Abstract
In general, Friedel-Crafts reaction is incompatible with amines due to the Lewis acidity of the catalysts. Recently, we reported that cyclic diaminocarbene-Gold(I) can be used as catalyst for the Friedel-Crafts alkylation between aromatic amines and alkenes. Herein, a systematically theoretical research was performed on this rare Friedel-Crafts reaction. The adopted calculation method is accurate enough to reproduce the crystal structure of the catalyst. It was found that the reactions followed the electrophilic aromatic substitution mechanism. The gold cation can activate the C=C double bond and generate the electrophilic group which can be attacked by the aromatic ring. The para-product is more energy favorable which agrees well with the experimental results. The reaction of α-methylstyrene follows the Markovnikov rule, and the activation energy to generate the branched product of methylstyrene is lower than that producing the linear product. However, the reaction of butanone follows the anti-Markovnikov rule, and the activation energy to generate the branched product of butanone is higher than that producing the linear product. These calculation results reveal the mechanism of this new Friedel-Crafts reaction. It can well explain the high para-selectivity and the substrate-dependent of the product structures in the experiment.
Similar content being viewed by others
Introduction
Friedel-Crafts (FC) reaction is an important method to incorporate carbon skeletons into aromatic system1,2. Great successes have been achieved for the hydroarylation of neutral arenes (such as toluene, anisole, and their homologues)3,4,5,6,7,8,9,10,11,12,13,14. Because the FC reactions typically require Lewis acid catalysts, for arenes containing nitrogen atom, the substrate scope of FC reactions are quite limited due to the coordination between amine and Lewis acid catalyst, except indole and pyrrole15,16,17,18,19,20,21,22,23. Being profited from the extremely weak basic properties24,25, acid-catalyzed additions of indole and pyrrole to alkenes have obtained great achievements15,16,17,18,19,20,21,22,23. However, the hydroarylation of alkaline arenes to alkenes still remains many challenges. Some researches have shown the possibility of hydroarylation between the parent anilines C6H5NH2 and alkenes26,27,28. However, the reaction of arenes with stronger basicity (such as N,N-dimethylaniline and N,N-diethylaniline29) still is a big problem, due to their ability to coordinate with Lewis acid catalyst which can lead to deactivation of the aromatic ring30. Furthermore, alkaline arenes can trap the proton in the C-H activation process and the reaction will be terminated as result26.
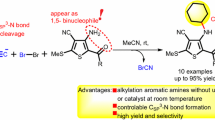
Recently, Bertrand et al. reported an anti-Bredt cyclic diaminocarbene which showed increased π-accepting character without diminishing its σ-donor property31,32. We found that Gold(I) compound derived from this new carbene can be used as effective catalyst for the FC reaction between alkenes and N,N-dialkylanilines33. Now, these new FC reactions are receiving more and more research interests34,35,36,37. As we known, most of the electrophilic substitution reactions followed the Markovnikov rule38. For the FC reaction of alkenes, the reactions following the Markovnikov rule should form branched product3,4. Only several examples were reported on the formation of linear product by anti-Markovnikov rule39,40. For the FC reactions between alkenes and N, N-dialkylanilines catalyzed by carbene Gold(I), both Markovnikov and anti-Markovnikov hydroarylations were observed and all these reactions gave high para-selectivity products (Fig. 1)33. The selectivity to the branched or linear product was highly depended on the structure of alkenes. The purpose of this paper is to find out the reaction mechanism of this rare FC reaction and understand the origin of the reaction selectivity.
The Friedel-Crafts reactions of N,N-Dimethylaniline and corresponding products (5a and 6a are the main products in the experiment33).
Results and Discussion
The structure of the catalyst
To examine the reliability of the theoretical method, the structure of the catalyst 1 was fully optimized with both B3LYP and M06-2X methods. The calculated results were compared with the x-ray experiment data (Table 1). The calculated structure parameters match well with the experiment data. Most of the calculation errors for the bond length and angle are smaller than 1.0% and the maximum error is 1.7%. It suggests that the calculation method used here is accurate enough to reproduce the crystal structure even though the catalyst contain gold atom. Indeed, methods based on DFT using pseudopotential basis set have been widely used for the calculation of compound containing gold41,42,43,44,45,46,47,48,49,50,51. Our previous experimental results on the hydration of alkynes catalyzed by gold(I) isocyanide were successfully explained by theoretical calculation based on DFT41.
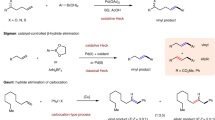
According to the experimental results, the conversion of the hydroarylation between N,N-diethylaniline and α-methylstyrene is zero when only 1 is used as catalyst. However, the conversion is 97% when chlorine scavenger reagent KBArF was added in the presence of 133. It suggests that the active center in the reaction should be anti-Bredt carbene-Au+ cation 1+ (Fig. 2). Similarly, other kinds of carbene-Au+ cations have been proposed to be the active catalytic species in a series of reactions52,53,54,55.
The reaction between 2 and 3
5a is the main product in the reaction between N,N-Dimethylaniline 2 and alkene 333. There has two possible pathways to produce 5a (Fig. 3). Pathway 1: the gold cation activates the alkene 3. Then, the electrophilic alkene attacks the aromatic ring. Pathway 2: the gold cation activates the N,N-Dimethylaniline 2.
As we known, FC reaction is a kind of electrophilic aromatic substitution reactions, in which the hydrogen atom of aromatic ring is replaced by an electrophile1,56,57,58,59. The complex 1+…3 can serve as the electrophilic reagent (Fig. 2). It has been known that the gold cation has strong ability to bind alkene60,61. The binding between alkene 3 and catalyst 1+ is highly exothermic (33.6 kcal/mol). The C=C bond length of alkene 3 was increased from 1.347 to 1.391 Å, which indicates that the C=C bond was activated after binding with 1+.
For pathway 1, both Cc and Cd of alkene 3 attacking the Ca of N,N-dimethylaniline 2 were taken into consideration, which can produce branched and linear product (5a and 5b) respectively. In the case of Cc attacking, the activation energy for the Ca–Cc bond formation (TS1A-1) is 27.4 kcal/mol and the Ca–Cc bond length in TS1A-1 is 1.998 Å (Energies obtained at M06-2X (6–311 + G*/LANL2DZ) by PCM calculation were discussed if not mentioned) (Fig. 4). At the same time, the C=C double bond of alkene 3 becomes almost single bond (bond length increases from 1.391 to 1.517 Å) in TS1A-1. An unstable intermediate (Int1A-1) is produced via TS1A-1 and the Ca–Cc bond length of Int1A-1 is further reduced to 1.647 Å. The formation of Ca–Cc bond makes the Ca-H bond active. Meanwhile, the Cc–Cd bond becomes single bond in Int1A-1 (length: 1.562 Å). Though TS2A-1 can lead to the final product 5a, a direct proton transfer from Ca to Cd is not energy favorable due to the high overall activation energy (40.8 kcal/mol).
(a) The pathway 1 to produce 5a by the Friedel-Crafts reaction between 2 and 3 without the assistance of aromatic amine (Energy in kcal/mol). Energies out of parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ). Energies in parenthesis were obtained at M06–2X (6–311 + G*/LANL2DZ) by PCM calculation. (b) The structures of the optimized transition states (Bond length in Å). All hydrogen atoms (except the transferred H) have been omitted for clarity.
It is worth to notice that there is plenty of aromatic amines in the microenvironment33. The aromatic amine is a good proton acceptor. It can abstract the proton of Ca-H in Int1A-1 with a barrier of 22.1 kcal/mol (TS2A-1′) (Fig. 5). Then, the intermediate Int2A-1′ can re-abstract the proton of ammonium via TS3A-1′ and the corresponding energy barrier is 14.7 kcal/mol. The re-abstracting proton process is vital for the catalytic cycle. If the catalyst cannot re-abstract the proton from the ammonium, the reaction will be terminated. As an example, when 25 mol% proton-trapping reagent, 2,6-di-t-butyl-4-methylpyridine, was added in the FC alkylation of aniline catalyzed by acid, no any alkylation reaction can be observed26. TS3A-1′ can lead to the final product 5a and the overall energy change from ReaA-1 to ProA-1′ is −24.6 kcal/mol.
(a) The pathway 1 to produce 5a by the Friedel-Crafts reaction between 2 and 3 with the assistance of aromatic amine (Energy in kcal/mol). Energies out of parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ). Energies in parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ) by PCM calculation. (b) The structures of the optimized transition states (Bond length in Å). All hydrogen atoms (except the transferred H) have been omitted for clarity.
As indicated above, N,N-dialkylaniline is not only reactant but also a promoter. It should be the reason why excess N,N-dialkylaniline is necessary for the reaction33. The rate determining step is the Ca–Cc bond formation process via the transition state TS1A-1 (barrier: 27.4 kcal/mol). This barrier height is reasonable considering that the reaction requires a temperature of 135 °C33.
Producing 5a by the pathway that the gold cation activates the N,N-Dimethylaniline 2 and the Ca carbon of 2 attacks the Cc of alkene 3 is not possible because of the extremely high activation energy (Figs 3 and 6). The binding energy between 2 and 1+ is −35.3 kcal/mol. This binding can increase the positive charge on the Ca–H proton from 0.112 to 0.214. It makes the Ca–H proton quite active. The external N,N-dialkylaniline 2 can abstract the Ca–H proton with quite low activation barrier (12.8 kcal/mol, TS1A-2). However, the following Ca–Cc bond formation is extremely energy-unfavorable and the corresponding activation barrier is 60.1 kcal/mol. It indicates that producing 5a by gold cation activated N,N-Dimethylaniline 2 is not possible. Hence, this pathway is not taken into consideration for the producing of 5b, 5c, 6a and 6b.
(a) The pathway 2 to produce 5a by the Friedel-Crafts reaction between 2 and 3 (Energy in kcal/mol). Energies out of parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ). Energies in parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ) by PCM calculation. (b) The structures of the optimized transition states (Bond length in Å). All hydrogen atoms (except the transferred H) have been omitted for clarity.
If the alkene terminal carbon Cd can attack the Ca of N,N-dialkylaniline 2 (Fig. 7), anti-Markovnikov product 5b should be obtained. The activation energy for the Ca-Cd bond formation is only 22.5 kcal/mol (TS1B). Though the barrier height of TS1B is lower than that of TS1A-1, the proton abstracting process via TS2B is not easy for this anti-Markovnikov reaction. The overall activation energy for the process to produce 5b is 29.5 kcal/mol. Furthermore, the energy change from ReaB to ProB is only −1.5 kcal/mol. Hence, comparing with the producing of 5a, the generation of the linear product 5b is difficult because the higher activation energy and quite small free energy change. Indeed, no 5b was observed for the FC reaction between 2 and 3 in our previous experiment33.
(a) The pathway to produce 5b by the Friedel-Crafts reaction between 2 and 3 (Energy in kcal/mol). Energies out of parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ). Energies in parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ) by PCM calculation. (b) The structures of the optimized transition states (Bond length in Å). All hydrogen atoms (except the transferred H) have been omitted for clarity.
Most of the known FC reactions gave both para- and ortho-products1,62,63. Interestingly, for the reaction between N,N-Dimethylaniline 2 and alkene 3, quite high para-selectivity was obtained and 5a was isolated with 93% yield33. Due to the steric hindrance of the –N(CH3)2 group, the Cc of alkene 3 attacking the Cb carbon of 2 is difficult (Fig. 8). The energy barrier for the Cb-Cc bond formation is 31.8 kcal/mol (TS1C). Furthermore, the access of extra aromatic amine is not easy due to the steric hindrance, which makes the abstracting proton from Cb not easy comparing with the process to produce 5a. The free energy change from Reac to Proc is −7.9 kcal/mol. Considering that the barrier of the rate determining step to produce 5c is 4.4 kcal/mol higher than that to produce 5a, observing a minute quantity of 5c as byproduct is reasonable33.
(a) The pathway to produce 5c by the Friedel-Crafts reaction between 2 and 3 (Energy in kcal/mol). Energies out of parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ). Energies in parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ) by PCM calculation. (b) The structures of the optimized transition states (Bond length in Å). All hydrogen atoms (except the transferred H) have been omitted for clarity.
The reaction between 2 and 4
The reaction of olefine ketone 4 is quite different from that of aromatic alkene 3. For aromatic alkene 3, the Ca of N,N-Dimethylaniline 2 and Cc of 3 forms C–C bond and the producing of 5a follows the Markovnikov rule. However, the reaction of olefine ketone 4 follows the anti-Markovnikov rule. The C–C bond was formed between the Ca of 2 and the terminal carbon Cd of 4 to produce 6a (Fig. 1)33. To fully understand this difference, both Cc and Cd of 4 attacking the Ca of 2 were taken into consideration, which can produce branched and linear product (6b and 6a) respectively. The binding between 4 and 1+ can form the electrophilic group and this process releases 25.5 kcal/mol energy. The C=C bond length of olefine ketone 4 was increased from 1.341 to 1.375 Å during the formation of 1+…4 (Fig. 2).
In the case of Cd attacking, the activation energy for the Ca-Cd bond formation (TS1D) is only 15.2 kcal/mol and the Ca-Cd bond length is 2.117 Å in TS1D (Fig. 9). During the Ca-Cd bond formation, the C=C double bond of 4 was increased from 1.375 to 1.439 Å. For the reaction of aromatic alkene 3, this bond was increased by 0.126 Å (from ReaA-1 to TS1A-1). As we know, the structure distortion can induce energy change and enhance the reaction barrier64. Correspondingly, the energy barrier of TS1D is 12.2 kcal/mol lower than that of the same process to produce 5a (TS1A). The smaller structure change is responsible for the low barrier of TS1D. Similarly, proton abstracting by aromatic amine and re-abstracting the proton of ammonium happen for the producing of 6a. The proton re-abstracting process from the ammonium is the rate determining step and the corresponding activation energy is 21.1 kcal/mol (TS3D).
(a) The pathway to produce 6a by the Friedel-Crafts reaction between 2 and 4 (Energy in kcal/mol). Energies out of parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ). Energies in parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ) by PCM calculation. (b) The structures of the optimized transition states (Bond length in Å). All hydrogen atoms (except the transferred H) have been omitted for clarity.
If the Cc of olefine ketone 4 can attack the Ca of N,N-dialkylaniline 2 (Fig. 10), Markovnikov product 6b should be observed in the experiment. In the case of Cc attacking, the activation energy for the Ca–Cc bond formation is quite high (TS1E: 30.8 kcal/mol). At the same time, the C=C double bond of 3 becomes almost single bond in TS1E (increasing from 1.375 to 1.461 Å). The proton abstracting by the aromatic amine and re-abstracting from the ammonium is easy to achieve in this pathway. However, the producing 6b is not competitive to 6a. The activation energy of the rate determining step to produce 6b is 9.7 kcal/mol higher than that of 6a. That is why the anti-Markovnikov linear product 6a was observed in the experiment33.
(a) The pathway to produce 6b by the Friedel-Crafts reaction between 2 and 4 (Energy in kcal/mol). Energies out of parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ). Energies in parenthesis were obtained at M06-2X (6–311 + G*/LANL2DZ) by PCM calculation. (b) The structures of the optimized transition states (Bond length in Å). All hydrogen atoms (except the transferred H) have been omitted for clarity.
Conclusion
The Friedel-Crafts alkylation of N,N-dimethylaniline with alkenes catalyzed by cyclic diaminocarbene-Gold(I) complex were theoretically investigated. The calculation method adopted here is accurate enough to reproduce the crystal structure of the catalyst. The gold cation can activate the C=C double bond to produce the electrophilic [R-C=C…Au-L]+. Then, the [R-C=C…Au-L]+ attacks the aromatic ring, following the electrophilic aromatic substitution mechanism. Being different from previous result that alkaline arenes will trap the proton and the reaction will be terminated as result26, herein, it was found that the alkaline N,N-dimethylaniline can assist the reaction. Based on the obtained reaction mechanism, we can well understand why the reaction was high para-selevtivity, and why branched and linear products were obtained for different substrates: (1) Producing the para-product is more energy favorable comparing with the ortho-product. (2) The reaction of α-methylstyrene follows the Markovnikov rule. The activation energy to generate the branched product of α-methylstyrene is lower than that producing the linear product. Besides, the reaction leading to branched product is highly exothermic. (3) The reaction of butanone follows the anti-Markovnikov rule. The activation energy to generate the branched product of butanone is higher than that producing the linear product.
These theoretical results are quite useful for designing more effective catalysts for this rare FC reaction using alkaline arenes as substrates. Based on the understanding of the reaction mechanism, the development of none-noble metal catalyst for this FC reaction is on going in our group.
Computational Methods
All the structures were fully optimized with B3LYP based on Density Functional Theory (DFT) (See SI for structure details). This method is a good choice for the calculation of organometallic systems65,66,67,68,69,70,71,72,73,74,75. The following combination of basis sets were used for geometric configuration optimization and frequency calculation: 6–31G basis set for all atoms except Au and LANL2DZ basis set for Au (abbreviated as 6–31G/LANL2DZ)41,75. LANL2DZ basis set includes the relativistic effect of the heavy element65,76. The calculation method adopted here can well reproduce the crystal structure of the carbene-gold used for the FC reaction of basic arenes with alkenes (Table 1). Because B3LYP method often suffers from incorrect energies, especially for systems containing non covalent bonds. A higher level method M06-2X was used to generate more accurate energies. The energy calculations were performed using 6–311 + G* basis set for all atoms except Au and LANL2DZ basis set for Au (abbreviated as 6–311 + G*/LANL2DZ). The influence of solvent was performed in condensed phase with the Polarizable Continuum Model (PCM) using 6–311 + G*/LANL2DZ basis set. This method creates the solute cavity via a set of overlapping spheres. Aniline was used as solvent to simulate the environment of N,N-dimethylaniline.
The computed stationary points have been characterized as minima or transition states by diagonalizing the Hessian matrix and analyzing the vibrational normal modes. In this way, the stationary points can be classified as minima if no imaginary frequencies are shown or as transition states if only one imaginary frequency is obtained. The particular nature of the transition states has been determined by analyzing the motion described by the eigenvector associated with the imaginary frequency. All calculations were performed with the Gaussian 03 suite of programs77.
References
Sartori, G. & Maggi, R. Use of solid catalysts in Friedel-Crafts acylation reactions. Chem. Rev. 106, 1077–1104 (2006).
Poulsen, T. B. & Jørgensen, K. A. Catalytic Asymmetric Friedel-Crafts Alkylation Reactions: Copper Showed the Way. Chem. Rev. 108, 2903–2915 (2008).
Nicolaou, K. C., Reingruber, R., Sarlah, D. & Brase, S. Enantioselective intramolecular Friedel-Crafts-Type α-arylation of aldehydes. J. Am. Chem. Soc. 131, 2086–2087 (2009).
Xiao, Y. P., Liu, X. Y. & Che, C. M. Highly efficient gold (III)-catalyzed intermolecular hydroarylation of unactivated alkenes with arenes under mild conditions. J. Organomet. Chem. 694, 494–501 (2009).
Hu, F. et al. Graphene-catalyzed direct Friedel-Crafts alkylation reactions: mechanism, selectivity, and synthetic utility. J. Am. Chem. Soc. 137, 14473–14480 (2015).
Zhou, Y., Li, X., Hou, S. & Xu, J. X. Facile synthesis of dihydrochalcones via the AlCl3-promoted tandem Friedel-Crafts acylation and alkylation of arenes with 2-alkenoyl chlorides. J. Mol. Catal. A: Chem. 365, 203–211 (2012).
Kheira, H., Li, P. & Xu, J. X. Synthesis of indenes via aluminum chloride-promoted tandem Friedel-Crafts alkylation of arenes and cinnamaldehydes. J. Mol. Catal. A: Chem. 391, 168–174 (2014).
Rafiee, E. & Khodayari, M. Synthesis and characterization of a green composite of H3PW12O40 and starch-coated magnetite nano particles as a magnetically-recoverable nano catalyst in Friedel-Crafts alkylation. J. Mol. Catal. A: Chem. 398, 336–343 (2015).
Yamazaki, T., Makihara, M. & Komura, K. Zeolite catalyzed highly selective synthesis of 2-methoxy-6-acetylnaphthalene by Friedel-Crafts acylation of 2-methoxynaphthalene in acetic acid reaction media. J. Mol. Catal. A: Chem. 426, 170–176 (2017).
Sohtome, Y. et al. Entropy-Controlled Catalytic Asymmetric 1,4-Type Friedel-Crafts Reaction of Phenols Using Conformationally Flexible Guanidine/Bisthiourea Organocatalyst. Angew. Chem. Int. Ed. 49, 7299–7303 (2010).
Niggemann, M. & Meel, M. J. Calcium‐Catalyzed Friedel-Crafts Alkylation at Room Temperature. Angew. Chem. Int. Ed. 49, 3684–3687 (2010).
Schafer, G. & Bode, J. W. Friedel-Crafts Benzylation of Activated and Deactivated Arenes. Angew. Chem. Int. Ed. 50, 10913–10916 (2011).
Wilsdorf, M., Leichnitz, D. & Reissig, H. Trifluoromethanesulfonic Acid Catalyzed Friedel–Crafts Alkylations of 1, 2, 4-Trimethoxybenzene with Aldehydes or Benzylic Alcohols. Org. Lett. 15, 2494–2497 (2013).
Kato, M. et al. Entropy-Driven 1,2-Type Friedel-Crafts Reaction of Phenols with N-tert-Butoxycarbonyl Aldimines. Chem. Eur. J. 21, 18606–18612 (2015).
Pan, S., Ryu, N. & Shibata, T. Ir (I)-Catalyzed C-H Bond Alkylation of C2-Position of Indole with Alkenes: Selective Synthesis of Linear or Branched 2-Alkylindoles. J. Am. Chem. Soc. 134, 17474–17477 (2012).
Kieffer, M. E., Repka, L. M. & Reisman, S. E. Enantioselective synthesis of tryptophan derivatives by a tandem Friedel-Crafts conjugate addition/asymmetric protonation reaction. J. Am. Chem. Soc. 134, 5131–5137 (2012).
Sevov, C. S. & Hartwig, J. F. Iridium-catalyzed intermolecular asymmetric hydroheteroarylation of bicycloalkenes. J. Am. Chem. Soc. 135, 2116–2119 (2013).
Wu, K., Jiang, Y., Fan, Y., Sha, D. & Zhang, S. Double Axially Chiral Bisphosphorylimides Catalyzed Highly Enantioselective and Efficient Friedel-Crafts Reaction of Indoles with Imines. Chem. Eur. J. 19, 474–478 (2013).
Bohstrom, Z. & Holmberg, K. Friedel-Crafts acylation of 2-methylindole with acetic anhydride using mesoporous HZSM-5. J. Mol. Catal. A: Chem. 366, 64–73 (2013).
Bohstrom, Z., Harelind, H. & Holmberg, K. Friedel-Crafts alkylation of sodium salicylate with 4-tert butylbenzyl chloride performed in aqueous dispersions of mesoporous oxides. J. Mol. Catal. A: Chem. 366, 171–178 (2013).
Rao, P. C. & Mandal, S. Friedel-Crafts Alkylation of Indoles with Nitroalkenes through Hydrogen-Bond-Donating Metal-Organic Framework. ChemCatChem 9, 1172–1176 (2017).
Trost, B. M. & Muller, C. Asymmetric Friedel-Crafts Alkylation of Pyrroles with Nitroalkenes Using a Dinuclear Zinc Catalyst. J. Am. Chem. Soc. 130, 2438–2439 (2008).
Majer, J., Kwiatkowski, P. & Jurczak, J. Enantioselective Friedel-Crafts Reaction of Acylpyrroles with Glyoxylates Catalyzed by BINOL-Ti (IV) Complexes. Org. Lett. 13, 5944–5947 (2011).
Van Order, R. B. & Lindwall, H. G. Indole. Chem. Rev. 30, 69–96 (1942).
Baltazzi, E. Recent Advances in the Chemistry of Pyrrole. Chem. Rev. 63, 511–556 (1963).
Anderson, L. L., Arnold, J. & Bergman, R. G. Proton-catalyzed hydroamination and hydroarylation reactions of anilines and alkenes: a dramatic effect of counteranions on reaction efficiency. J. Am. Chem. Soc. 127, 14542–14543 (2005).
Kaspar, L. T., Fingerhut, B. & Ackermann, L. Titanium-Catalyzed Intermolecular Hydroamination of Vinylarenes. Angew. Chem. Int. Ed. 44, 5972–5974 (2005).
Prades, A., Corberan, R., Poyatos, M. & Peris, E. A simple catalyst for the efficient benzylation of arenes by using alcohols, ethers, styrenes, aldehydes, or ketones. Chem. Eur. J. 15, 4610–4613 (2009).
Folkers, E. & Runquist, O. Correlation of base strengths of aliphatic and N-substituted anilines. J. Org. Chem. 29, 830–832 (1964).
Beller, M., Thiel, O. R. & Trauthwein, H. Catalytic alkylation of aromatic amines with styrene in the presence of cationic rhodium complexes and acid. Synlett 2, 243–245 (1999).
Martin, D., Lassauque, N., Donnadieu, B. & Bertrand, G. A Cyclic Diaminocarbene with a Pyramidalized Nitrogen Atom: A Stable N-Heterocyclic Carbene with Enhanced Electrophilicity. Angew. Chem. Int. Ed. 51, 6172–6175 (2012).
Martin, D., Canac, Y., Lavallo, V. & Bertrand, G. Comparative reactivity of different types of stable cyclic and acyclic mono-and diamino carbenes with simple organic substrates. J. Am. Chem. Soc. 136, 5023–5030 (2014).
Hu, X. B., Martin, D., Melaimi, M. & Bertrand, G. Gold-catalyzed hydroarylation of alkenes with dialkylanilines. J. Am. Chem. Soc. 136, 13594–13597 (2014).
Perez, M., Mahdi, T., Hounjet, L. & Stephan, D. W. Electrophilic phosphonium cations catalyze hydroarylation and hydrothiolation of olefins. Chem. Commun. 51, 11301–11304 (2015).
Okumura, S. et al. para-Selective Alkylation of Benzamides and Aromatic Ketones by Cooperative Nickel/Aluminum Catalysis. J. Am. Chem. Soc. 138, 14699–14704 (2016).
Song, G., Luo, G., Oyamada, J., Luo, Y. & Hou, Z. M. ortho-Selective C–H addition of N, N-dimethyl anilines to alkenes by a yttrium catalyst. Chem. Sci. 7, 5265–5270 (2016).
Li, W. & Werner, T. B(C6F5)3-Catalyzed Michael Reactions: Aromatic C–H as Nucleophiles. Org. Lett. 19, 2568–2571 (2017).
Beller, M., Seayad, J., Tillack, A. & Jiao, H. Catalytic Markovnikov and anti-Markovnikov Functionalization of Alkenes and Alkynes: Recent Developments and Trends. Angew. Chem. Int. Ed. 43, 3368–3398 (2004).
Xu, W. G. & Yoshikai, N. Highly Linear Selective Cobalt-Catalyzed Addition of Aryl Imines to Styrenes: Reversing Intrinsic Regioselectivity by Ligand Elaboration. Angew. Chem. Int. Ed. 53, 14166–4170 (2014).
Schramm, Y., Takeuchi, M., Semba, K., Nakao, Y. & Hartwig, J. F. Anti-Markovnikov Hydroheteroarylation of Unactivated Alkenes with Indoles, Pyrroles, Benzofurans, and Furans Catalyzed by a Nickel-N-Heterocyclic Carbene System. J. Am. Chem. Soc. 137, 12215–12218 (2015).
Xu, Y. et al. Hydration of alkynes at room temperature catalyzed by gold (I) isocyanide compounds. Green Chem. 17, 532–537 (2015).
Zhou, A. et al. Atom-economic generation of gold carbenes: gold-catalyzed formal [3 + 2] cycloaddition between ynamides and isoxazoles. Chem. Sci. 6, 1265–1271 (2015).
Hansmann, M. M., Rominger, F. & Hashmi, A. S. K. Gold–allenylidenes–an experimental and theoretical study. Gold–allenylidenes–an experimental and theoretical study. Chem. Sci. 4, 1552 (2013).
Hansmann, M. M., Tsupova, S., Rudolph, M., Rominger, F. & Hashmi, A. S. K. Gold-Catalyzed Cyclization of Diynes: Controlling the Mode of 5-endo versus 6-endo Cyclization-An Experimental and Theoretical Study by Utilizing Diethynylthiophenes. Chem. Eur. J. 20, 2215–2223 (2014).
Hansmann, M. M., Pernpointner, M., Dopp, R. & Hashmi, A. S. K. A Theoretical DFT-Based and Experimental Study of the Transmetalation Step in Au/Pd-Mediated Cross‐Coupling Reactions. Chem. Eur. J. 19, 15290–15303 (2013).
Vilhelmsen, M. H. & Hashmi, A. S. K. Reaction Mechanism for the Dual Gold‐Catalyzed Synthesis of Dibenzopentalene: A DFT Study. Chem. Eur. J. 20, 1901–1908 (2014).
Hashmi, A. S. K., Pernpointner, M. & Hansmann, M. M. Theoretical insights into the superior activity of gold catalysts and reactions of organogold intermediates with electrophiles. Faraday Discuss. 152, 179–184 (2011).
Jerabek, P., Roesky, H. W., Bertrand, G. & Frenking, G. Coinage Metals Binding as Main Group Elements: Structure and Bonding of the Carbene Complexes [TM(cAAC)2] and [TM(cAAC)2]+ (TM = Cu, Ag, Au). J. Am. Chem. Soc. 136, 17123–17135 (2014).
Caramori, G. F. et al. Cyclic trinuclear copper (I), silver (I), and gold (I) complexes: a theoretical insight. Dalton Trans. 44, 377–385 (2015).
Puls, A. et al. A Novel Concept for the Synthesis of Multiply Doped Gold Clusters [(M@ AunM′m) Lk]q+. Angew. Chem. Int. Ed. 53, 4327–4331 (2014).
Dietz, O., Rayon, V. M. & Frenking, G. Molecular structures, bond energies, and bonding analysis of group 11 cyanides TM (CN) and isocyanides TM(NC)(TM = Cu, Ag, Au). Inorg. Chem. 42, 4977–4984 (2003).
Zeng, X. M., Frey, G. D., Kinjo, R., Donnadieu, B. & Bertrand, G. Synthesis of a Simplified Version of Stable Bulky and Rigid Cyclic (Alkyl)(amino)carbenes, and Catalytic Activity of the Ensuing Gold(I) Complex in the Three-Component Preparation of 1,2-Dihydroquinoline Derivatives. J. Am. Chem. Soc. 131, 8690–8696 (2009).
Zeng, X. M., Kinjo, R., Donnadieu, B. & Bertrand, G. Serendipitous discovery of the catalytic hydroammoniumation and methylamination of alkynes. Angew. Chem. Int. Ed. 49, 942–945 (2010).
Kinjo, R., Donnadieu, B. & Bertrand, G. Gold-Catalyzed Hydroamination of Alkynes and Allenes with Parent Hydrazine. Angew. Chem. Int. Ed. 50, 5560–5563 (2011).
Ye, L. W., Wang, Y., Aue, D. H. & Zhang, L. M. Experimental and Computational Evidence for Gold Vinylidenes: Generation from Terminal Alkynes via a Bifurcation Pathway and Facile C–H Insertions. J. Am. Chem. Soc. 134, 31–34 (2012).
Vos, A. M., Schoonheydt, R. A., Proft, F. D. & Geerlings, P. DFT study on the electrophilic aromatic substitution catalyzed by Lewis acids. J. Catal. 220, 333–346 (2003).
Wang, S. C. & Tantillo, D. J. Substituent Effects on Tandem Alkenyl Migration/Electrophilic Aromatic Substitution Reactions: A Theoretical Study. J. Org. Chem. 72, 8394–8401 (2007).
Zhu, H. & Meyer, M. P. Cationic intermediates in Friedel-Crafts acylation: structural information from theory and experiment. Chem. Commun. 47, 409–411 (2011).
Shravani, M., Balaiah, S., Srinivas, K., Bhanuprakash, K. & Huc, I. Unusual Regioselective Electrophilic Substitutions in Quinoline Foldamers: Conceptual DFT and Frontier Molecular Orbital Analysis Reveal the Crucial Role of Folding and Substituents. ChemPhysChem 13, 3526–3534 (2012).
Fedorov, A., Moret, M. & Chen, P. Gas-phase synthesis and reactivity of a gold carbene complex. J. Am. Chem. Soc. 130, 8880–8881 (2008).
Fedorov, A., Batiste, L., Bach, A., Birney, D. M. & Chen, P. Potential Energy Surface for (Retro-) Cyclopropanation: Metathesis with a Cationic Gold Complex. J. Am. Chem. Soc. 133, 12162–12171 (2011).
Khenkin, A. M. & Neumann, R. Nitrosonium Salts, NO+X− (X = B(3,5-diCF3Ph)4 − or PW12O40 3−), as Electrophilic Catalysts for Alkene Activation in Arene Alkylation and Dimerization Reactions. J. Am. Chem. Soc. 130, 11876–11877 (2008).
Mo, X., Yakiwchuk, J., Dansereau, J., McCubbin, J. A. & Hall, D. G. Theory of 1,3-dipolar cycloadditions: distortion/interaction and frontier molecular orbital models. J. Am. Chem. Soc. 137, 9694–9703 (2015).
Ess, D. H. & Houk, K. N. Unsymmetrical Diarylmethanes by Ferroceniumboronic Acid Catalyzed Direct Friedel-Crafts Reactions with Deactivated Benzylic Alcohols: Enhanced Reactivity due to Ion-Pairing Effects. J. Am. Chem. Soc. 130, 10187–10198 (2008).
Oyamada, J. & Hou, Z. M. Regioselective C–H Alkylation of Anisoles with Olefins Catalyzed by Cationic Half-Sandwich Rare Earth Alkyl Complexes. Angew. Chem. Int. Ed. 51, 12828–12832 (2012).
Noey, E. L., Luo, Y., Zhang, L. M. & Houk, K. N. Mechanism of Gold(I)-Catalyzed Rearrangements of Acetylenic Amine-N-Oxides: Computational Investigations Lead to a New Mechanism Confirmed by Experiment. J. Am. Chem. Soc. 134, 1078–1084 (2012).
Mandal, D. & Shaik, S. Interplay of Tunneling, Two-State Reactivity, and Bell-Evans-Polanyi Effects in C-H Activation by Nonheme Fe (IV) O Oxidants. J. Am. Chem. Soc. 138, 2094–2097 (2016).
Qi, Z. et al. Mechanism, reactivity, and regioselectivity in rhodiumcatalyzed asymmetric ring-opening reactions of oxabicyclic alkenes: a DFT Investigation. Sci. Rep. 7, 40491 (2017).
Pierens, G. K., Venkatachalam, T. K. & Reutens, D. C. NMR and DFT investigations of structure of colchicine in various solvents including density functional theory calculations. Sci. Rep. 7, 5605 (2017).
Thangavel, S. et al. Catalytic oxidation of primary aromatic alcohols using half sandwich Ir (III), Rh (III) and Ru (II) complexes: A practical and theoretical study. J. Mol. Catal. A: Chem. 423, 160–171 (2016).
Borisov, Y. A. & Akhrem, I. S. DFT mechanistic study of reactions of С6H6 and 1, 3, 5-Ad3C6H3 with CBr3. The first example of hydride transfer from aromatic C–H bond to electrophile. J. Mol. Catal. A: Chem. 426, 610–617 (2017).
Ustynyuk, L. Y. & Bulychev, B. M. Activation effect of metal chlorides in post-metallocene catalytic systems for ethylene polymerization: A DFT study. J. Organoment. Chem. 793, 160–170 (2015).
Ustynyuk, L. Y. DFT modeling of the post-titanocene catalytic system LTiCl2-Bu2Mg-Et2AlCl for alkene polymerization: The role of alkyl bridge Mg-C-Ti and β-agostic C-H-Ti bonds in the formation of active centers. J. Mol. Catal. A: Chem. 426, 600–609 (2017).
Zhao, T. X., Hu, X. B., Wu, D. S., Li, R. & Yang, G. Q. Direct Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol at Room Temperature Using Imidazolium Hydrogen Carbonate Ionic Liquid as a Recyclable Catalyst and Dehydrant. ChemSusChem 10, 2046–2052 (2017).
Hu, X. B., Sun, Y., Mao, J. Y. & Li, H. R. Theoretical study on the structure-reactivity relationships of acetylacetone-Fe catalyst modified by ionic compound in C–H activation reaction. J. Catal. 272, 320–332 (2010).
Takagi, N. et al. How Can We Understand Au8 Cores and Entangled Ligands of Selenolate- and Thiolate-Protected Gold Nanoclusters Au24(ER)20 and Au20(ER)16 (E = Se, S; R = Ph, Me)? A Theoretical Study. J. Am. Chem. Soc. 137, 8593–8602 (2015).
Frisch, M. J. et al. Gaussian 03, Revision B.02; Gaussian, Inc., Pittsburgh PA (2003).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21676134).
Author information
Authors and Affiliations
Contributions
X.B.H. proposed the idea and wrote the paper. H.Z.W. and T.X.Z. performed the calculation and analyzed the data. All authors discussed together.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, H., Zhao, T. & Hu, X. Friedel-Crafts Reaction of N,N-Dimethylaniline with Alkenes Catalyzed by Cyclic Diaminocarbene-Gold(I) Complex. Sci Rep 8, 11449 (2018). https://doi.org/10.1038/s41598-018-29854-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29854-0
- Springer Nature Limited
This article is cited by
-
Regioselective and solvent-free arylation of β-nitrostyrenes with mono- and dialkyl anilines
Research on Chemical Intermediates (2021)