Abstract
An isotropic periodic sum (IPS) is a powerful technique to reasonably calculate intermolecular interactions for wide range of molecular systems under periodic boundary conditions. A linear-combination-based IPS (LIPS) has been developed to attain computational accuracy close to an exact lattice sum, such as the Ewald sum. The algorithm of the original LIPS method has a high computational cost because it needs long-range interaction calculations in real space. This becomes a performance bottleneck for long-time molecular simulations. In this work, the combination of an LIPS and fast Fourier transform (FFT) was developed, and evaluated on homogeneous and heterogeneous molecular systems. This combinational approach of LIPS/FFT attained computational efficiency close to that of a smooth particle mesh Ewald while maintaining the same high accuracy as the original LIPS. We concluded that LIPS/FFT has great potential to extend the capability of IPS techniques for the fast and accurate computation of many types of molecular systems.
Similar content being viewed by others
Introduction
Molecular dynamics (MD) simulations continue to evolve. Recent advances in computer power and algorithmic developments have made it possible to simulate a wide range of molecular systems with reasonable time and length scales for scientific and industrial applications1,2,3,4,5.
The computational accuracy of MD has been supported by established molecular models and intermolecular interaction calculation methods6,7,8,9,10,11,12,13,14. Importantly, the accuracy of long-range interaction calculations strongly affects to the accuracy of MD. Under the periodic boundary conditions (PBCs), long-range interactions are ordinally calculated using truncation or lattice sum methods. The simplicity and calculation efficiency are merits of truncation methods, but truncation of long-range interactions can be serious defects on simulating Lennard-Jones (LJ) liquids15,16,17, aqueous18,19,20,21,22,23,24,25,26,27,28, and macromolecular systems29,30,31,32,33,34,35,36,37,38,39,40,41,42,43. The reaction field method44,45, the Wolf method and its modifications46,47, and the smoothing and/or shifting cutoff methods are kinds of truncation methods that have serious defects for various systems (see above citations). The Ewald sum48 is used as the most accurate lattice sum method. It incorporates PBCs using the discrete Fourier transform (DFT), but the reciprocal term is computationally expensive. The particle mesh Ewald (PME) and its modifications49,50 are particle-particle/particle-mesh approaches that use fast Fourier transforms (FFT) for the Ewald sum, and the smooth PME (SPME)50 is now the de facto standard lattice sum method. The SPME perform well for intermediate-sized systems (\(N\sim {10}^{3}-{10}^{5}\), where N is the number of particles)51. However, the SPME does not have a high performance for large systems (\(N\sim {10}^{6}\) or larger), because it is difficult for FFT to attain strong scaling on massively parallel machines52,53. The Barnes-Hut tree-code54 and the fast multipole method55 are lattice sum methods that use hierarchical tree structures. These tree-based method can attain stronger scaling than FFT because it does not contain reciprocal space calculations that require all-to-all communications on massively parallel machines53. The tree-based method has been applied for long-range interaction calculations of molecular simulations52,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70. The Gaussian split Ewald sum71 is a combination between the Ewald sum and Poisson equation solver, and can avoid all-to-all communications on massively parallel machines. Importantly, all the lattice sum methods are potentially subject to the symmetry effect that comes from the lattice-like repetition of the unit cell for PBC. This effect is especially troublesome when simulating macromolecular systems, where conformational distributions are sensitive to image interactions38,40,72,73,74,75,76,77,78,79,80. More research into the lattice sum method is required, and the symmetry effect should be carefully considered.
To avoid the symmetry effect, the isotropic periodic sum (IPS) technique is a promising approach. It was first developed by Wu and Brooks81. The IPS has become a powerful technique to reasonably calculate intermolecular interactions for a wide range of molecular systems, including net-charge systems under PBCs. It has been applied to solids82, liquids81,83,84,85,86, solid–liquid87,88 and liquid-vapor interfaces89,90, liquid crystals91, proteins81,92, lipids89,93, combined quantum mechanics/molecular mechanics methods94, and constant pH MD simulations95,96,97. Improved methods have been developed for large-scale systems that exploit the possibility of parallel computing98,99.
An extended IPS technique, that is, the linear-combination-based IPS (LIPS) was developed to improve the accuracy of the IPS for both homogeneous and heterogeneous systems7,100,101,102,103. The LIPS provides periodic reaction fields that can design pseudo pair potentials in the range of extended IPS theory. This pseudo pair potential has the high accuracy that achieves computational results close to an exact lattice sum. For example, in the phase transition of liquid crystal systems, the LIPS and SPME with fine grid spacing are the only techniques that can reasonably estimate the solid–liquid-crystalline phase transition temperature104. The LIPS has great potential as one of the best possible approaches to contribute to a further substantial advance in IPS techniques. However, the algorithm of the original LIPS has a high computational cost because it requires long-range interaction calculations in real space. This becomes a performance bottleneck for long-time molecular simulations using the LIPS. Several possible approaches to raise the computational efficiency of the LIPS were suggested in the original paper100, but have not been applied.
In the present work, the combination of the LIPS and fast Fourier transform (FFT), that is, LIPS/FFT, is developed as a substantial advance in the computational efficiency of the LIPS for intermediate-sized systems \((N\sim {10}^{3}\,\mbox{--}\,{10}^{5})\). The performance of LIPS/FFT is evaluated on homogeneous and heterogeneous polar molecular systems. LIPS/FFT attains computational efficiency close to the SPME while maintaining the high accuracy of the LIPS. We conclude that LIPS/FFT has great potential to extend the capability of IPS techniques for the fast and accurate computation of many types of molecular systems.
Methodology
LIPS/FFT method
In the LIPS method, pseudo pair potentials are designed using extended IPS theory, which provides periodic reaction fields100,101. Several types of pseudo pair potentials have been developed100,101, and these can be expressed as the following equation generally:
where uLIPS is the LIPS pseudo pair potential; r is the interaction distance; Rc is the cutoff radius of the LIPS, which is closely related to the production of periodic reaction fields; u is an original pair potential, such as Coulomb interaction; and ϕ is the effective potential from the periodic reaction field. The LIPS pseudo pair potential has high accuracy. The previous paper demonstrated that one of the pseudo pair potentials, LIPS-SW, had the same accuracy as the SPME with fine grid spacing (less than 0.1 nm), for the estimation of the solid–liquid-crystalline phase transition temperature104. To attain high accuracy, the LIPS potentials require a large \({R}_{{\rm{c}}}\) condition Rc = L/2, where L is the length of the longest side of the simulation box. This means long-range interaction calculations in real space, and becomes a performance bottleneck for MD simulations using the LIPS. To avoid the aforementioned difficulty and attain advanced computational efficiency, the combination of the LIPS and FFT is developed as follows: (i) The original LIPS pair potential is divided into short-range, long-range and boundary pair potentials uS, uL and uB, respectively. For the exact implementation within CHARMM105,106,107 (version c40b2), the following expressions are used:
where rc (<Rc) is the short-range cutoff radius introduced for convenience. (ii) LIPS potential energy ULIPS is calculated as the summation of the short-range, long-range, and boundary potential energy, US, UL and UB, respectively.
(iii) The short-range potential energy US is simply calculated in real space:
where rij is the interaction distance between particles i and j. (iv) The long-range potential energy is calculated in reciprocal space using FFT. The details are very similar to those of the SPME50. For any simulation box, the charge distribution q and potential energy distribution Φ can be exactly defined using matrices that contain positional information for point charges. For the advanced calculation efficiency of FFT, the point charges have to be redistributed to predefined grid points. To do this, the following charge array Q is introduced:
where k1, k2, and k3 are integers that correspond to predefined grid points; n1, n2, and n3 are integers that correspond to the real space summation; \({M}_{n}\) is the cardinal b-spline function of the n-th order; w1i, w2i, and w3i are real numbers; and K1, K2, and K3 are positive integers that correspond to the total number of grid points. Thus, the Fourier transform of q is approximated by Q as follows:
where F is the operator of the discrete Fourier transform (DFT); m1, m2, and m3 are integers that correspond to the reciprocal space summation; and
The term Φ for long-range pair potential at the grid points also has to be defined. Thus, the following energy array Φ* is introduced:
The convolution between \(\,\,Q\,\) and Φ*, \(\,Q\ast {{\rm{\Phi }}}^{\ast }\), is expressed as follows:
where j1, j2, and j3 are integers. With the fine grid spacing conditions, \(\,Q\ast {{\rm{\Phi }}}^{\ast }\) can be used as the approximation of qΦ. For advanced computational efficiency, the convolution calculation using FFT is essential. \(\,Q\ast {{\rm{\Phi }}}^{\ast }\) can be simply calculated using DFT and inverse DFT (IDFT):
where F−1 is the operator of IDFT. Therefore, long-range potential energy UL can be calculated as follows:
where B = |b1|2 ⋅ |b2|2 ⋅ |b3|2. The reciprocal long-range force is calculated using the differential of Eq. 13 with respect to position vector of charges ri:
where rαi is the α (=x, y, z) component of the coordinate of molecule i, ri. The term ∂Q/∂rαi can be calculated based on Eq. 7 from the property of the b-spline functions:
where w is a real number. (v) The boundary potential energy UB is simply calculated in real space:
The potential energy and force of LIPS/FFT is calculated from the aforementioned five steps. Note that a similar approach was performed by Wu and Brooks for the combination of the IPS and FFT (IPS/DFFT)92.
Simulation conditions
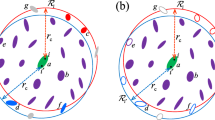
To evaluate the capability of LIPS/FFT on homogeneous and heterogeneous polar molecular systems, MD simulations for bulk water and water-vapor interfacial systems were performed. For a careful evaluation of the effect of IPS techniques on long-range interaction calculations, all the following three simulation conditions were met. (i) LIPS/FFT was applied only for electrostatic interactions. LIPS-SW101 and LIPS-5th100 were used for the pseudo pair potential of LIPS/FFT. For comparison, IPS/DFFT and the SPME were also applied only for electrostatic interactions. The IPS method for non-polar systems (IPSn)81 and polar systems (IPSp)84 were used for IPS/DFFT. For all the aforementioned methods, the short-range cutoff radius for real space interaction calculations and the order for b-spline interpolations were set to 1.0 nm and 8, respectively. The factor α for the SPME48,49,50 was set to 3.71692 nm−1, which corresponds to the short-range cutoff radius. For the treatment of Lennard-Jones (LJ) interactions, the shifting/switching functions to the LJ forces were used105,106,107, with the non-bonded cutoff and inner-cutoff equal to 1.0 nm and 0.99 nm, respectively. (ii) The large cutoff radii Rc less than or equal to L/2 were mainly used under cubic simulation boxes as a result of considering previous reports for the symmetry effect79,80. Even though IPS techniques are conceptually different from the lattice sum, LIPS/FFT and IPS/DFFT at infinite Rc is almost equal to the lattice sum. Moreover, these two methods at Rc > L/2 contain at least the nearest copy boxes of PBCs without any processing by IPS techniques. These facts imply the concern that the combination of IPS techniques and FFT cannot avoid the effect from PBCs when requiring large cutoff radii Rc > L/2 for accuracy. For a secure application, therefore, LIPS/FFT and IPS/DFFT should be used at Rc ≤ L/2. (iii) Fine grid spacing less than or equal to 0.1 nm was used. as a result of considering the previous study for liquid-crystalline phase transition phenomena104, which demonstrated that even using the SPME, fine grid spacing conditions (less than 0.1 nm) were required for the accurate estimation of the phase transition temperature of macromolecular systems. LIPS/FFT and IPS/DFFT under fine grid spacing conditions should be evaluated for future applications to complex macromolecular systems.
Bulk water systems were simulated with a constant molecular number, volume, and temperature condition108,109,110. The number of water molecules was 6,192, the size of the simulation box was 5.71 nm × 5.71 nm × 5.71 nm, and the temperature was 298.15 K. The extended simple point charge (SPC/E) model111 was used for water molecules. The atoms in each water molecule were constrained by the SHAKE algorithm112. The Verlet leapfrog integrator113 was used with a time step of 2 fs. All simulation systems were equilibrated prior to data acquisition, and the elapsed time after the equilibration was 1 ns.
The water-vapor interfacial systems were simulated with the constant molecular number, volume, and temperature condition. The number of water molecules was 13,500, the size of simulation box was 10.8 nm × 10.8 nm × 10.8 nm, and the temperature was 298.15 K. The SPC/E model was used for water molecules. The atoms in each water molecule were constrained by the SHAKE algorithm. The Verlet leapfrog integrator was used with a time step of 2 fs. All simulation systems were equilibrated prior to data acquisition, and the elapsed time after the equilibration was 15 ns.
All the aforementioned simulations were performed using CHARMM105,106,107 (version c40b2) modified for LIPS/FFT implementation.
Results and Discussion
To directly evaluate the accuracy of the electrostatic forces calculated by LIPS/FFT, the following two steps were performed: (i) The instantaneous value of the electrostatic forces was calculated for each method using exactly the same coordinates of molecular systems that had been equilibrated using the SPME. Note that the coordinates were not equilibrated using any IPS techniques but using the SPME. (ii) The instantaneous value was compared with that of the SPME using the root-mean-squared deviation of the forces, δf, and the largest error of the forces, ef,max, defined as follows:
where fα,i and fα,i,SPME are instantaneous forces calculated by LIPS/FFT and the SPME, respectively. For the calculation of δf, grid spacing Δ for the SPME was set to 0.05 nm. δf and ef,max for IPS/DFFT were also calculated for comparison. Figure 1(a) shows the Rc dependences of δf (top) and ef,max (bottom) for bulk water systems for two grid spacing conditions: Δ = 0.1 nm and 0.05 nm. Overall, the Rc dependences of δf and ef,max were similar to each other method, with few exceptions. However, the results show that LIPS/FFT was in better agreement with the SPME than IPS/DFFT. With increasing Rc, δf and ef,max decreased, except for the case using IPSn/DFFT with Δ = 0.1 nm. This indicates that only IPSn/DFFT with Δ = 0.1 nm barely improved its accuracy with increasing Rc. This very slow decay of δf and ef,max was caused by the cutoff boundary condition of the IPSn pseudo pair potential. This boundary condition was too simple to accurately estimate polar molecular systems100. Using finer grid spacings can improved the accuracy of IPSn/DFFT to a level close to the other methods. With decreasing Δ, δf and ef,max decreased, except for the case that used IPSp/DFFT. This indicates that only IPSp/DFFT could not improve its accuracy by choosing fine grid spacing less than 0.1 nm. The IPSp pseudo pair potential included a screening effect from the countercharge modeled on the typical structure of polar molecular systems. This screening effect smeared information for the charge distribution embedded in the fine grids, and then inhibited the accuracy improvement of IPSp/DFFT. Figure 1(b) shows the Rc dependences of δf (top) and ef,max (bottom) for water-vapor interfacial systems for two grid spacing conditions: Δ = 0.1 nm and 0.05 nm. It is obvious that LIPS/FFT was in much better agreement with the SPME than IPS/DFFT. δf and ef,max started to drastically decrease when Rc exceeded the thickness of the water slab (∼4 nm), with a few exceptions. This fast decay was because the sphere-like cutoff territory with large Rc became able to contain the major part of the characteristic structure of molecular systems. For LIPS/FFT, δf at Rc > 4 nm decayed in proportion to \({R}_{{\rm{c}}}^{-3.4}\). For IPSp/DFFT, δf decay was clearly slower than it was for LIPS/FFT; δf at Rc > 4 nm decayed in proportion to \({R}_{{\rm{c}}}^{-1.9}\). For IPSn/DFFT, δf decay was very slow. Using finer grid spacings could improve the accuracy of IPSn/DFFT; however, the slow decay tendency would still remain. Even using Δ = 0.05 nm, δf at Rc > 4 nm barely decay. With decreasing Δ, δf and ef,max decreased, except for the case using IPSp/DFFT. Similar to the results of bulk water systems, only IPSp/DFFT could not improve its accuracy by choosing fine grid spacing less than 0.1 nm. The screening effect of IPSp inhibited accurate calculation using IPS/DFFT regardless of the homogeneity/heterogeneity of the molecular structure of systems.
To evaluate the accuracy of LIPS/FFT for estimating homogeneous/heterogeneous polar molecular systems, some physical properties for bulk water and water-vapor systems were calculated. The potential energy U is one of the fundamental thermodynamic properties. Figure 2(a) shows U for bulk water systems at Δ = 0.1 nm. In comparison with the results of the SPME, the accuracy for estimating U is in the following order: LIPS-5th/FFT (=SPME) = LIPS-SW/FFT = IPSp/DFFT > IPSn/DFFT. This order follows the results of δf and ef,max. The radial distribution function g(r) is a critical property showing local configuration of molecular systems. The conventional expression give,
where ni(r) is the number of molecules in the region between r and r + Δr from molecule i. Figure 2(b) shows the oxygen-oxygen g(r) for bulk water systems at Rc = 2.8 nm and Δ = 0.1 nm. In comparison with the results of the SPME, the accuracy for estimating g(r) is in the following order: LIPS-5th/FFT (=SPME) = LIPS-SW/FFT = IPSp/DFFT > IPSn/DFFT. This order follows the results of δf and ef,max. The velocity auto-correlation function C(t) explains microscopic motion of molecular systems114. The conventional expression give,
where vi is the velocity of molecule i. Figure 2(c) shows C(t) for bulk water systems at Rc = 2.8 nm and Δ = 0.1 nm. All the results were almost same as that of the SPME. The self-diffusion coefficient D is one of the representative dynamic properties. D can be determined either by the Einstein relation or Green-Kubo formula, which are basically equivalent. Here we used the Einstein relation,
where t is time. The slope of the mean-squared displacement of a diffusing particle in the long-time limit was calculated for the diffusion coefficient. Figure 2(d) shows D for bulk water systems at Δ = 0.1 nm. All the results were almost same as that of the SPME. The mass density profile ρ(z) is one of the fundamental thermodynamic properties showing the mass distribution of heterogeneous systems. Figure 2(e) shows ρ(z) for water-vapor interfacial systems at Rc = L/2 and Δ = 0.1 nm. All the results were almost same as that of the SPME. The electrostatic potential profile ψ(z) is well known as one of the properties sensitive to the cutoff radius84,90,92,100,101,102,103, and thus should be calculated to evaluate the accuracy of the truncation methods. ψ(z) was calculated using the double integration of the Poisson equation:
where z is the direction normal to the interface and ρc is the charge density profile for the z-direction. ψ(0) on the left-hand side indicates a vacuum for liquid-vapor interfacial systems. Figure 2(f) shows ψ(z) for water-vapor interfacial systems at Rc = L/2 and Δ = 0.1 nm. The results of the electrostatic potential profile ψ(z) for water-vapor interfacial systems do not always follow the results of δf and ef,max. In comparison with the results of the SPME, the accuracy for estimating ψ(z) is in the following order: LIPS-SW/FFT (=SPME) > LIPS-5th/FFT = IPSn/DFFT > IPSp/DFFT. However, from Fig. 1(b), the accuracy for estimating δf and ef,max at Rc = L/2 and Δ = 0.1 nm is in the following order: LIPS-5th/FFT > LIPS-SW/FFT > IPSp/DFFT > IPSn/DFFT. There are two possible reasons for this difference. One is the influence of pseudo pair potentials at a relatively short interaction distance. For the pseudo pair potentials, the deviation from the Coulomb potential simply increased with an increase of the interaction distance. In a relatively short interaction distance (r < Rc/2), the deviation was in the following order: LIPS-SW < LIPS-5th = IPSn < IPSp. This fact corresponds to the results of ψ(z), but not to that of δf and ef,max because the deviation at a short interaction distance was concealed in δf and ef,max, which reflected the total deviation. The other reason is the influence of the difference between the instantaneous and equilibrated values. δf and ef,max are the instantaneous values calculated using coordinates equilibrated by the SPME, whereas ψ(z) is the physical property calculated from the molecular structure equilibrated by each method. This difference may affect the evaluation of accuracy. Note that the results of ψ(z) at Rc = L/2 and Δ = 0.05 nm are almost the same as those plotted in Fig. 2(f), and consistent with the accuracy of δf and ef,max at the same Rc and Δ conditions (data not shown).
(a) Potential energy U for bulk water systems at Δ = 0.1 nm. (b) Radial distribution function g(r) for bulk water systems at Rc = 2.8 nm and \({\rm{\Delta }}=0.1\) nm. (c) Velocity auto-correlation function C(t) for bulk water systems at Rc = 2.8 nm and Δ = 0.1 nm. (d) Diffusion coefficient D for bulk water systems at Δ = 0.1 nm. (e) Mass density profiles ϕ(z) for water-vapor interfacial systems at Rc = L/2 and Δ = 0.1 nm. (f) Electrostatic potential profiles ψ(z) for water-vapor interfacial systems at Rc = L/2 and Δ = 0.1 nm.
To evaluate the computational efficiency of LIPS/FFT, CPU time tCPU was measured for water-vapor interfacial systems with different N. An Intel(R) Xeon(R) CPU E5-2690 v2 (10 cores/20 threads, 3.00 GHz) was used for the measurement. L when N = 12000, 40500, and 96000 was 7.2 nm, 10.8 nm, and 14.4 nm, respectively. Rc and Δ were set to L/2 and 0.1 nm, respectively. Figure 3 shows the N dependence of tCPU. The results clearly demonstrate that LIPS/FFT attained almost the same computational efficiency as the SPME. tCPU for every method satisfied O(N logN) scaling, which means ideal computational efficiency using the FFT algorithm.
Conclusion
We developed a combination of the LIPS and FFT, LIPS/FFT, for a substantial advance in the computational efficiency of the LIPS for intermediate-sized systems \((N\sim {10}^{3}\,\mbox{--}\,{10}^{5})\). The performance of LIPS/FFT was evaluated on homogeneous/heterogeneous polar molecular systems. LIPS/FFT attained computational efficiency almost the same as that of the SPME while maintaining the advanced accuracy of the original LIPS. Furthermore, static and dynamic properties calculated by LIPS-SW/FFT was almost the same as that by the SPME. This indicates that LIPS-SW/FFT accurately estimated not only instantaneous values but also equilibrated values. This high accuracy was observed within Rc ≤ L/2. It clearly demonstrated that LIPS/FFT could overcome the concern that the combination of IPS techniques and FFT could not avoid the symmetry effect when requiring large cutoff radii Rc > L/2 for accuracy. One of the potential advantages of IPS techniques is the capability to simulate complex ionic systems that are difficult to accurately simulate using conventional lattice sum methods79,80,115. LIPS/FFT can enhance this advantage because it has both high accuracy and computational efficiency, while avoiding the symmetry effect. We conclude that the developed LIPS/FFT has great potential to extend the capability of IPS techniques for the fast and accurate computation of many types of molecular systems that involve highly complex ionic structure and dynamics, such as counterion condensation, ion conduction, and electrochemical migration. In further studies, the capability of LIPS/FFT for complex ionic systems will be intensively evaluated.
References
Perilla, J. R. et al. Molecular dynamics simulations of large macromolecular complexes. Current opinion in structural biology 31, 64–74 (2015).
Dror, R. O., Dirks, R. M., Grossman, J., Xu, H. & Shaw, D. E. Biomolecular simulation: a computational microscope for molecular biology. Annual review of biophysics 41, 429–452 (2012).
Dror, R. O. et al. Structural basis for nucleotide exchange in heterotrimeric g proteins. Science 348, 1361–1365 (2015).
Chung, H. S., Piana-Agostinetti, S., Shaw, D. E. & Eaton, W. A. Structural origin of slow diffusion in protein folding. Science 349, 1504–1510 (2015).
Lindorff-Larsen, K., Maragakis, P., Piana, S. & Shaw, D. E. Picosecond to millisecond structural dynamics in human ubiquitin. The Journal of Physical Chemistry B 120, 8313–8320 (2016).
Schlick, T. Molecular modeling and simulation: an interdisciplinary guide: an interdisciplinary guide, vol. 21 (Springer Science & Business Media, 2010).
Cisneros, G. A., Karttunen, M., Ren, P. & Sagui, C. Classical electrostatics for biomolecular simulations. Chemical Reviews 114, 779–814 (2014).
MacKerell Jr, A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. The journal of physical chemistry B 102, 3586–3616 (1998).
Klauda, J. B. et al. Update of the charmm all-atom additive force field for lipids: validation on six lipid types. The journal of physical chemistry B 114, 7830–7843 (2010).
Vanommeslaeghe, K. et al. Charmm general force field: A force field for drug-like molecules compatible with the charmm all-atom additive biological force fields. Journal of computational chemistry 31, 671–690 (2010).
Cornell, W. D. et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. Journal of the American Chemical Society 117, 5179–5197 (1995).
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the amber ff99sb protein force field. Proteins: Structure, Function, and Bioinformatics 78, 1950–1958 (2010).
Jorgensen, W. L., Madura, J. D. & Swenson, C. J. Optimized intermolecular potential functions for liquid hydrocarbons. Journal of the American Chemical Society 106, 6638–6646 (1984).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the opls all-atom force field on conformational energetics and properties of organic liquids. Journal of the American Chemical Society 118, 11225–11236 (1996).
Smit, B. Phase diagrams of lennard-jones fluids. The Journal of Chemical Physics 96, 8639–8640 (1992).
Trokhymchuk, A. & Alejandre, J. Computer simulations of liquid/vapor interface in lennard-jones fluids: Some questions and answers. The Journal of chemical physics 111, 8510–8523 (1999).
Lopez-Lemus, J. & Alejandre, J. Thermodynamic and transport properties of simple fluids using lattice sums: bulk phases and liquid-vapour interface. Molecular Physics 100, 2983–2992 (2002).
Neumann, M. & Steinhauser, O. The influence of boundary conditions used in machine simulations on the structure of polar systems. Molecular Physics 39, 437–454 (1980).
Alper, H. E. & Levy, R. M. Computer simulations of the dielectric properties of water: Studies of the simple point charge and transferrable intermolecular potential models. The Journal of Chemical Physics 91, 1242–1251 (1989).
Kitchen, D. et al. Conserving energy during molecular dynamics simulations of water, proteins, and proteins in water. Journal of Computational Chemistry 11, 1169–1180 (1990).
Tasaki, K., McDonald, S. & Brady, J. Observations concerning the treatment of long-range interactions in molecular dynamics simulations. Journal of computational chemistry 14, 278–284 (1993).
Smith, P. E. & van Gunsteren, W. F. Consistent dielectric properties of the simple point charge and extended simple point charge water models at 277 and 300 k. The Journal of chemical physics 100, 3169–3174 (1994).
Feller, S., Pastor, R., Rojnuckarin, A., Bogusz, S. & Brooks, B. Effect of electrostatic force truncation on interfacial and transport properties of water. The Journal of Physical Chemistry 100, 17011–17020 (1996).
van der Spoel, D., van Maaren, P. J. & Berendsen, H. J. A systematic study of water models for molecular simulation: derivation of water models optimized for use with a reaction field. The Journal of chemical physics 108, 10220–10230 (1998).
Mark, P. & Nilsson, L. Structure and dynamics of liquid water with different long-range interaction truncation and temperature control methods in molecular dynamics simulations. Journal of Computational Chemistry 23, 1211–1219 (2002).
Yonetani, Y. A severe artifact in simulation of liquid water using a long cut-off length: appearance of a strange layer structure. Chemical Physics Letters 406, 49–53 (2005).
van der Spoel, D. & van Maaren, P. The origin of layer structure artifacts in simulations of liquid water. Journal of Chemical Theory and Computation 2, 1–11 (2006).
Yonetani, Y. Liquid water simulation: A critical examination of cutoff length. The Journal of Chemical Physics 124, 204501 (2006).
Loncharich, R. & Brooks, B. The effects of truncating long-range forces on protein dynamics. Proteins: Structure, Function, and Bioinformatics 6, 32–45 (1989).
Schreiber, H. & Steinhauser, O. Cutoff size does strongly influence molecular dynamics results on solvated polypeptides. Biochemistry 31, 5856–5860 (1992).
Schreiber, H. & Steinhauser, O. Molecular dynamics studies of solvated polypeptides: why the cut-off scheme does not work. Chemical physics 168, 75–89 (1992).
Schreiber, H. & Steinhauser, O. Taming cut-off induced artifacts in molecular dynamics studies of solvated polypeptides* 1:: The reaction field method. Journal of molecular biology 228, 909–923 (1992).
Saito, M. Molecular dynamics simulations of proteins in water without the truncation of long-range coulomb interactions. Molecular Simulation 8, 321–333 (1992).
Guenot, J. & Kollman, P. Conformational and energetic effects of truncating nonbonded interactions in an aqueous protein dynamics simulation. Journal of computational chemistry 14, 295–311 (1993).
Saito, M. Molecular dynamics simulations of proteins in solution: artifacts caused by the cutoff approximation. The Journal of chemical physics 101, 4055–4061 (1994).
Oda, K., Miyagawa, H. & Kitamura, K. How does the electrostatic force cut-off generate non-uniform temperature distributions in proteins? Molecular Simulation 16, 167–177 (1996).
Norberg, J. & Nilsson, L. On the truncation of long-range electrostatic interactions in dna. Biophysical journal 79, 1537–1553 (2000).
Patra, M. et al. Molecular dynamics simulations of lipid bilayers: major artifacts due to truncating electrostatic interactions. Biophysical Journal 84, 3636–3645 (2003).
Beck, D., Armen, R. & Daggett, V. Cutoff size need not strongly influence molecular dynamics results for solvated polypeptides. Biochemistry 44, 609–616 (2005).
Monticelli, L., Simões, C., Belvisi, L. & Colombo, G. Assessing the influence of electrostatic schemes on molecular dynamics simulations of secondary structure forming peptides. Journal of Physics: Condensed Matter 18, S329 (2006).
Reif, M., Kräutler, V., Kastenholz, M., Daura, X. & Hünenberger, P. Molecular dynamics simulations of a reversibly folding β-heptapeptide in methanol: influence of the treatment of long-range electrostatic interactions. The Journal of Physical Chemistry B 113, 3112–3128 (2009).
Mazars, M. Long ranged interactions in computer simulations and for quasi-2d systems. Physics Reports 500, 43–116 (2011).
Piana, S. et al. Evaluating the effects of cutoffs and treatment of long-range electrostatics in protein folding simulations. PLoS One 7, e39918 (2012).
Barker, J. & Watts, R. Monte carlo studies of the dielectric properties of water-like models. Molecular Physics 26, 789–792 (1973).
Watts, R. Monte carlo studies of liquid water. Molecular Physics 28, 1069–1083 (1974).
Wolf, D., Keblinski, P., Phillpot, S. & Eggebrecht, J. Exact method for the simulation of coulombic systems by spherically truncated, pairwise 1/r summation. The Journal of chemical physics 110, 8254–8282 (1999).
Fennell, C. J. & Gezelter, J. D. Is the ewald summation still necessary? pairwise alternatives to the accepted standard for long-range electrostatics. The Journal of chemical physics 124, 234104 (2006).
Ewald, P. The calculation of optical and electrostatic grid potential. Ann. Phys 64, 253–87 (1921).
Darden, T., York, D. & Pedersen, L. Particle mesh ewald: An N log (N) method for ewald sums in large systems. The Journal of chemical physics 98, 10089–10092 (1993).
Essmann, U. et al. A smooth particle mesh ewald method. The Journal of Chemical Physics 103, 8577–8593 (1995).
Frenkel, D. & Smit, B. Understanding molecular simulation: from algorithms to applications, vol. 1 (Academic Pr, 2002).
Kia, A., Kim, D. & Darve, E. Fast electrostatic force calculation on parallel computer clusters. Journal of Computational Physics 227, 8551–8567 (2008).
Yokota, R., Barba, L. A., Narumi, T. & Yasuoka, K. Petascale turbulence simulation using a highly parallel fast multipole method on gpus. Computer Physics Communications 184, 445–455 (2013).
Barnes, J. & Hut, P. A hierarchical 0 (n log n) force-calculation algorithm. Nature 324, 4 (1986).
Greengard, L. & Rokhlin, V. A fast algorithm for particle simulations. Journal of Computational Physics 73, 325–348 (1987).
Niedermeier, C. & Tavan, P. A structure adapted multipole method for electrostatic interactions in protein dynamics. The Journal of chemical physics 101, 734 (1994).
Zhou, R. & Berne, B. A new molecular dynamics method combining the reference system propagator algorithm with a fast multipole method for simulating proteins and other complex systems. The Journal of chemical physics 103, 9444 (1995).
Petersen, H. Accuracy and efficiency of the particle mesh ewald method. The Journal of chemical physics 103, 3668 (1995).
Niedermeier, C. & Tavan, P. Fast version of the structure adapted multipole method–efficient calculation of electrostatic forces in protein dynamics. Molecular simulation 17, 57–66 (1996).
Pollock, E. & Glosli, J. Comments on p 3 m, fmm, and the ewald method for large periodic coulombic systems. Computer Physics Communications 95, 93–110 (1996).
Lambert, C. G., Darden, T. A. & Board, J. A. Jr A multipole-based algorithm for efficient calculation of forces and potentials in macroscopic periodic assemblies of particles. Journal of Computational Physics 126, 274–285 (1996).
Lim, K. et al. Molecular dynamics for very large systems on massively parallel computers: the mpsim program. Journal of Computational Chemistry 18, 501–521 (1997).
Eichinger, M., Grubm’ueller, H., Heller, H. & Tavan, P. Famusamm: An algorithm for rapid evaluation of electrostatic interactions in molecular dynamics simulations. Journal of Computational Chemistry 18, 1729–1749 (1997).
Figueirido, F., Levy, R., Zhou, R. & Berne, B. Large scale simulation of macromolecules in solution: Combining the periodic fast multipole method with multiple time step integrators. The Journal of Chemical Physics 106, 9835–9849 (1997).
Dimitrov, D. & Raev, N. Molecular dynamics simulations of the electrical double layer at the 1 m kcl solution | hg electrode interface. Journal of Electroanalytical Chemistry 486, 1–8 (2000).
Wang, Z., Lupo, J., Patnaik, S. & Pachter, R. Large scale molecular dynamics simulations of a 4-n-pentyl-4′-cyanobiphenyl (5cb) liquid crystalline model in the bulk and as a droplet. Computational and Theoretical Polymer Science 11, 375–387 (2001).
Mathias, G., Egwolf, B., Nonella, M. & Tavan, P. A fast multipole method combined with a reaction field for long-range electrostatics in molecular dynamics simulations: The effects of truncation on the properties of water. The Journal of Chemical Physics 118, 10847 (2003).
Mathias, G. & Tavan, P. Angular resolution and range of dipole–dipole correlations in water. The Journal of chemical physics 120, 4393 (2004).
Deng, S. & Cai, W. Extending the fast multipole method for charges inside a dielectric sphere in an ionic solvent: High-order image approximations for reaction fields. Journal of computational physics 227, 1246–1266 (2007).
Lorenzen, K., Schwörer, M., Tröster, P., Mates, S. & Tavan, P. Optimizing the accuracy and efficiency of fast hierarchical multipole expansions for md simulations. Journal of Chemical Theory and Computation 8, 3628–3636 (2012).
Shan, Y., Klepeis, J. L., Eastwood, M. P., Dror, R. O. & Shaw, D. E. Gaussian split ewald: A fast ewald mesh method for molecular simulation. The Journal of chemical physics 122, 054101 (2005).
Roberts, J. & Schnitker, J. How the unit cell surface charge distribution affects the energetics of ion–solvent interactions in simulations. The Journal of Chemical Physics 101, 5024–5031 (1994).
Roberts, J. & Schnitker, J. Boundary conditions in simulations of aqueous ionic solutions: a systematic study. The Journal of Physical Chemistry 99, 1322–1331 (1995).
Luty, B. & Van Gunsteren, W. Calculating electrostatic interactions using the particle-particle particle-mesh method with nonperiodic long-range interactions. The Journal of Physical Chemistry 100, 2581–2587 (1996).
Hünenberger, P. H. & McCammon, J. A. Ewald artifacts in computer simulations of ionic solvation and ion–ion interaction: a continuum electrostatics study. The Journal of chemical physics 110, 1856–1872 (1999).
Hünenberger, P. & McCammon, J. Effect of artificial periodicity in simulations of biomolecules under ewald boundary conditions: a continuum electrostatics study. Biophysical Chemistry 78, 69–88 (1999).
Weber, W., Hünenberger, P. & McCammon, J. Molecular dynamics simulations of a polyalanine octapeptide under ewald boundary conditions: influence of artificial periodicity on peptide conformation. The Journal of Physical Chemistry B 104, 3668–3675 (2000).
Patra, M., Karttunen, M., Hyvönen, M., Falck, E. & Vattulainen, I. Lipid bilayers driven to a wrong lane in molecular dynamics simulations by subtle changes in long-range electrostatic interactions. The Journal of Physical Chemistry B 108, 4485–4494 (2004).
Ye, X., Cai, Q., Yang, W. & Luo, R. Roles of boundary conditions in dna simulations: analysis of ion distributions with the finite-difference poisson-boltzmann method. Biophysical journal 97, 554–562 (2009).
Lu, X. & Cui, Q. Charging free energy calculations using the generalized solvent boundary potential (gsbp) and periodic boundary condition: a comparative analysis using ion solvation and oxidation free energy in proteins. The Journal of Physical Chemistry B 117, 2005–2018 (2013).
Wu, X. & Brooks, B. Isotropic periodic sum: A method for the calculation of long-range interactions. The Journal of Chemical Physics 122, 044107 (2005).
Ojeda-May, P. & Pu, J. Assessing the accuracy of the isotropic periodic sum method through madelung energy computation. The Journal of chemical physics 140, 164106 (2014).
Takahashi, K., Yasuoka, K. & Narumi, T. Cutoff radius effect of isotropic periodic sum method for transport coefficients of lennard-jones liquid. The Journal of Chemical Physics 127, 114511 (2007).
Wu, X. & Brooks, B. Isotropic periodic sum of electrostatic interactions for polar systems. The Journal of Chemical Physics 131, 024107 (2009).
Takahashi, K., Narumi, T. & Yasuoka, K. Cutoff radius effect of the isotropic periodic sum method in homogeneous system. ii. water. The Journal of Chemical Physics 133, 014109 (2010).
Takahashi, K., Narumi, T. & Yasuoka, K. Cut-off radius effect of the isotropic periodic sum method for polar molecules in a bulk water system. Molecular Simulation 38, 397–403 (2012).
Nakamura, H., Ohto, T. & Nagata, Y. Polarizable site charge model at liquid/solid interfaces for describing surface polarity: Application to structure and molecular dynamics of water/rutile tio2 (110) interface. Journal of Chemical Theory and Computation 9, 1193–1201 (2013).
Ohto, T. et al. Influence of surface polarity on water dynamics at the water/rutile tio2 (110) interface. Journal of physics. Condensed matter: an Institute of Physics journal 26, 244102–244102 (2014).
Klauda, J., Wu, X., Pastor, R. & Brooks, B. Long-range lennard-jones and electrostatic interactions in interfaces: application of the isotropic periodic sum method. The Journal of Physical Chemistry B 111, 4393–4400 (2007).
Takahashi, K. Z., Narumi, T. & Yasuoka, K. Cutoff radius effect of the isotropic periodic sum and wolf method in liquid–vapor interfaces of water. The Journal of Chemical Physics 134, 174112 (2011).
Nozawa, T., Takahashi, K. Z., Kameoka, S., Narumi, T. & Yasuoka, K. Application of isotropic periodic sum method for 4-pentyl-4′-cyanobiphenyl liquid crystal. Molecular Simulation 41, 927–935 (2015).
Wu, X. & Brooks, B. Using the isotropic periodic sum method to calculate long-range interactions of heterogeneous systems. The Journal of Chemical Physics 129, 154115 (2008).
Venable, R., Chen, L. & Pastor, R. Comparison of the extended isotropic periodic sum and particle mesh ewald methods for simulations of lipid bilayers and monolayers. The Journal of Physical Chemistry B 113, 5855–5862 (2009).
Ojeda-May, P. & Pu, J. Isotropic periodic sum treatment of long-range electrostatic interactions in combined quantum mechanical and molecular mechanical calculations. Journal of Chemical Theory and Computation 10, 134–145 (2014).
Wu, X. & Brooks, B. R. A virtual mixture approach to the study of multistate equilibrium: Application to constant ph simulation in explicit water. PLoS Comput Biol 11, e1004480 (2015).
Wu, X., Lee, J. & Brooks, B. R. Origin of pka shifts of internal lysine residues in snase studied via equal-molar vmms simulations in explicit water. The Journal of Physical Chemistry B (2016).
Lee, J., Miller, B. T. & Brooks, B. R. Computational scheme for ph-dependent binding free energy calculation with explicit solvent. Protein Science 25, 231–243 (2016).
Takahashi, K. Z., Narumi, T. & Yasuoka, K. A combination of the tree-code and ips method to simulate large scale systems by molecular dynamics. Journal of Chemical Physics 135, 174108 (2011).
Wu, X., Pickard, F. C. IV & Brooks, B. R. Isotropic periodic sum for multipole interactions and a vector relation for calculation of the cartesian multipole tensor. The Journal of Chemical Physics 145, 164110 (2016).
Takahashi, K. Z., Narumi, T., Suh, D. & Yasuoka, K. An improved isotropic periodic sum method using linear combinations of basis potentials. Journal of Chemical Theory and Computation 8, 4503–4516 (2012).
Takahashi, K. Z. Design of a reaction field using a linear-combination-based isotropic periodic sum method. Journal of computational chemistry 35, 865–875 (2014).
Takahashi, K. Z. & Yasuoka, K. A determination of liquid–vapour interfacial properties for methanol using a linear-combination-based isotropic periodic sum. Molecular Simulation 41, 795–800 (2015).
Nozawa, T., Yasuoka, K. & Takahashi, K. Z. Critical test of isotropic periodic sum techniques with group-based cut-off schemes. Scientific reports 8, 4185 (2018).
Nozawa, T., Takahashi, K. Z., Narumi, T. & Yasuoka, K. Comparison of the accuracy of periodic reaction field methods in molecular dynamics simulations of a model liquid crystal system. Journal of computational chemistry 36, 2406–2411 (2015).
Brooks, B. R. et al. Charmm: a program for macromolecular energy, minimization, and dynamics calculations. Journal of computational chemistry 4, 187–217 (1983).
MacKerell, A. D. et al. Charmm: the energy function and its parameterization. Encyclopedia of computational chemistry (1998).
Brooks, B. R. et al. Charmm: the biomolecular simulation program. Journal of computational chemistry 30, 1545–1614 (2009).
Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Molecular Physics 52, 255–268 (1984).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. The Journal of Chemical Physics 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Physical Review A 31, 1695–1697 (1985).
Berendsen, H., Grigera, J. & Straatsma, T. The missing term in effective pair potentials. Journal of Physical Chemistry 91, 6269–6271 (1987).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. Journal of Computational Physics 23, 327–341 (1977).
Hockney, R. W. The potential calculation and some applications. Methods in Computational Physics 9, 135–211 (1970).
Balucani, U., Brodholt, J. & Vallauri, R. Analysis of the velocity autocorrelation function of water. Journal of Physics: Condensed Matter 8, 6139 (1996).
Hub, J. S., de Groot, B. L., Grubm’uller, H. & Groenhof, G. Quantifying artifacts in ewald simulations of inhomogeneous systems with a net charge. Journal of chemical theory and computation 10, 381–390 (2014).
Acknowledgements
We sincerely thank Dr. Ryuji Sakamaki of X-Ability Co., Ltd. for his great effort in implementing LIPS/FFT within CHARMM (version c40b2). KZT was supported in part by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) Grant Number 16H06071. TN was supported in part by Grant-in-Aid for JSPS Fellows, and Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant-in-Aid for the Program for Leading Graduate Schools.
Author information
Authors and Affiliations
Contributions
K.Z.T. designed the project and developed the LIPS/FFT method; K.Z.T. and T.N. performed MD simulations and data analyses; K.Z.T. wrote the paper, and all authors contributed to the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahashi, K.Z., Nozawa, T. & Yasuoka, K. A fast and accurate computational method for the linear-combination-based isotropic periodic sum. Sci Rep 8, 11880 (2018). https://doi.org/10.1038/s41598-018-30364-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30364-2
- Springer Nature Limited