Abstract
The growth of animal consumers is affected by the balance of elements in their diet with the transition between limitation by one element to another known as the threshold elemental ratio (TER). Precise estimates of TERs with known levels of uncertainty have yet to be generated for most zooplankton consumers. We determined the TER for carbon (C) and phosphorus (P) in for a common lake zooplankter, Daphnia magna, using experimental measurements and theoretical considerations. Daphnia growth responses to food C:P ratios across a relatively narrow range (80–350) generated an empirical estimate of TERC:P of 155 ± 14. While this TER matched our modelled estimate of TERC:P (155 ± 16), it was lower than previous estimates of this dietary transition point. No threshold was found when we examined daphnid body C:N or C:P ratios in response to changing food C:P ratios, which indicates P-limitation at even lower food C:P ratios. Our results provide strong evidence that D. magna is likely to experience acute P-limitation when food C:P ratios exceed even relatively low ratios (~155). Our model further demonstrated that while physiological adjustments may reduce the likelihood of P-limitation or reduce its intensity, these changes in animal material processing would be accompanied by reduced maximum growth rates.
Similar content being viewed by others
Introduction
Nutrition is a central determinant of organismal performance due to its effects on life-history traits such as growth, reproduction, and survival1,2. Given the potential ecological consequences of inadequate nutrition on organisms and their role in ecosystems3, there is a continuing need to determine if, and when, animal consumers experience nutritional deficiency in their food sources2. One well-developed approach to assessing the frequency or intensity of nutrient limitation acting on consumers has been to calculate threshold elemental ratios (TER)4,5. The TER is the nutritional mixture where animals grow fastest and is characterized by high dietary and environmental supplies of all needed nutrients. It follows that when elemental ratios in food are above or below this ‘threshold’ ratio6, animal growth will be reduced. TERs are thus roughly equivalent to ideal or optimal nutrient ratios for primary producers7 and the ‘stoichiometric knife-edge’ for consumers8,9 as they delineate food conditions under which the organism is limited by one element or another.
Modelled estimates of TERs produced for aquatic consumers vary substantially within and among taxa10. Within taxa, this variability in TER estimates creates uncertainty about the nutritional requirements for maximal growth. For example, Daphnia, a well-studied zooplankter, is widely recognized to be sensitive to low food phosphorus (P) content11,12,13. Despite this, the TER where animals switch from carbon (C) to P limitation remains uncertain with modelled TERC:P estimates ranging from 100 to nearly 400 (Table 1). This wide range of TERC:P estimates partly reflects differences in assumptions regarding nutrient acquisition and in parameter values that represent the maximal efficiency of C and P assimilation. Generally, models that use low C assimilation efficiencies elevate TERC:P and predict a lower likelihood of P-limitation in animal consumers1,10. Such uncertainty in TERC:P and the food conditions under which animals experience dietary P-limitation has implications for understanding of zooplankton nutrition in lakes. For example, TERC:P estimates on the low end of the range (~150 or lower) lead to predictions of more frequent and severe P-limitation of Daphnia whereas higher TERC:P estimates (~250 and above) indicate P-limitation of daphnids should be uncommon and, when present, to be quite mild (e.g.,14).
Uncertainty about the frequency and intensity of dietary P-limitation may also partly reflect difficulties in empirically assessing animal growth responses to subtle changes in food quality. Growth responses to marginally poor nutrition may be unapparent due to animal acclimation, which allows for continued growth even if acute dietary limitation is present15. Physiological acclimation may reduce effect sizes of poor food quality despite a proximate nutritional deficiency16. In addition, precise manipulation of food quality across a narrow gradient can be technically difficult to achieve and may produce relatively small effects relative to residual experimental error. Past assessments of stoichiometric food quality and its effects generally focused on broad nutritional gradients and severe forms of nutritional limitation to avoid these experimental limitations. However, the use of a few but widely spaced food C:P ratios in growth response experiments would appear less capable of generating precise estimates of food C:P ratio above which P-limitation occurs in Daphnia or other invertebrate consumers.
Given the myriad of stoichiometric dynamics that emerge from nutrient-stressed consumers3,17,18, there is an obvious need to have a deeper understanding of the conditions that lead to nutritional stress. For example, incorporation of elemental limitation into predator-prey models has largely assumed linear relationships between food quality and animal life-history traits and distinct transition points between different types of elemental limitation4,18. These sharp transitions may be ‘modulated’ if consumers alter physiological processes, including ingestion and digestion, in response to poor stoichiometric food quality (e.g.,19). While ‘stoichiometric modulation’ has been proposed to decouple growth from food C:nutrient ratios16, its consequences on an animal’s likelihood of nutrient limitation and its growth rate remain unclear. One approach to studying the implications of consumer physiological performance is to explicitly connect models of TER and animal growth.
Here we estimated the TERC:P of Daphnia magna using a combination of growth experiments and individual mass balance modelling. With our experiments, we grew animals on algal food across a relatively narrow food C:P ratio range (~100 to ~300). We used these data to empirically determine the precise threshold food C:P ratio where growth rates of Daphnia become limited by food P content. Animal body P content, an additional indicator of consumer P nutrition, was measured across this food C:P ratio gradient and compared against animal growth responses. Using updated parameter values from these experiments, we calculated the TERC:P and maximal growth rates for Daphnia using an established individual mass balance model10 and extended this model to estimate error around the TERC:P. Our primary objective was to estimate, empirically and theoretically, the TERC:P of Daphnia as a means to understand the nature and frequency of P-limitation likely to be experienced by this important zooplankton consumer. Further, we compared our empirical and modeling based TERC:P estimates, contrasted these estimates with previously derived literature values and discussed physiological reasons for this variation.
Methods
Growth experiments
Mass specific growth rate (MSGR) experiments on D. magna were completed over a relatively narrow range of dietary C:P ratios to empirically identify the TERC:P. For a food source, we grew Scendesmus obliquus (purchased as S. acutus from the Canadian Phycological Culture Centre 10) under different P supplies. Algae were grown in semi-continuous cultures at 20 °C under constant aeration and light intensity of ~150 μmol s−1 m−2. We diluted cultures daily with sterilized algal media20 enriched with different P concentrations. Collected algae was examined under the microscope and found primarily (>95%) unicellular regardless of the P media used to grow the cells. Using this approach, we cultured algae having a range of C:P ratios (80–350). Algae was collected each day and concentrated by centrifuging (Sorvall Legend TR) at 4066 g for 15 minutes at room temperature (~20 °C). For each experimental run, we produced algal food at one or more “elevated” ratio (C:P ratios >120) and a low C:P ratio (C:P ratio ~100). To verify algal nutrient content and density, we saved a small subsample by filtering each concentrated algal sample onto pre-weighed Whatman GF/C filters (2 ashed for CN and 2 unashed for P analysis). Filters were dried for a minimum of 2 h at 60 °C before being reweighed for mass determination and subsequent elemental analysis.
Daphnia magna (clone DG106) mothers were grown in jars containing 400 mL of P-free COMBO media21 that was changed at least three times a week and contained high quantities of high P algae (C:P ~80–100). Experiments were set up by pooling neonates under 16 h from broods 2–5 from these cultured mothers. These neonates were subsequently triple rinsed in P-free COMBO to remove all the residue food and nutrients. For growth experiments, we saved five aggregate samples of 20 neonates each that were subsequently dried to determine initial mass. The remaining neonates were placed into individual tubes containing 40 mL of P-free COMBO and fed 6 mg C L−1 of the prescribed diet. On day three of each experiment, we transferred all animals into new media containing a higher food concentration (8 mg C L−1). After 6 days of growth, animals were saved individually for MSGR (n = 8). Samples of 4 pooled individuals were also saved for body P content (n = 5, total of 20 animals) and CN analysis (n = 3, total of 12 animals). All samples were dried at 60 °C for a minimum of 72 h before being weighed. We repeated this experiment 18 times with 41 different food C:P ratios between June and August of 2015.
Elemental analysis
We measured the P content of algae and animals after digesting filters or animal bodies with potassium persulfate using the molybdate-blue method22. Particulate C and N content was measured with a CN analyzer (Vario Elementar EL III).
Statistics
MSGR was calculated by subtracting the natural log of average neonate mass of all the experiments from the natural log of the individual mass of each experimental animal and dividing by the duration of the experiment (6 days). Using least squares based optimization, a piecewise linear curve was fitted to the data of animal MSGR vs algal C:P ratios. Linear fits were made to assess relationships between Daphnia body C:N and C:P ratios and algal C:P ratios as piecewise regression yielded no improvement over simple linear regression for these two response variables.
Model description
We used an individual mass balance model to estimate the TER for C:P and maximum growth rate of D. magna. Building off of previously published approaches4,5,10, we assumed balanced growth at the point of the threshold such that the rate of C increase must be matched by the rate of P increase scaled by the body C:P ratio:
with \(\frac{{{\rm{Q}}}_{{\rm{C}}}}{{{\rm{Q}}}_{{\rm{P}}}}\) = body C:P ratio of Daphnia. Under steady state conditions, the incorporation of C and P into new body production will be equal and equivalent to their delivery rates from ingested food after digestion and absorption such that:
with IC = maximum ingestion rate, AC = assimilation efficiency of C, RC = respiration rate, fP/C = food P:C ratio, and AP = assimilation efficiency of P. We rearranged Equation 2 to isolate the food C:P ratio, which is equivalent to the TERC:P:
Using Equation 3, we calculated TERC:P using parameter values from the literature and the current study (Table 2). To assess uncertainty in this TER, we propagated error associated with each parameter value (pi) to calculate the standard deviation of the TERC:P (δTER) using standard error propagation methods:
where \(\frac{\partial {\rm{G}}}{\partial {{\rm{p}}}_{{\rm{i}}}}\) indicates the partial derivative of a function with respect to the specified parameter and Δpi indicates the empirical error in the parameter value.
We further examined the links between TERC:P and maximum growth rates using modified Gaussian functions which take into account the functional relationship between TERC:P and maximum growth rates in the individual mass balance model. This allowed us to determine the probability density in the “TERC:P vs maximum growth space”. To do so, we used the following formulation:
where m is the maximum growth rate being considered and TER is the threshold being considered. The f(m) term is the model functional relationship between the threshold C:P and m given by:
with the mo term indicating the calculated maximum growth rate according to the empirical growth parameters as follows:
The σTER value is the standard deviation for TER and σm is standard deviation for maximum growth. These were propagated from the empirical parameters according to equation 4. Combining all of this into a normalized 2D Guassian results in equation 5. Along with this, we determined a confidence region for the relationship by tracing the specific probability density at which 95% of the values are expected to be contained in a standard 2D Guassian distribution. As we used a normalized Equation 5, the integral of the probability density over the region is equal to 0.95, meaning with the parameters and parameter errors given, there is a 95% chance that the region contains the true TER and maximum growth combination.
Data accessibility
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Growth and body elemental composition responses
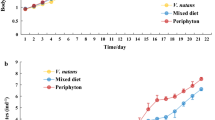
Daphnia growth rate decreased with higher food C:P ratios (Fig. 1). Using piecewise linear regression, we found a break-point in growth rates at a food C:P ratio of 155 ± 14. Above this break-point, daphnid growth rates decreased more steeply with increasing food C:P ratios. Animals reduced their growth rate by 25–30% at food C:P ratios of 200–300, compared to the growth rates for food C:P <130 (Fig. 1). At food C:P ratios lower than the breakpoint ratio of 155, daphnid growth rates still decreased with increasing C:P, however, this was at a significantly lower rate (Fig. 1).
Daphnia body C:N and C:P ratios both increased with food C:P ratios across the 100 to 300 range (Fig. 2). We found no apparent break-point that would be consistent with a TER at any point along the food C:P ratio gradient. Both ratios were linearly related to food C:P ratios and were not accompanied by non-normal residual error. Daphnid body C:P ratios displayed a steeper slope with more variation explained against food C:P ratios compared to body C:N ratios (Fig. 2).
Modelled threshold elemental ratios and growth rates
The TERC:P calculated using Equation 3 and its standard deviation with the error propagation approach (Equation 4) was found to be 155 ± 16. As dictated in Equation 7, increasing maximum growth rate in our model was accompanied by lower TERC:P, which is apparent in our probabilistic density analysis (Fig. 3). In this analysis, the TERC:P associated with our observed maximum growth rates (~0.52 day−1) had a value of ~140. While our analysis also found that higher TERC:P are possible given the parameter values and their error used in our model, these parameter value sets were associated with relatively slow maximum growth rates (<0.4 day−1) of Daphnia that are well below those measured here and previously reported in the literature. We calculated relatively high TERC:P (>200) when using lower AC values (0.50) but found low maximum growth rates (<0.3 day−1) in these model runs (Fig. 3).
Modelled threshold elemental ratios of carbon and phosphorus in Daphnia. (a) Probability vs TERC:P and maximum growth rates estimated by individual mass-balance model using parameter values and error found in Table 2. The z scale (amount of shading) indicates how probable it is that with the specified parameters, Daphnia will experience the TERC:P and maximum growth combination indicated by the axis values. The solid line surrounding the region of high probability represents the 95% confidence region (b) Direct slice of plot a (indicated by dotted line) through a maximum growth rate of 0.52 as indicated by the dotted line in a. (c) TERC:P versus maximum growth rates for calculated with different assimilation efficiencies. 95% confidence region for each are circled Ac = 0.5 (left), Ac = 0.68 (middle) and Ac = 0.8 (right).
Discussion
We found that the TERC:P of Daphnia magna to be ~155 based on both growth measurements and with refined parameter values in an individual mass balance model10. Our empirical estimate of Daphnia’s TERC:P was generated using a fine-scale food quality gradient that allowed us to carefully examine animal growth responses to poor food quality near the TER. Our modelled TERC:P estimate included updated parameter values and propagated error terms, which provided an estimate of the threshold with a known level of certainty. Our current estimates are similar to TERC:P estimates produced by similar modelling efforts (Table 1) but are substantially lower than TERC:P estimates from two other studies (385, Urabe and Watanabe11; 300, Brett et al.20). A low TERC:P of ~155, as found here, indicates that D. magna may be especially prone to experience P-limitation in its food10 and that food with even moderately low food C:P ratios (200) will have negative effects on its growth and reproduction.
Our results demonstrate slower growth in Daphnia consuming slightly elevated food C:P ratios (~200) compared to those animals feeding on food C:P ratios at or below the TERC:P (~150). This reduction in growth rates and the changes observed in animal body P content with food C:P ratios above the threshold is consistent with expectations and previous observations of P-limited growth in animals23,24 at higher food C:P ratios. At or near the calculated TERC:P , our growth experiments found no evidence of an obvious stoichiometric ‘knife-edge’ as even below our regression break-point, Daphnia growth rates continued to increase with decreasing food C:P ratios. As we did not reduce food C:P ratios below 80, we cannot rule out possible declines in growth rates with lower food C:P ratios (<60−70). It may be that the stoichiometric knife-edge is not a sharp transition point and instead manifests itself as a stoichiometric ‘hill-top’. This lack of abrupt changes in growth above and below the threshold may be because of physiological acclimation to nutrient-deficient food, which allows an organism to sustain growth even when presented with slightly imbalanced food1.
We used a mass balance formulation to calculate a theoretical estimate of the TERC:P for Daphnia. This model formulation has been previously used with Daphnia in several earlier studies to estimate TERC:P of 200 or below (Table 1). Our current model formulation, simplistic compared to some of these studies (e.g.,25), is derived from Frost et al.10 under the assumptions of high food abundance and no possible limitation by a third element. The current application of this model of Daphnia TERC:P differed from past studies by incorporating uncertainty in measured parameter values and yielded a calculated TERC:P with a 95% confidence interval between 123–187. We further constrained the probable range of TERC:P range by examining the relationship between the modelled TERC:P and the maximum MSGR predicted by the model. Given that measured maximum growth rates are typically ~0.50 day−1, we excluded TERC:P estimates that were associated with much faster or slower growth. The TERC:P estimate (~140) associated with this growth rate is nearly the same as our experimentally determined point and to the average modelled TERC:P as described above.
We found that an elevated TERC:P (e.g., >200) could be calculated using this TER model formulation but these higher threshold ratios were associated with unrealistic sets of parameter values. Combinations of parameters that yielded elevated TERC:P for Daphnia were generally inconsistent with parameter values reported in the literature and when combined to calculate growth yielded unrealistically low maximum specific growth rates. For example, lower ingestion rates, higher respiration rates, and low assimilation efficiency of C all reduce the net intake and incorporation of C by Daphnia and, in the TER model, parameter values associated with these physiological states yielded higher estimates of the TERC:P. Such changes in animal acquisition of C would severely constrain maximal animal growth rates below that typically measured for laboratory animals and are inconsistent with observations of physiological processes in well-nourished, fast growing Daphnia. This theoretical coupling of TER and growth rates thus provides a quantitative approach to assessing whether parameters used to calculate thresholds are realistic and to verify that TER estimates for a given species are consistent with the known biology of the species under consideration. These connections also indicate that species-specific differences in nutrient acquisition, while largely beyond the scope of this study, should be examined for their connections to maximum attainable growth rates.
We estimated the TERC:P using the mass balance formulations, which have been extensively analyzed by previous studies10,25,26. The past analyses have indicated that caution is warranted when using this approach due, in part, to parameters in the model being themselves sensitive to food quality10,25. For example, sustained P-limitation can dramatically reduce carbon use efficiency15,23, which, if represented in a TER model, yields a relatively high TERC:P and the contradictory conclusion that P-limitation is unlikely for this taxon14. A previous examination of this problem recommended using parameter values that were based on first principles and well-grounded in the biology of the organism25. Another solution is to only use parameter values estimated on animals growing maximally under conditions of high quantities of good food quality, which thus match the conditions assumed to be operating at the TER. Estimates of TER that incorporate parameters derived from animals growing under food or nutrient-limiting conditions should thus be considered unreliable and may account for some of the previously reported differences in thresholds estimated for Daphnia and other taxa.
Modelled TER estimates are also sensitive to physiological parameters that are known to vary with organismal traits, life-history, trophic position, environmental conditions, and other forms of physiological stress6,10,27. Against this, it is valid to question whether a TERC:P determined under single set of laboratory conditions can be applied to a broader context. Laboratory derived TERC:P estimates provide baseline information on consumer nutritional requirements, in the absence of other environmental factors, that can be used understand the nutritional circumstances under which an organism would be nutrient-limited in the field. One could imagine incorporating additional sources of variation such as multiple limiting elements, physico-chemical conditions (e.g., temperature), and other food traits (e.g, digestibility or lipid composition) to yield multidimensional TER estimates28. Future work should examine the sensitivity of TER to these other conditions and couple this information with data from emerging nutritional indicators2 to better define conditions, dietary or otherwise, that separate multiple forms of nutritional limitation on ecologically important consumer taxa. In addition, the applicability of growth-related TER estimates to other response variables such as age at first reproduction and reproductive effort would be worthwhile given the possibly different nutritional demands of these alternative life-history traits3. Nonetheless, while physiological parameters (e.g., feeding rates and body stoichiometry) can vary among species and TER estimates should be viewed as species-specific, mass balance constraints underlying the TER formulation place limits on TER and growth rates that are unavoidable regardless of taxonomic identity.
This study aimed to better understand the TERC:P of Daphnia magna by combining growth experiments and metabolic models. Our work found a relatively high demand for P for Daphnia that makes it especially susceptible to dietary P- limitation. While our model results demonstrate physiological adjustments could potentially modulate these P demands and reduce the likelihood of P-limited growth, this type of acclimation would necessarily be accompanied by dramatically slower growth. Consequently, there is a link between increasing the acquisition of the limiting element relative to other elements and the ability of an animal to grow maximally. This trade-off appears to complement other molecular complexities29 that dictate strong connections between fast growth and elevated P demands in organisms. Future work should try to couple these alternative views of animal growth physiology to better understand how material constraints affect growth processes and nutrient dynamics as advocated by the theory of ecological stoichiometry3.
References
Frost, P. C., Evans-White, M. A., Finkel, Z. V., Jensen, T. C. & Matzek, V. Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 109, 18–28 (2005).
Wagner, N. D., Hillebrand, H., Wacker, A. & Frost, P. C. Nutritional indicators and their uses in ecology. Ecol. Lett. 16, 535–544 (2013).
Sterner, R. W. & Elser, J. J. Ecological stoichiometry: the biology of elements from molecules to the biosphere. (Princeton University Press, 2002).
Sterner, R. Modelling interactions of food quality and quantity in homeostatic consumers. Freshw. Biol. 38, 473–481 (1997).
Frost, P. C. & Elser, J. J. Growth responses of littoral mayflies to the phosphorus content of their food. Ecol. Lett. 5, 232–240 (2002).
Anderson, T. R. & Hessen, D. O. Threshold elemental ratios for carbon versus phosphorus limitation in Daphnia. Freshw. Biol. 50, 2063–2075 (2005).
Klausmeier, C. A., Litchman, E., Daufresne, T. & Levin, S. A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429, 171–174 (2004).
Boersma, M. & Elser, J. J. Too much of a good Thing: On Stoichiometrically balanced diets and maximal growth. Ecology 87, 1325–1330 (2006).
Elser, J. J. et al. Life on the stoichiometric knife-edge: effects of high and low food C: P ratio on growth, feeding, and respiration in three Daphnia species. Inland Waters 6, 136–146 (2016).
Frost, P. C. et al. Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol. Lett. 9, 774–779 (2006).
Urabe, J., Clasen, J. & Sterner, R. W. Phosphorus limitation of Daphnia growth: Is it real? Limnol. Oceanogr. 42, 1436–1443 (1997).
Boersma, M. The nutritional quality of P-limited algae for. Daphnia. Limnol. Oceanogr. 45, 1157–1161 (2000).
Frost, P. C. et al. Transgenerational effects of poor elemental food quality on Daphnia magna. Oecologia 162, 865–872 (2010).
Brett, M. T., Müller-Navarra, D. C. & Sang-Kyu, P. Empirical analysis of the effect of phosphorus limitation on algal food quality for freshwater zooplankton. Limnol. Oceanogr. 45, 1564–1575 (2000).
He, X. & Wang, W.-X. Stoichiometric regulation of carbon and phosphorus in P-deficient Daphnia magna. Limnol. Oceanogr. 53, 244–254 (2008).
Mulder, K. & Bowden, W. B. Organismal stoichiometry and the adaptive advantage of variable nutrient use and production efficiency in Daphnia. Ecol. Model. 202, 427–440 (2007).
Elser, J. J. & Urabe, J. The stoichiometry of consumer-driven nutrient recycling: Theory, observations, and consequences. Ecology 80, 735–751 (1999).
Andersen, T., Elser, J. J. & Hessen, D. O. Stoichiometry and population dynamics. Ecol. Lett. 7, 884–900 (2004).
Mitra, A. A multi-nutrient model for the description of stoichiometric modulation of predation in micro- and mesozooplankton. J. Plankton Res. 28, 597–611 (2006).
Sterner, R. W., Hagemeier, D. D., Smith, W. L. & Smith, R. F. Phytoplankton nutrient limitation and food quality for. Daphnia. Limnol. Oceanogr. 38, 857–871 (1993).
Kilham, S. S., Kreeger, D. A., Lynn, S. G., Goulden, C. E. & Herrera, L. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377, 147–159 (1998).
American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 18th Edn., Washington DC. (ISBN 0-87553-207-1, 1992).
DeMott, W. R., Gulati, R. D. & Siewertsen, K. Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna. Limnol. Oceanogr. 43, 1147–1161 (1998).
Sterner, R. W. & Schulz, K. L. Zooplankton nutrition: recent progress and a reality check. Aquat. Ecol. 32, 261–279 (1998).
Anderson, T. R., Hessen, D. O., Elser, J. J. & Urabe, J. Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am. Nat. 165, 1–15 (2005).
Shimizu, Y. & Urabe, J. Regulation of phosphorus stoichiometry and growth rate of consumers: theoretical and experimental analyses with Daphnia. Oecologia 155, 21–31 (2008).
Doi, H. et al. Integrating elements and energy through the metabolic dependencies of gross growth efficiency and the threshold elemental ratio. Oikos 119, 752–765 (2010).
Frost, P. C., Xenopoulos, M. A. & Larson, J. H. The stoichiometry of dissolved organic carbon, nitrogen, and phosphorus release by a planktonic grazer, Daphnia. Limnol. Oceanogr. 49, 1802–1808 (2004).
Elser, J. J. et al. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540–550 (2000).
Urabe, J. & Watanabe, Y. Possibility of N or P limitation for planktonic cladocerans: An experimental test. Limnol. Oceanogr. 37, 244–251 (1992).
Acknowledgements
We thank Andrea Conine for help running experiments and laboratory analysis. Support for this research was provided by the Natural Sciences and Engineering Research Council of Canada through funds provided to Hamza Khattak and Paul Frost. All applicable institutional and Canadian guidelines for the care and use of Daphnia were followed.
Author information
Authors and Affiliations
Contributions
H.K., C.P., N.D.W. and P.C.F. conceived and designed the experiments. H.K., N.D.W. and C.P. conducted the experiments, analyzed the data and P.C.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khattak, H.K., Prater, C., Wagner, N.D. et al. The threshold elemental ratio of carbon and phosphorus of Daphnia magna and its connection to animal growth. Sci Rep 8, 9673 (2018). https://doi.org/10.1038/s41598-018-27758-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27758-7
- Springer Nature Limited
This article is cited by
-
A critical assessment of the stoichiometric knife-edge: no evidence for artifacts caused by the experimental P-supplementation of algae
Aquatic Ecology (2021)
-
Transgenic and cell wall-deficient Chlamydomonas reinhardtii food affects life history of Daphnia magna
Journal of Applied Phycology (2020)