cell suspension culture. Molecules 17, 14310–14322 (2012).
Andujar, I., Rios, J. L., Giner, R. M. & Recio, M. C. Pharmacological properties of shikonin - a review of literature since 2002. Planta Med 79, 1685–1697 (2013).
Papageorgiou, V. P., Assimopoulou, A. N. & Ballis, A. C. Alkannins and shikonins: a new class of wound healing agents. Curr Med Chem 15, 3248–3267 (2008).
Kourounakis, A. P., Assimopoulou, A. N., Papageorgiou, V. P., Gavalas, A. & Kourounakis, P. N. Alkannin and shikonin: effect on free radical processes and on inflammation - a preliminary pharmacochemical investigation. Arch Pharm (Weinheim) 335, 262–266 (2002).
Jang, S. Y., Lee, J. K., Jang, E. H., Jeong, S. Y. & Kim, J. H. Shikonin blocks migration and invasion of human breast cancer cells through inhibition of matrix metalloproteinase-9 activation. Oncol Rep 31, 2827–2833 (2014).
Masuda, Y. et al. Beta-hydroxyisovalerylshikonin induces apoptosis in human leukemia cells by inhibiting the activity of a polo-like kinase 1 (PLK1). Oncogene 22, 1012–1023 (2003).
Chen, X., Yang, L., Oppenheim, J. J. & Howard, M. Z. Cellular pharmacology studies of shikonin derivatives. Phytother Res 16, 199–209 (2002).
Masuda, Y. et al. Involvement of tumor necrosis factor receptor-associated protein 1 (TRAP1) in apoptosis induced by beta-hydroxyisovalerylshikonin. J Biol Chem 279, 42503–42515 (2004).
Kroemer, G. & Pouyssegur, J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13, 472–482 (2008).
Wei, Y., Li, L., Wang, D., Zhang, C. Y. & Zen, K. Importin 8 regulates the transport of mature microRNAs into the cell nucleus. J Biol Chem 289, 10270–10275 (2014).
Bensinger, S. J. & Christofk, H. R. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol 23, 352–361 (2012).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Elstrom, R. L. et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64, 3892–3899 (2004).
Wei, Y. et al. Shikonin Inhibits the Proliferation of Human Breast Cancer Cells by Reducing Tumor-Derived Exosomes. Molecules 21 (2016).
Altenberg, B. & Greulich, K. O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 84, 1014–1020 (2004).
Eigenbrodt, E., Reinacher, M., Scheefers-Borchel, U., Scheefers, H. & Friis, R. Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Crit Rev Oncog 3, 91–115 (1992).
Gupta, V. & Bamezai, R. N. Human pyruvate kinase M2: a multifunctional protein. Protein Sci 19, 2031–2044 (2010).
Salani, B. et al. IGF1 regulates PKM2 function through Akt phosphorylation. Cell Cycle 14, 1559–1567 (2015).
Wang, C. et al. Inactivation of Spry2 accelerates AKT-driven hepatocarcinogenesis via activation of MAPK and PKM2 pathways. J Hepatol 57, 577–583 (2012).
Jin, F. et al. The miR-125a/HK2 axis regulates cancer cell energy metabolism reprogramming in hepatocellular carcinoma. Sci Rep 7, 3089 (2017).
Li, P. et al. Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Res 25, 588–603 (2015).
Peng, F. et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene 37, 1062–1074 (2018).
Acknowledgements
This work is supported by grants from PLA Nanjing Military Region and the program for New Century Excellent Talents in University from the Ministry of Education, China (NCET-12-0261).
Author information
Authors and Affiliations
Contributions
Xiaoyue Zhao, Yanan Zhu, Jianhua Hu and Longwei Jiang performed the experiments, analyzed the data and prepared the manuscript. Ke Zen, Shaochang Jia and Limin Li designed the study and were also involved in data analysis. Ke Zen and Limin Li wrote the manuscript. All authors reviewed and approved the text and figures.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, X., Zhu, Y., Hu, J. et al. Shikonin Inhibits Tumor Growth in Mice by Suppressing Pyruvate Kinase M2-mediated Aerobic Glycolysis. Sci Rep 8, 14517 (2018). https://doi.org/10.1038/s41598-018-31615-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31615-y
- Springer Nature Limited
Keywords
This article is cited by
-
Mitochondrial respiratory chain component NDUFA4: a promising therapeutic target for gastrointestinal cancer
Cancer Cell International (2024)
-
Wheat germ agglutinin modified mixed micelles overcome the dual barrier of mucus/enterocytes for effective oral absorption of shikonin and gefitinib
Drug Delivery and Translational Research (2024)
-
Anoikis and cancer cell differentiation: novel modes of shikonin derivatives anticancer action in vitro
Molecular Biology Reports (2024)
-
Pien Tze Huang regulates phosphorylation of metabolic enzymes in mice of hepatocellular carcinoma
Scientific Reports (2023)
-
Nanosystem-mediated lactate modulation in the tumor micro environment for enhanced cancer therapy
Nano Research (2023)