Abstract
Contact heat evoked potentials (CHEPs) have become an acknowledged research tool in the assessment of the integrity of the nociceptive system and gained importance in the diagnostic work-up of patients with suspected small fiber neuropathy. For the latter, normative values for CHEP amplitude and latency are indispensable for a clinically meaningful interpretation of the results gathered in patients. To this end, CHEPs were recorded in 100 healthy subjects over a wide age range (20–80 years) and from three different dermatomes of the lower extremities (L2, L5, and S2). A normal baseline (35–52 °C) and increased baseline stimulation (42–52 °C) were applied. Statistical analysis revealed significant effects of stimulation site, stimulation intensity, and sex on CHEP parameters (N2 latency, N2P2 amplitude, and NRS). Significant positive correlations of body height with N2 latency, and pain ratings with N2P2 amplitudes were observed. This is the first time that normative values have been obtained from multiple dermatomes of the lower extremities. The present dataset will facilitate the clinical application of CHEPs in the neurophysiological diagnosis of small fiber neuropathy and by discerning pathological findings help establish a proximal-distal gradient of nerve degeneration in polyneuropathies.
Similar content being viewed by others
Introduction
Contact heat stimulation activates small diameter A-delta and C fiber nociceptors within the epidermis1,2. The recorded cortical potential is related to conduction within peripheral A-delta fibers, relayed to central spinothalamic projections, thalamus and cortex3,4,5. Contact heat evoked potentials (CHEPs) have been employed to document damage along the entire nociceptive neuraxis in a wide range of neurological diseases6,7,8,9,10.
The use of CHEPs in the neurophysiological assessment of disorders affecting the lower extremities has been challenged by a poor signal-to-noise ratio and technical drawbacks2,11,12. While several studies have reported normative values of CHEPs from the upper extremities11,13,14,15, few have addressed the lower extremity12.
The availability of such normative data may help close an important diagnostic gap in increasingly prevalent conditions, such as small fiber neuropathies16. Small fiber pathologies often pose a challenge as conventional neurophysiology does not yield conclusive results17,18.
The effect of stimulus intensity on the acquisition of CHEPs from the lower extremities has only recently been assessed systematically12. In that study, we demonstrated the superiority of the increased baseline (IB) protocol (42–52 °C) for the acquisition of CHEPs from lower extremities with higher signal persistence12. However, normative values for the lower extremities only exist for the normal baseline (NB) protocol (35–51 °C)11,13.
A recent multicenter study provides a large data set for commonly used stimulation sites11. However, the study protocol only included one site from the lower extremities, thus precluding its use for the assessment of length-dependency in polyneuropathies. As small fiber neuropathies often present in a distal-symmetrical fashion owing to a length-dependency of fiber degeneration19, normative values from proximal and distal sites are needed in order to establish a neurophysiological gradient.
With the IB protocol being the preferable stimulation paradigm for CHEP acquisition from the lower extremities12, the need for a comprehensive set of normative values for both stimulation protocols is addressed here. Beyond the proof of feasibility, the present study provides normative values across a wide range of age groups. In particular, the inclusion of an older population is of high clinical relevance, as this cohort is epidemiologically most often affected by polyneuropathies. As age already has a physiological impact on CHEP parameters, a robust and reliable stimulation paradigm (i.e., IB stimulation) is a requisite for a diagnostically meaningful approach.
Material and Methods
Subjects
Hundred healthy subjects (47 men and 53 women) from three predefined age groups (20–40, 41–60, and 61–80 years) were included. Inclusion criteria were native language either English or German. Exclusion criteria included pregnancy, intake of psychoactive medication, and any neurological condition.
All participants provided written informed consent prior to the assessments and all procedures described below were in accordance with the Declaration of Helsinki. The study has been approved by the local ethics board ‘Kantonale Ethikkommission Zürich, KEK’ (EK-04/2006, PB_2016-02051, clinicaltrial.gov number: NCT02138344).
Study design
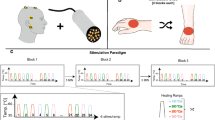
Subjects medical history was assessed and subsequently nerve conduction as well as somatosensory evoked potentials were recorded in order to exclude neuropathy. All subjects underwent a clinical sensory examination of mechanoreception and nociception, both of which were semi-quantitatively assessed according to the grading system of the International Standards for Neurological Classification of Spinal Cord Injury20. Afterwards subjects lay down in a supine position and three stimulation sites from the lower extremity were examined: the L2 dermatome at the inner side of the thigh, the L5 dermatome at the dorsum of the foot, and the S2 dermatome 5 cm above the popliteal fossa (Fig. 1C). The order of the tested dermatome and body side was randomized for each subject. CHEPs were recorded employing two different stimulation protocols: (1) the conventional normal baseline protocol (NB) followed by (2) the increased baseline protocol (IB). The two protocols differ by their applied baseline temperature, i.e., 35 °C for the normal and 42 °C for the IB protocol, while the peak temperature of 52 °C was the same for both protocols (Fig. 1D)21,22. A summary of the study protocol is illustrated in Fig. 1.
Summary of the study design: (A) clinical screening block including medical history, clinical sensory examination, somatosensory evoked potential (SEP), and nerve conduction study (NCS). (B) EEG setup for the CHEPs recording. (C) Stimulation sites of the CHEPs thermode. (D) Illustration of both stimulation protocols.
Acquisition of CHEPs
The CHEPs measurement set-up has been published elsewhere4,5,15,21,23. Briefly, the acquisition of CHEPs was performed using a contact heat stimulator with the established PATHWAY Pain & Sensory Evaluation System (Medoc Ltd., Ramat Yishai, Israel). The thermode surface of 27 mm consists of a heating thermo-foil covered with a layer of thermo-conductive plastic. The nominal heating rate of the thermode is 70 °C/s (thermo-foil), with a cooling rate of 40 °C/s (peltier element).
Cortical potentials to the noxious heat stimuli were recorded with 9 mm Ag/AgCl cup electrodes filled with conductive adhesive gel. The recording sites on the scalp were prepared with Nuprep (D.O. Weaver & Co. Aurora, CO) and alcohol. Cup electrodes were positioned on the vertex (Cz) referenced to the earlobes (A1-A2) according to the 10–20 system (Fig. 1B). The vertex position is considered as the most reliable position to record N2 and P2 potentials24. All signals were sampled at 2000 Hz using a preamplifier (20000x, bandpass filter 1–300 Hz, ALEA Solutions, Zurich, Switzerland). Data were recorded with 100 ms pre-trigger and a one second post-trigger in a customized program based on LabView (V2.04 CHEP, ALEA Solutions, Zurich, Switzerland).
Prior to the CHEP recordings, a familiarization procedure comprising a heat stimulus at the contralateral leg was applied. Contact heat stimuli were applied with an inter-stimulus interval of 8–12 sec. After each stimulus the thermode was marginally repositioned within the tested area to avoid peripheral receptor fatigue and habituation1. In addition, cued by an auditory signal provided four seconds after heat stimulus, subjects were asked to rate the perceived intensity of each stimulus using a numeric rating scale (NRS) ranging from 0 (no pain) to 10 (worst pain imaginable). The verbal instructions for the subjects comprised the following points: keep eyes open and fix a point on the ceiling, remain relaxed and quiet during the assessment, rate the perceived heat stimulus after the auditory signal on a scale ranging from 0 (no pain) to 10 (worst pain imaginable).
Data analysis and statistics
In both stimulation protocols, stimuli were applied with the goal of 15 artifact-free signals without exceeding the total number of 20 trials. Signals were visually analyzed and trials with obvious muscle or ocular artifacts were discarded. The remaining signals were averaged and the N2P2 amplitude was visually inspected by two independent examiners. The whole EEG analysis was performed using a customized program based on LabView (V2.04 CHEP, ALEA Solutions, Zurich, Switzerland).
R software (version 3.3.1) and SPSS software (version 16) for Windows was used to conduct all statistical analyses and generate the graphs. The data were tested for normal distribution using the Shapiro-Wilk test and by visually inspecting histograms and Q-Q plots. While N2 latencies and NRS were normally distributed, N2P2 amplitudes were not. Statistical significance was set at α < 0.05 and was adjusted for multiple comparisons using Tukey contrasts.
To establish normative values, descriptive statistics (i.e., mean and 95% CI) were calculated. Sex difference in body height was tested using an independent t-test.
The main effects of stimulation protocol (i.e., NB, IB), stimulation sites (i.e., L2, L5, & S2), and age group were investigated by building a linear mixed model with protocol site and age group as fixed factors and random subject effects. Post-hoc tests were used to examine differences in CHEP parameters between stimulation sites under both stimulation paradigms.
Exploration of the effects of subject demographics such as age and body height as well as perceived pain during testing on CHEP parameters was performed using pairwise Spearman correlations. An additional general linear mixed model was set up to test the effect of sex and height on CHEP parameters. N2 latency, N2P2 amplitude, and NRS were set as dependent variables, while sex was included as a fixed factor and height as covariate. Examination of model diagnostics, in particular residuals of dependent variables, indicated that a logarithmic (log) transformation for amplitude data was necessary to meet model requirements for both general linear mixed models used.
Results
Subjects
A total of 100 healthy subjects participated in the study. Three had to be excluded due to the results of the clinical screenings, i.e., suspected neurological condition. The remaining subjects included 45 men and 52 women with a mean age of 47.6 ± 17.2 years. The subjects had a mean height of 171.6 ± 8.7 cm and men were significantly taller than women (p < 0.001).
Main effects of stimulation protocol, stimulation site and age on CHEP parameters
The dataset of 97 included subjects was used to establish normative values for CHEPs for lower extremities. Figure 2 illustrates a representative example of averaged CHEP signals for all three tested sites and both stimulation protocols.
Representative example of CHEPs recordings from the lower extremities (female, 51 years) using the normal and increased baseline protocol for each tested site (L2, S2 & L5). Averaged signals of the normal baseline protocol are displayed in black, while averaged signals of the increased baseline protocol are shown in blue.
The normative values (mean ± 95% CI) of the investigated parameters (N2 latencies, N2P2 amplitudes, and pain ratings (NRS)) for each stimulation site, stimulation protocol and age group are summarized in Table 1. The middle-aged and the elderly subject group showed significantly longer latencies and smaller amplitudes compared to the young group (see Table 2).
Figure 3 shows N2 latencies and N2P2 amplitudes for each tested site, stimulation protocol, grouped by age and sex. The linear mixed model revealed significant main effects of stimulation protocol, site and age (groups) on all investigated CHEP parameters. Sex had no significant effect on N2 latencies (F = 1.0, p = 0.3), and NRS (F = 1.2, p = 0.3) when corrected for height. However, log(N2P2 amplitudes) were significantly higher in females compared to males (F = 4.9, p = 0.029). Further post-hoc tests primarily displayed significant differences between the L5 dermatome and the two further proximally located stimulation sites (L2 & S2), while the L2 and S2 dermatomes were comparable. Main effects and dermatome-wise comparisons are summarized in Table 2. In detail, the L5 dermatome featured longer N2 latencies and decreased N2P2 amplitudes.
Correlations of age, body height, and perceived pain
The Spearman correlation analysis of CHEP latencies and amplitudes with age, body height, and pain ratings consistently disclosed significant negative correlations between age and N2P2 amplitudes (all p-values < 0.001). Significant positive correlations of age and N2 latencies emerged in the L5 and the S2 dermatome in both protocols (p < 0.01 in L5 NB, L5 IB & S2 NB; p < 0.001 in S2 IB). Body height was positively correlated with N2 latencies at all stimulation sites and for both stimulation protocols except S2 IB (p < 0.05 in L2 IB, L5 NB & L5 IB; p < 0.01 in L2 NB & S2 NB). In addition, pain ratings consistently correlated positively with amplitudes (p < 0.05 in L2 NB, L2 IB, L5 NB, L5 IB; p < 0.01 in S2 NB & S2 IB). All correlation matrices are illustrated in Fig. 4.
Discussion
In the present study, we provide normative values for CHEPs from three stimulation sites on the lower extremities. These stimulation sites were chosen to allow for proximal-to-distal comparisons in length-dependent small fiber neuropathies. In addition, as each site reflects a specific spinal segment (dermatome), the normative values may also facilitate diagnoses in pathologies of the lumbar cord or in radiculopathies25,26.
In line with previous studies, age, and height had a significant influence on CHEP parameters11,27,28,29. The effect of age has been extensively debated by other authors27,30,31. Interestingly, in a study on laser evoked potentials, age had a significant influence on amplitudes but not latency27. The authors emphasized a central mechanism of amplitude attenuation, whereas the peripheral afferent input remains unaltered27. In contrast, a recent study from our group showed that latency was affected by age, yet only under the IB protocol15.
In the present study, age exerted a significant effect under both stimulation protocols. The discrepancy between the age-mediated effect on upper and lower extremities might be explained in terms of predominant vulnerability of fibers from the lower extremities during ageing, as most neuropathies manifest first in the lower extremities32. Subclinical dysfunctions may then contribute to increased latencies with ageing. Furthermore, conduction length from the lower extremities is generally longer possibly potentiating any jitter introduced by slight demyelination.
Regarding length-dependency, stimulating the dorsum of the foot (L5 dermatome) yielded significantly longer N2 latencies and smaller N2P2 amplitudes than proximal (S2 or L2) stimulation. Moreover, stimulus intensity was perceived as less painful after distal stimulation. These results are readily explained by the longer peripheral conduction length, leading to temporal dispersion of the afferent volley2, and the proximal-to-distal gradient in skin innervation33. The fact that differences in latency between distal and proximal stimulation sites persist under both stimulation protocol is of clinical relevance. This possibly facilitates the detection of distally-accentuated impairments of axonal segments in length-dependent polyneuropathies.
In studies using laser- or contact heat stimulation, N2P2 amplitudes usually correlate well within subjects with ratings of pain intensity21. Higher NRS scores were associated with larger N2P2 amplitudes across all stimulation sites and under both stimulation paradigms. In line with the literature, females reported higher ratings to the noxious heat stimuli34. Sex-related effects were also observed for N2 latencies and N2P2 amplitudes. As in previous studies15, we again draw upon longer conduction distances in the male subjects due to significantly greater height in order to explain these findings.
Applying the IB protocol led to shorter latencies and higher amplitudes for all stimulation sites and across all age groups15. The latency shift and amplitude increase were in a comparable range with data acquired for the upper extremities15. Stimulus characteristics should be taken into account when comparing results from different laboratories11,27. In slight contrast to previous studies applying CHEPs to the lower extremities11,13, pain ratings and some amplitudes (S2 and L2 dermatome) within the older population tend to be higher in the present study. These results can be explained in terms of improved temporal and spatial summation due to a more synchronized afferent volley using IB stimulation. A similar increase in amplitudes and subjective pain ratings was demonstrated for the stimulation of cervical dermatomes15. In line with results from other groups, sex differences with females displaying larger N2P2 amplitudes could also be reproduced in our data set.
For CHEPs, bearing the inherent advantage of being able to control the baseline temperature, the IB protocol is well-established and the underlying mechanisms have been extensively studied15,21,22. Increasing the baseline temperature of stimulation shortens stimulus duration, decreases time to threshold for receptor activation and consequently leads to a more synchronized afferent volley with an improved spatio-temporal summation at central synapses22. Recently, we have demonstrated that using the IB protocol for the acquisition of CHEPs from the lower extremities can improve persistence of the cortical potential in a clinically meaningful manner12. Our findings are in line with previous studies, Lagerburg et al. also reported improved acquisition when increasing the baseline temperature for stimulation in cases where there was no cortical response with NB stimulation13. Based on these observations, IB stimulation should be preferred over conventional stimulation whenever possible12.
Histological studies showed that both N2P2 amplitude and N2 latency correlate well with intra-epidermal nerve fiber density7,35,36. However, in the clinical routine N2 latency usually emerges as the more robust readout15,27,37, and has therefore been proposed as a more sensitive measure of pathology15,27 compared to amplitude. In line with literature15, amplitudes in the present study were also highly variable (i.e., high standard deviation) between subjects. Amplitudes are less reproducible over time for both upper and lower extremities12,37, and are susceptible to attention and arousal effects38,39.
Currently, the diagnostic approach to a patient with suspected small fiber neuropathy usually includes bedside examination of sensory function for both mechano- and nociception19. Additional confirmatory tests, like quantitative sensory testing or skin biopsies are usually recommended to substantiate the clinical diagnosis19. CHEPs are so far not routinely used, however, would provide an objective readout of A-delta fiber function11. Here, we present normative values of CHEPs for the foot dorsum, a very distal and commonly affected area in small fiber neuropathies19, and two more proximal stimulation sites. Toe and foot involvement occur early during disease progression in many peripheral neuropathies32. The relative sparing of proximal sites may facilitate the monitoring of symptom progression over time in a distal-to-proximal fashion.
CHEPs do not only bear potentially high diagnostic yield in length-dependent polyneuropathies, but also in patients with non-length dependent patterns of sensory abnormalities. For the latter, CHEPs can be employed as a sensitive measure of spinal pathology, i.e. myelopathy5,9. Such concomitant spinal pathology cannot be detected by skin biopsies, nor be adequately localized using quantitative sensory testing18. Hence, CHEPs may supplement the neurophysiological test battery as a non-invasive, objective and clinically applicable technique.
Limitations
A major limitation of this study is the collection of normative data at only one center. Therefore, the use of the acquired normative values for CHEPs in lower extremities is limited to clinical sites using the exact same CHEP acquisition equipment. This makes the generalizability of the data weaker compared to multicenter normative data sets.
Conclusion
In this study we provide normative values for the acquisition of CHEPs from lower extremities in a large cohort of healthy subjects across different age groups. Age, height and sex have substantial impact on the latency and amplitude of CHEPs. Latencies exhibit length-dependency allowing for an appropriate diagnosis of a proximal-distal gradient in peripheral neuropathies.
Increasing stimulation intensity markedly shortens latencies and increases amplitudes through reduced signal dispersion along the afferent fibers. This comprehensive set of normative values will improve the neurophysiological diagnosis of patients with small fiber neuropathies or neuropathic pain conditions affecting the lower extremities.
References
Greffrath, W., Baumgartner, U. & Treede, R. D. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain 132, 301–311, https://doi.org/10.1016/j.pain.2007.04.026 (2007).
Magerl, W., Ali, Z., Ellrich, J., Meyer, R. A. & Treede, R. D. C- and A delta-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain 82, 127–137 (1999).
Baumgartner, U., Greffrath, W. & Treede, R. D. Contact heat and cold, mechanical, electrical and chemical stimuli to elicit small fiber-evoked potentials: merits and limitations for basic science and clinical use. Neurophysiol Clin 42, 267–280, https://doi.org/10.1016/j.neucli.2012.06.002 (2012).
Haefeli, J., Kramer, J. L., Blum, J. & Curt, A. Assessment of Spinothalamic Tract Function Beyond Pinprick in Spinal Cord Lesions: A Contact Heat Evoked Potential Study. Neurorehabil Neural Repair 28, 494–503, https://doi.org/10.1177/1545968313517755 (2014).
Jutzeler, C. R. et al. Improved Diagnosis of Cervical Spondylotic Myelopathy with Contact Heat Evoked Potentials. Journal of neurotrauma 34, 2045–2053, https://doi.org/10.1089/neu.2016.4891 (2017).
Atherton, D. D. et al. Use of the novel Contact Heat Evoked Potential Stimulator (CHEPS) for the assessment of small fibre neuropathy: correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol 7, 21, https://doi.org/10.1186/1471-2377-7-21 (2007).
Wu, S. W. et al. Biomarkers of neuropathic pain in skin nerve degeneration neuropathy: contact heat-evoked potentials as a physiological signature. Pain 158, 516–525, https://doi.org/10.1097/j.pain.0000000000000791 (2017).
Ulrich, A., Min, K. & Curt, A. High sensitivity of contact-heat evoked potentials in “snake-eye” appearance myelopathy. Clin Neurophysiol 126, 1994–2003, https://doi.org/10.1016/j.clinph.2014.12.020 (2015).
Ulrich, A., Haefeli, J., Blum, J., Min, K. & Curt, A. Improved diagnosis of spinal cord disorders with contact heat evoked potentials. Neurology 80, 1393–1399, https://doi.org/10.1212/WNL.0b013e31828c2ed1 (2013).
Schestatsky, P., Llado-Carbo, E., Casanova-Molla, J., Alvarez-Blanco, S. & Valls-Sole, J. Small fibre function in patients with meralgia paresthetica. Pain 139, 342–348, https://doi.org/10.1016/j.pain.2008.05.001 (2008).
Granovsky, Y. et al. Normative data for Adelta contact heat evoked potentials in adult population: a multicenter study. Pain 157, 1156–1163, https://doi.org/10.1097/j.pain.0000000000000495 (2016).
Rosner, J. et al. Contact heat evoked potentials: Reliable acquisition from lower extremities. Clin Neurophysiol 129, 584–591, https://doi.org/10.1016/j.clinph.2017.12.034 (2018).
Lagerburg, V. et al. Contact heat evoked potentials: normal values and use in small-fiber neuropathy. Muscle Nerve 51, 743–749, https://doi.org/10.1002/mus.24465 (2015).
Chen, I. A. et al. Contact heat evoked potentials in normal subjects. Acta neurologica Taiwanica 15, 184–191 (2006).
Jutzeler, C. R., Rosner, J., Rinert, J., Kramer, J. L. & Curt, A. Normative data for the segmental acquisition of contact heat evoked potentials in cervical dermatomes. Sci Rep 6, 34660, https://doi.org/10.1038/srep34660 (2016).
Chan, A. C. & Wilder-Smith, E. P. Small fiber neuropathy: Getting bigger! Muscle Nerve 53, 671–682, https://doi.org/10.1002/mus.25082 (2016).
Botez, S. A. & Herrmann, D. N. Pitfalls of diagnostic criteria for small fiber neuropathy. Nature clinical practice. Neurology 4, 586–587, https://doi.org/10.1038/ncpneuro0920 (2008).
Devigili, G. et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 131, 1912–1925, https://doi.org/10.1093/brain/awn093 (2008).
Terkelsen, A. J. et al. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. The Lancet. Neurology 16, 934–944 (2017).
Kirshblum, S. C. et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 34, 535–546, https://doi.org/10.1179/204577211X13207446293695 (2011).
Kramer, J. L., Haefeli, J., Curt, A. & Steeves, J. D. Increased baseline temperature improves the acquisition of contact heat evoked potentials after spinal cord injury. Clin Neurophysiol 123, 582–589, https://doi.org/10.1016/j.clinph.2011.08.013 (2012).
Kramer, J. L., Haefeli, J., Jutzeler, C. R., Steeves, J. D. & Curt, A. Improving the acquisition of nociceptive evoked potentials without causing more pain. Pain 154, 235–241, https://doi.org/10.1016/j.pain.2012.10.027 (2013).
Haefeli, J. S., Blum, J., Steeves, J. D., Kramer, J. L. & Curt, A. E. Differences in spinothalamic function of cervical and thoracic dermatomes: insights using contact heat evoked potentials. J Clin Neurophysiol 30, 291–298, https://doi.org/10.1097/WNP.0b013e31827ed9ee (2013).
Wydenkeller, S., Wirz, R. & Halder, P. Spinothalamic tract conduction velocity estimated using contact heat evoked potentials: what needs to be considered. Clin Neurophysiol 119, 812–821, https://doi.org/10.1016/j.clinph.2007.12.007 (2008).
Quante, M., Lorenz, J. & Hauck, M. Laser-evoked potentials: prognostic relevance of pain pathway defects in patients with acute radiculopathy. Eur Spine J 19, 270–278 (2010).
Hullemann, P. et al. Laser-evoked potentials in painful radiculopathy. Clin Neurophysiol 128, 2292–2299 (2017).
Truini, A. et al. Laser-evoked potentials: normative values. Clin Neurophysiol 116, 821–826, https://doi.org/10.1016/j.clinph.2004.10.004 (2005).
Cruccu, G. et al. Assessment of trigeminal small-fiber function: brain and reflex responses evoked by CO2-laser stimulation. Muscle Nerve 22, 508–516 (1999).
Chao, C. C., Hsieh, S. T., Chiu, M. J., Tseng, M. T. & Chang, Y. C. Effects of aging on contact heat-evoked potentials: the physiological assessment of thermal perception. Muscle Nerve 36, 30–38 (2007).
Gibson, S. J. & Helme, R. D. Age-related differences in pain perception and report. Clin Geriatr Med 17, 433–456 (2001).
Gagliese, L. & Melzack, R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev 24, 843–854 (2000).
Merkies, I. S., Faber, C. G. & Lauria, G. Advances in diagnostics and outcome measures in peripheral neuropathies. Neurosci Lett 596, 3–13 (2015).
Lauria, G. Innervation of the human epidermis. A historical review. Italian journal of neurological sciences 20, 63–70 (1999).
Derbyshire, S. W., Nichols, T. E., Firestone, L., Townsend, D. W. & Jones, A. K. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain 3, 401–411 (2002).
Casanova-Molla, J., Grau-Junyent, J. M., Morales, M. & Valls-Sole, J. On the relationship between nociceptive evoked potentials and intraepidermal nerve fiber density in painful sensory polyneuropathies. Pain 152, 410–418, https://doi.org/10.1016/j.pain.2010.11.012 (2011).
Chao, C. C., Hsieh, S. C., Tseng, M. T., Chang, Y. C. & Hsieh, S. T. Patterns of contact heat evoked potentials (CHEP) in neuropathy with skin denervation: correlation of CHEP amplitude with intraepidermal nerve fiber density. Clin Neurophysiol 119, 653–661, https://doi.org/10.1016/j.clinph.2007.11.043 (2008).
Kramer, J. L. et al. Test-retest reliability of contact heat-evoked potentials from cervical dermatomes. J Clin Neurophysiol 29, 70–75, https://doi.org/10.1097/WNP.0b013e318246ada2 (2012).
Beydoun, A., Morrow, T. J., Shen, J. F. & Casey, K. L. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalography and clinical neurophysiology 88, 173–181 (1993).
Garcia-Larrea, L., Peyron, R., Laurent, B. & Mauguiere, F. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport 8, 3785–3789 (1997).
Acknowledgements
This work was supported by the Swiss Spinal Cord Injury Cohort Study Nested Project Grant (J.R. and C.R.J., 2016-N-005) and the Swiss National Science Foundation (A.C., grant number 320030_169250). J.R. is supported by funding of the Hartmann Müller Stiftung (grant number 1997). P.S.S. was supported by a Blusson Integrated Cures Partnership International Award (Rick Hansen Foundation and International Collaboration on Repair Discoveries [ICORD]). J.L.K.K. is supported by a Michael Smith Foundation for Health Research and Rick Hansen Scholar award and project funding from the Natural Sciences and Engineering Research Council of Canada (Discovery Grant). C.R.J. is funded by postdoctoral research fellowships from the International Foundation for Research in Paraplegia (IRP, F16-01769), Swiss National Science Foundation (SNSF, P2EZP3_172162), and Craig H. Neilsen Foundation (460378).
Author information
Authors and Affiliations
Contributions
J. Rosner contributed substantially to the conception and design of the study, the data acquisition, analysis, and interpretation. Furthermore, he drafted the research article. P.H. was substantially involved in the data collection, data analysis, and drafting the research article. J. Rinert, P.S.S. and L.S. were involved in the data collection, data analysis, and revising the research article. J.L.K.K. and C.R.J. contributed substantially to the data analysis and interpretation, and were involved in revising the research article. A.C. contributed substantially to the data analysis and interpretation and participated in revising the research article critically for important intellectual content. M.H. made substantial contributions to study conception and design, data acquisition, analysis and interpretation as well as participated in revising the research article critically for important intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosner, J., Hostettler, P., Scheuren, P.S. et al. Normative data of contact heat evoked potentials from the lower extremities. Sci Rep 8, 11003 (2018). https://doi.org/10.1038/s41598-018-29145-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29145-8
- Springer Nature Limited
This article is cited by
-
Interpersonal physiological and psychological synchrony predict the social transmission of nocebo hyperalgesia between individuals
Communications Psychology (2024)
-
EEG-based sensory testing reveals altered nociceptive processing in elite endurance athletes
Experimental Brain Research (2023)
-
Improved acquisition of contact heat evoked potentials with increased heating ramp
Scientific Reports (2022)
-
Cold evoked potentials elicited by rapid cooling of the skin in young and elderly healthy individuals
Scientific Reports (2022)