Abstract
Streptococcus anginosus is increasingly recognized as an opportunistic pathogen. However, our knowledge about virulence determinants in this species is scarce. One exception is the streptolysin-S (SLS) homologue responsible for the β-hemolytic phenotype of the S. anginosus type strain. In S. anginosus the expression of the hemolysin is reduced in the presence of high glucose concentrations. To investigate the genetic mechanism of the hemolysin repression we created an isogenic ccpA deletion strain. In contrast to the wild type strain, this mutant exhibits hemolytic activity in presence of up to 25 mM glucose supplementation, a phenotype that could be reverted by ccpA complementation. To further demonstrate that CcpA directly regulates the hemolysin expression, we performed an in silico analysis of the promoter of the SLS gene cluster and we verified the binding of CcpA to the promoter by electrophoretic mobility shift assays. This allowed us to define the CcpA binding site in the SLS promoter region of S. anginosus. In conclusion, we report for the first time the characterization of a potential virulence regulator in S. anginosus.
Similar content being viewed by others
Introduction
Bacteria of the Streptococcus anginosus group (SAG) are considered commensals of mucosal membranes1 but an increasing number of reports in recent years demonstrate their clinical importance2,3,4,5. In a large population based investigation the incidence rate of invasive SAG infections (8.65/100 000 population) even exceeds the combined incidence rate of group A and B streptococcal invasive infections (7.40/100 000 population)6. Bacteria of the SAG (Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius) can frequently be isolated from abscesses and blood samples5,7 and are associated with different clinical pictures1,7,8. The knowledge about the virulence gene repertoire of these species is rare and mainly relies on interpretation of genome data9. One exception is the gene locus responsible for the β-hemolytic phenotype of the S. anginosus type strain which displays high homologies to streptolysin-S (SLS) of Streptococcus pyogenes10,11. SLS of S. pyogenes is considered a major virulence factor that is cytolytic for a variety of eukaryotic cells and has been shown to play an important role in in vivo models of skin and soft tissue infections12. The SLS molecule is a posttranslationally modified peptide of 2.7 kDa that is present in numerous pathogenic streptococci and other Gram-positive pathogens such as clostridia and listeria. It belongs to the TOMM (thiazole/oxazole-modified microcins) family of virulence peptides13. SLS of S. anginosus is able to lyse erythrocytes of different origins including human, sheep and chicken and it is inhibited in the presence of high glucose levels in the growth medium14.
Bacteria tightly regulate the uptake and consumption of different carbohydrates as the simultaneous utilization of all accessible sugars would be energetically inefficient. This regulatory process leading to a hierarchical metabolism of sugars is called carbon catabolite repression (CCR)15. In Gram-positive bacteria the catabolite control protein A (CcpA) is the major player in CCR16 although CcpA independent CCR mechanisms are well documented17,18,19. In order to be able to bind to DNA, CcpA needs to be activated by a phosphorylated form (serine-46) of the histidine-containing phosphocarrier protein (HPr). The availability of glucose and other preferred sugars leads to increased fructose-1,6-bisphosphate (FBP) levels in the cell. Accumulation of FBP activates the kinase activity of the HPr kinase/phosphorylase which phosphorylates the serine-46 residue of HPr. Thus high FBP levels indirectly activate CcpA via HPr20. Activated CcpA binds to catabolite responsive elements (cre) located in the promoter region of target genes predominantly resulting in the downregulation of gene expression. Studies in Bacillus subtilis and other Gram-positive bacteria have determined the consensus sequence of the cre sites which consist of highly degenerate pseudo-palindromes21,22,23,24. It was demonstrated that CCR is one of the most important regulatory processes in different bacteria with 5–10% of genes affected by CCR23,25,26. CcpA thereby mainly represses the expression of genes involved in consumption of alternative sugars and it activates expression of genes needed for glucose metabolism27. However, CcpA was also demonstrated to regulate virulence gene expression in different bacterial pathogens and thus represents an important link between virulence and metabolism24,28,29,30,31. In S. pyogenes CcpA controls SLS expression by binding to a cre site in the bacterial promoter24.
Since the S. anginosus SLS genes are repressed by high glucose levels14, we investigated if CcpA is responsible for this effect. Through the generation of various deletion mutants and EMSA assays, we show that SLS expression in this species is under control of the CcpA regulator and we identified the CcpA binding site in the SLS promoter region of S. anginosus. Thus we are able to demonstrate for the first time the regulation of a putative virulence gene in the emerging pathogen S. anginosus.
Results
CcpA affects SLS expression
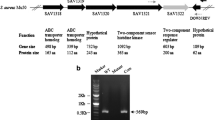
In previous experiments we noticed that the SLS expression of S. anginosus was reduced with increasing glucose concentrations in the growth medium14. To elucidate the molecular mechanism behind this phenomenon and the potential role of CcpA on reducing the SLS expression, a S. anginosus ΔccpA strain was constructed (Fig. 1a). It carries a 100 bp deletion resulting in a frameshift and a truncated CcpA protein. This strain was transformed with the promoter reporter plasmid pBSU409::sagprom11 that contains the EGFP under the control of the SLS promoter10. To determine the influence of sugar on SLS expression in a ccpA negative background, the activity of the promoter was quantified at a range of different glucose concentrations in the medium (Fig. 1b). The dose-dependent reduction of SLS expression observed in the S. anginosus type strain is absent in the S. anginosus ΔccpA strain that showed a constant relative fluorescence over all tested glucose concentrations.
(a) Schematic representation of the 100 bp deletion in the S. anginosus ∆ccpA strain. The site of deletion is marked with black lines and the deleted base pairs in bold. The 100 bp deletion results in a frameshift and a premature stop codon (*). (b) Effect of glucose supplementation on SLS promoter activity. The activity of the SLS promoter was determined using an EGFP reporter plasmid. The relative mean fluorescence intensity (MFI) of cells grown in THY medium supplemented with the indicated glucose concentrations is shown in comparison to the positive control. Negative control: S. anginosus pBSU409; positive control: S. anginosus pBSU409::cfbprom; WT: S. anginosus type strain; ΔccpA: S. anginosus ΔccpA strain. The mean values and standard deviations of five independent experiments are shown. Mann-Whitney-U test was performed to illustrate significant difference to S. anginosus pBSU409::sagprom (p < 0.05).
Effect of ccpA knockout on SLS activity
To investigate the effect of the reduced SLS expression on the hemolysin activity, a functional hemolytic assay with human erythrocytes was performed with cells growing in the presence of different glucose concentrations (Fig. 2). The S. anginosus type strain incubated in medium supplemented with up to 12.5 mM glucose showed regular β-hemolysis, while cells grown in medium with the supplementation of 25 mM glucose were non-hemolytic. In contrast to the wild type, the ΔccpA strain was hemolytic in the presence of all tested glucose concentrations. To verify that the ccpA deletion is responsible for this phenotypic difference a ccpA complementation strain was created that contains the ccpA gene, carrying a silent mutation, in the original locus followed by an erythromycin resistance cassette. This S. anginosus ΔccpA::ccpA strain exhibits the same hemolytic phenotype as the wild type strain, showing a lack of hemolysis for cells that were grown in medium supplemented with 25 mM of glucose.
Hemolytic activity of S. anginosus strains for human erythrocytes. Bacteria were grown in THY medium supplemented with the indicated glucose concentrations. The hemolytic behavior of the S. anginosus type strain (a), the S. anginosus ΔccpA strain (b) and the complemented S. anginosus ΔccpA::ccpA strain (c) is illustrated for different bacterial cell dilutions. Positive control: ddH2O; negative control: assay buffer. The mean values and standard deviations of five independent experiments are shown.
In silico prediction of CcpA binding sites
CcpA is an extensively investigated regulator in Gram-positive bacteria and consensus sequences of its DNA binding site, the catabolite responsive element (cre), have been determined (http://regprecise.lbl.gov/RegPrecise/collection_tf.jsp?collection_id = 163). Recently a second CcpA binding site (cre2) was identified in Streptococcus suis23. To substantiate the involvement of CcpA in the control of SLS, we screened the SLS promoter for the presence of potential cre sites allowing three mismatches to the consensus sequences. This led to the identification of three putative CcpA binding sites in the SLS promoter of the S. anginosus type strain (Fig. 3a). One of these cre sites (creA) is located 167 bp upstream of the transcription start site and shows homologies to the cre2 site. The creB sequence consists of a cre homologue overlapping a potential cre2 site and the third sequence (creC) is located in between the −35 and −10 region of the SLS promoter. It contains three mismatches to less conserved nucleotides of the consensus cre site (Fig. 3b).
(a) The locations of the putative cre sites are illustrated in respect to the transcription start site (arrow). A: creA, B: creB, C: creC. (b) Comparison of the in silico predicted cre site in the SLS promoter and the published consensus cre sites. The size of the single nucleotides corresponds to their conservation. The creB site consists of a cre homologue overlapping a potential cre2 site. Mismatches to the consensus sequences are underlined and bold.
CreC site mutation abolishes glucose dependent SLS repression
To investigate the influence of the in silico predicted cre sites on the observed glucose dependent repression of the SLS promoter activity, we mutated the putative cre sites in the reporter plasmid pBSU409::sagprom. The sequences of creA and creB were separately deleted and the altered promoters were cloned in front of egfp in the reporter plasmid (Fig. 4a). Since the creC site overlaps the bacterial promoter, we could not create a deletion of this sequence. Instead we mutated four putative important nucleotides of creC that should diminish the binding of CcpA to this site. All of the three different reporter plasmids carrying the mutated SLS promoters were transformed separately into the S. anginosus type strain and the promoter activity was determined for cells grown in medium with increasing glucose supplementations (Fig. 4b). The strain carrying the reporter plasmid with the creA deletion showed a similar behavior like the strain containing the wild type promoter with a concentration dependent reduction of promoter activity. Deletion of creB in the promoter results in a lower promoter activity compared to the wild type promoter without glucose supplementation and showed constant expression of egfp without significant differences for varying glucose concentrations. Compared to the wild type promoter, the promoter activity with 25 mM glucose supplementation was significantly higher in the creB deletion. In contrast to the previous findings the mutation of creC completely abolishes the glucose dependent promoter repression. This strain shows a constantly high SLS promoter activity with increasing glucose concentrations in the growth medium most closely resembling the expression data obtained for the S. anginosus ΔccpA strain.
(a) Schematic representation of reporter plasmids with mutated SLS promoters used to investigate the in silico predicted cre sites. WT: promoter of SLS; creA_del: pBSU409::sagprom_creA_del (pBSU881); creB_del: pBSU409::sagprom_creB_del (pBSU880); creC_mut: pBSU409::sagprom_creC_mut (pBSU876). (c) The sequence of the predicted cre site (upper row) and the mutations of the sequence (bold and underlined) in pBSU409::sagprom_creC_mut (lower row) are shown. (b) Effect of glucose supplementation on the activity of mutated SLS promoters in the S. anginosus type strain. The relative mean fluorescence intensity of cells grown in THY medium supplemented with the indicated glucose concentrations is shown in comparison to the positive control. Positive control: S. anginosus pBSU409::cfbprom; Negative control: S. anginosus pBSU409. The mean values and standard deviations of five independent experiments are shown. Mann-Whitney-U test was performed to illustrate significant difference to S. anginosus pBSU409::sagprom (p < 0.05).
CcpA binds to creC site in vitro
The data obtained in the reporter plasmid assay prompted us to further characterize the interaction of CcpA with the potential cre binding sites. To investigate if CcpA is able to directly regulate the SLS expression by binding to the in silico predicted cre sequences, we performed electrophoretic mobility shift assays (EMSA) with purified His-tagged CcpA. Several studies reported that CcpA is able to bind to cre sites in the absence of HPr and the allosteric effector fructose-1,6-bisphosphate23,30,32,33. Therefore we performed the EMSA with CcpA alone and a promoter fragment harboring creC (GTTTACGCGAAAGCGCTTTTTTTATATA). Increasing amounts of CcpA (0.5–8 µg) induced a shift of labeled creC, indicating binding of CcpA to the DNA (Fig. 5). The addition of a 500-fold molar excess of unlabeled creC was able to inhibit the observed shift (Fig. 5, lane A) whereas the addition of the same amount of unlabeled mutated creC (GTTTACGCGAAGGATCCTTTTTTATATA) and unlabeled creB had no effect (Fig. 5, lane B and C). Thus, we were able to demonstrate that CcpA binds to the creC site located in the promoter of the SLS operon.
EMSA of creC using His-tagged CcpA. Increasing amounts of purified CcpA (0–8 µg) were used and assayed for binding to labeled creC (GTTTACGCGAAAGCGCTTTTTTTATATA). The specificity of binding was assayed using 500-fold molar excess of unlabeled creC (A), creC_mut (B GTTTACGCGAAGGATCCTTTTTTATATA) and creB (C; TGCTATAAGAACGCGCTTTTTATTTTGTTTTAGATGGT).
Discussion
Bacteria sense the environmental conditions in order to tightly regulate their gene expression. One global regulatory mechanism that bacteria exert to conserve energy is CCR. The bacteria thereby regulate the uptake and consumption of different carbohydrates by downmodulation of alternative sugar utilization pathways in the presence of preferred substrates. This CCR mechanism was also demonstrated to play an important role in disease progression and virulence gene expression in different Gram-positive pathogens. In Staphylococcus aureus CcpA affects the expression of important virulence factors and it is required for pathogenesis34,35. The enolase and suilysin expression is regulated by CcpA in Streptococcus suis and a ccpA mutant was attenuated in a murine infection model36. Additionally, the capsule biosynthesis is affected by CcpA in different pathogens31,34,37 and CcpA was demonstrated to regulate the SLS expression in S. pyogenes by binding to a cre site located in the SLS promoter24.
Despite the increased knowledge about the epidemiology of S. anginosus1,2,3,4,5,6, the pathogenicity mechanisms in this species are poorly investigated38. One exception is the SLS responsible for the β-hemolytic phenotype of the S. anginosus type strain. The SLS of S. anginosus is a broad-range hemolysin able to lyse erythrocytes of different origins. The activity of the hemolysin is temperature dependent and a reduced SLS expression was demonstrated in cells growing in the presence of high glucose concentrations11,14. Such an expression pattern indicates a CCR mechanism controlling the SLS expression. We therefore constructed a ccpA deletion mutant and investigated the activity of the SLS promoter using a GFP reporter system. The observed reductions of the promoter activity in the S. anginosus wild type strain is absent in the ccpA mutant indicating that the SLS operon is controlled by CcpA. Even without glucose supplementation, the mutant strain showed an increased promoter activity which could be explained by the THY growth medium that already contains 11 mM glucose.
To investigate the potential effect of the reduced hemolysin expression in the presence of high glucose concentrations on the hemolytic activity of S. anginosus cells we performed hemolysis assays with human erythrocytes. The wild type as well as the ∆ccpA strain showed hemolytic activity up to 12.5 mM glucose supplementation. The observed reduction of the promoter activity measured in the wild type strain under these conditions thereby seemed to be sufficient for complete lysis of the erythrocytes. The wild type cells growing with 25 mM glucose supplementation showed no hemolytic activity at all whereas the ∆ccpA strain was still hemolytic. The complemented S. anginosus ∆ccpA::ccpA showed the same behavior like the wild type strain, thus confirming the role of CcpA in the control of hemolysis.
To investigate if CcpA directly or indirectly regulates the hemolysin expression, we screened the SLS promoter for potential cre sites. To reduce the possibility of missing a putative cre site, we allowed three mismatches to the already degenerate consensus cre sites and we included the published cre2 site in our analysis although this site was so far only reported in S. suis23. The in silico prediction identified three putative cre sites in the SLS promoter including the creC site that overlaps the −35 region of the bacterial promoter10. The location of the cre site in respect to the transcriptional start site is determining the effect of CcpA on the transcription and a position overlapping the bacterial promoter would indicate that CcpA is a strong repressor of the indicated gene39. To investigate the influence of the in silico predicted cre sites on the observed glucose dependent repression of the SLS transcription, we performed the promoter reporter assay with plasmids containing mutated SLS promoters. The deletion of the creA site resulted in an expression pattern like the wild type promoter demonstrating that this site is no CcpA binding site. For the creB deletion a low but constant reporter expression was measured at all tested glucose concentrations. As the creC site overlaps the −35 region of the bacterial promoter we mutated potentially conserved nucleotides of its sequence instead of deleting the cre site as this would probably destroy the promoter. The expression pattern measured with the creC site mutation resembles the pattern observed in the ∆ccpA strain, with a trend towards increased expression with higher glucose concentrations which is not significant. Thus, indicating that the creC site is most relevant for the glucose dependent reduction of the SLS transcription.
To verify that CcpA directly regulates SLS expression by binding to the creC site, we performed band shift experiments using His-tagged CcpA alone as previous studies reported that CcpA is able to bind to cre sites in the absence of serine phosphorylated HPr23,30,32,33. Increasing amounts of CcpA were able to shift the labeled creC site. Excess amounts of unlabeled creC were able to compete for binding of CcpA to the labeled creC. The specificity of binding was tested with an unlabeled mutated creC sequence. CcpA was not able to bind to this site suggesting that the interaction of CcpA and creC is specific. The presence of more than one cre site in the promoter of a CcpA regulated gene was already described23,39. We observed in our promoter reporter assay that the putative creB site deletion resulted in an increased promoter activity with 25 mM glucose supplementation compared to the wild type. To analyze the potential binding of CcpA to this sequence we used the creB sequence as specific competitor in the band shift experiment but this sequence was not able to compete for CcpA binding to the creC motif. This indicates that under the conditions tested CcpA does not bind to creB. However, it cannot be ruled out that the creB site is involved in the regulation of the SLS expression in vivo as it was reported that serine phosphorylated HPr increases the affinity of CcpA to cre sites40,41.
In conclusion, we report the first investigation of the regulation of a potential virulence factor in S. anginosus and we demonstrate that CcpA directly regulates the SLS expression of S. anginosus by binding to a cre site overlapping the bacterial promoter.
Materials and Methods
Bacterial strains and growth conditions
The S. anginosus and Escherichia coli strains used in this study are summarized in Table 1. The S. anginosus type strain was incubated on Sheep blood agar plates (TSA + SB, Oxoid, Basingstoke, UK) at 37 °C and 5% CO2 atmosphere. Liquid cultures of S. anginosus were incubated in THY broth (Todd-Hewitt Broth [Oxoid] supplemented with 0.5% yeast extract [BD, Miami, USA]) and supplemented with appropriate antibiotics if necessary. S. anginosus strains carrying pAT28 derivatives were grown on THY agar plates supplemented with 120 µg ml−1 spectinomycin and S. anginosus strains containing pG+host5 vector were incubated on TSA + SB in the presence of 5 µg ml−1 erythromycin. E. coli strains were routinely incubated aerobically in LB medium at 37 °C and appropriate antibiotics were added if necessary. The E. coli DH5α strain served as cloning host for pAT28 plasmids and E. coli EC101 was used for pG+host5 vectors with 100 µg ml−1 spectinomycin and 400 µg ml−1 erythromycin respectively. E. coli BL21(DE3) containing a pET21a derivative was grown in presence of 100 µg ml−1 ampicillin.
General DNA techniques
Genomic DNA (GenEluteTM Bacterial Genomic DNA Kits, Sigma-Aldrich, St. Louis, MO) and plasmid DNA (QIAprep® Spin Miniprep Kit, Qiagen, Hilden, Germany) was isolated following standard procedures of the manufacturers. Taq polymerase (Roche, Mannheim, Germany) was used for Polymerase Chain Reactions (PCR) with an initial denaturation step of 3 min 94 °C, 30 amplification cycles of 1 min 94 °C, 30 sec. 50 °C, 1–4 min 72 °C and a final elongation of 7 min 72 °C. Oligonucleotides used in this study are summarized in Table 2.
Construction of ccpA deletion and complementation strain
The temperature sensitive pG+host5 vector was used for the creation of the S. anginosus ∆ccpA strain42. Briefly, the up- and downstream regions of the target were amplified using Primers 1/2 and 3/4. The resulting PCR products were fused in an overlap-extension PCR (OE-PCR) with Primer 1/4 and were subsequently cloned into the pG+host5 plasmid. The resulting deletion vector pG+host5-ccpA∆100nt was transformed into the S. anginosus type strain using the competence stimulating peptide CSP-1 as described previously43 and the regeneration of the transformants was performed for 3 h at 39 °C to directly obtain integration mutants. Cells were plated on TSA + SB plates supplemented with 5 µg ml−1 erythromycin and incubated at 37 °C for two days. Erythromycin resistant clones were tested via PCR for the integration of the plasmid at the desired locus. To induce loss of the chromosomal integration of pG+host5, cultures were grown for 8 h at 30 °C. Subsequently single clones were tested for erythromycin susceptibility and the generation of the desired 100 bp deletion was verified by sequencing.
The S. anginosus ∆ccpA::ccpA strain was constructed by a chromosomal integration of the ccpA gene at its native locus. A linear DNA fragment consisting of the ccpA gene and an erythromycin resistance gene flanked by the up- and downstream sequence of ccpA was constructed via OE-PCR. First, a silent point mutation was introduced into the ccpA gene to be able to distinguish the complemented strain and the type strain. Therefore two PCR products (F1: Primer 6/7, F2: Primer 8/9) were fused in an OE-PCR using Primer 6/9. The erythromycin resistance gene was amplified with Primer 10/11 and pAT18 as template and fused to the OE-PCR product with Primers 6/11. In a final OE-PCR the 500 bp downstream region of ccpA (Primer 12/13) was fused to the previously constructed DNA using Primer 6/13 generating the final ccpA complementation fragment. The linear PCR product was transformed into S. anginosus ∆ccpA using CSP-1 and the integration of the PCR product in the ccpA locus was verified by PCR and sequencing leading to the creation of S. anginosus ∆ccpA::ccpA.
Promoter reporter assay
To investigate the SLS promoter activity an EGFP reporter system was used as previously described11. Briefly, S. anginosus carrying pBSU409 derivatives were grown in THY medium with glucose supplementations of 0, 5, 12.5 and 25 mM for 16 h at 37 °C and the expression of EGFP was measured using fluorescence-activated cell sorting (FACSCalibur; Becton Dickinson Immunocytometry Systems, San Jose, CA). To determine the influence of CcpA on the SLS promoter activity the pBSU409::sagprom plasmid was transformed in S. anginosus ∆ccpA. For the characterization of potential cre sites mutated SLS promoters were constructed and cloned in front of egfp in pBSU409. The creA deletion was generated by OE-PCR. In a first step two PCR fragments were amplified using Primer 14/16 and 17/15 which were afterwards fused with Primer 14/15. The resulting SLS promoter with the creA deletion was cloned in pBSU409 leading to the formation of plasmid pBSU409::sagprom_creA_del. The same procedure was used for the creB deletion (Primer 14/18 and 19/15) and the creC mutation (Primer 14/20 and 21/15) leading to pBSU409::sagprom_creB_del and pBSU409::sagprom_creC_mut. The plasmids were transformed into the S. anginosus type strain and the SLS promoter activity was determined. The S. anginosus pBSU409 strain served as negative control44 and the S. anginosus pBSU409::cfbprom strain carrying the CAMP-factor promoter (cfb) of S. agalactiae was used as positive control45. Depicted are the mean fluorescence intensities of the different strains normalized to the values obtained for the positive control in the presence of indicated glucose concentration.
Hemolysis assay
The hemolysis assay was carried out with human erythrocytes. Peripheral blood was collected from healthy adult volunteers who gave written informed consent to donate blood for the use in the study. The ethics committee at the University of Ulm approved this procedure (Certificate No. 23/13) and the methods were carried out in accordance with the regulations. The hemolytic activity of the S. anginosus type strain, the S. anginosus ∆ccpA and the S. anginosus ∆ccpA::ccpA strain was measured as described previously with some modifications11. The cells were grown overnight in THY medium with glucose supplementations of 0, 5, 12.5 and 25 mM and treated as previously described. A serial dilution of the cells was generated and 100 µl of each dilution was incubated together with 100 µl of a 0.5% erythrocyte solution at 37 °C for 1 h in a 96-well plate with conical bottom. After centrifugation, 100 µl of the supernatant was transferred to a 96-well plate with flat bottom and the Absorption at 540 nm corresponding to free hemoglobin was measured in a plate reader. As positive control served ddH2O and PBS was used as negative control.
Expression and purification of His-tagged CcpA
The CcpA protein of S. anginosus was expressed using the pET21a system and E. coli BL21(DE3). For the generation of the expression plasmid, ccpA was amplified (Primer 22/23) and cloned into pET21a leading to the formation of pET21a-ccpA-N-His. Expression of recombinant N-terminal His-tagged CcpA was induced in mid-logarithmic growth of E. coli BL21 using 0.1 mM isopropyl-β-D-thiogalactopyranoside. Cells were harvested by centrifugation after 8 h incubation at 30 °C and bacterial pellets were stored at −20 °C. CcpA was purified under native conditions using the Protino® Ni-TED 1000 Packed Columns Kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) following the instructions of the manufacturer. After purification, the elution buffer was exchanged to TKED buffer (100 mM Tris-HCl, 150 mM KCl, 1 mM EDTA, 0.1 mM dithiothreitol)32 using Amicon® Ultra Centrifugal filters (30 K, Merck Millipore Ltd, Carrigtwohill, Ireland) and the protein concentration was determined using the Quick StartTM Bradford Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Electrophoretic mobility shift assay (EMSA)
Double stranded DNA probes were constructed by annealing sense and antisense oligonucleotide 24/25 (creC), 26/27 (creC_mut) and 28/29 (creB). Briefly, equal molar amounts of sense and antisense oligonucleotide were mixed in Annealing Buffer (10 mM Tris-HCl, 1 mM EDTA, 50 mM NaCl, pH 8.0) and annealing was performed in a PCR machine (5 min 95 °C, 70 cycles of 1 min reducing the temperature by 1 °C per cycle). Oligonucleotides were purchased from metabion international AG (Planegg, Germany) with oligonucleotide 24 (IRD700_cre_fwd) labeled with the fluorescent dye IRDye700 at the 5′ end. EMSA was performed with a constant amount of labeled oligonucleotide probe (5 pmol) and increasing amounts of CcpA (0–8 µg). Each reaction was incubated for 30 min at room temperature in EMSA binding buffer (20 mM Tris-HCl, 1.5 mM dithiothreitol, 1 mM EDTA, 75 mM NaCl, 1 µg poly(dI-dC), 5% glycerol, pH 8.0). The DNA-protein complexes were separated on native 6% polyacrylamide gels in 0.5 × TBE buffer and were analyzed using the Odyssey Clx near-infrared fluorescence imaging system (LI-COR Corporate Offices, Lincoln, NE).
Equipment and Settings
Analysis of EMSA pictures was performed using the Image Studio Version 5.2 (LI-COR Corporate Offices, Lincoln, NE). The 700 nm channel was used in the DNA Gel Setup with a Scan resolution of 42 µm and a Focus Offset of 2.0 mm.
Bioinformatical and statistical analysis
Nucleotide sequences were retrieved from the GenBank database (http://www.ncbi.nlm.nih.gov/). For statistical analysis the nonparametric Mann-Whitney U test was performed and datasets were considered significant with a p-value smaller than 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Whiley, R. A., Beighton, D., Winstanley, T. G., Fraser, H. Y. & Hardie, J. M. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J Clin Microbiol 30, 243–244 (1992).
Ruoff, K. L. Streptococcus anginosus (“Streptococcus milleri”): the unrecognized pathogen. Clin Microbiol Rev 1, 102–108 (1988).
Reissmann, S. et al. Contribution of Streptococcus anginosus to infections caused by groups C and G streptococci, southern India. Emerg Infect Dis 16, 656–663, https://doi.org/10.3201/eid1604.090448 (2010).
Kobo, O., Nikola, S., Geffen, Y. & Paul, M. The pyogenic potential of the different Streptococcus anginosus group bacterial species: retrospective cohort study. Epidemiol Infect 145, 3065–3069, https://doi.org/10.1017/S0950268817001807 (2017).
Laupland, K. B., Pasquill, K., Parfitt, E. C., Dagasso, G. & Steele, L. Streptococcus anginosus group bloodstream infections in the western interior of British Columbia, Canada. Infect Dis (Lond), 1-6, https://doi.org/10.1080/23744235.2017.1416163 (2017).
Laupland, K. B., Ross, T., Church, D. L. & Gregson, D. B. Population-based surveillance of invasive pyogenic streptococcal infection in a large Canadian region. Clin Microbiol Infect 12, 224–230, https://doi.org/10.1111/j.1469-0691.2005.01345.x (2006).
Claridge, J. E. 3rd, Attorri, S., Musher, D. M., Hebert, J. & Dunbar, S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis 32, 1511–1515, https://doi.org/10.1086/320163 (2001).
Siegman-Igra, Y., Azmon, Y. & Schwartz, D. Milleri group streptococcus–a stepchild in the viridans family. Eur J Clin Microbiol Infect Dis 31, 2453–2459, https://doi.org/10.1007/s10096-012-1589-7 (2012).
Olson, A. B. et al. Phylogenetic relationship and virulence inference of Streptococcus Anginosus Group: curated annotation and whole-genome comparative analysis support distinct species designation. BMC Genomics 14, 895, https://doi.org/10.1186/1471-2164-14-895 (2013).
Tabata, A. et al. Novel twin streptolysin S-like peptides encoded in the sag operon homologue of beta-hemolytic Streptococcus anginosus. J Bacteriol 195, 1090–1099, https://doi.org/10.1128/JB.01344-12 (2013).
Asam, D., Mauerer, S., Walheim, E. & Spellerberg, B. Identification of beta-haemolysin-encoding genes in Streptococcus anginosus. Mol Oral Microbiol 28, 302–315, https://doi.org/10.1111/omi.12026 (2013).
Datta, V. et al. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol Microbiol 56, 681–695, https://doi.org/10.1111/j.1365-2958.2005.04583.x (2005).
Molloy, E. M., Cotter, P. D., Hill, C., Mitchell, D. A. & Ross, R. P. Streptolysin S-like virulence factors: the continuing sagA. Nat Rev Microbiol 9, 670–681, https://doi.org/10.1038/nrmicro2624 (2011).
Asam, D., Mauerer, S. & Spellerberg, B. Streptolysin S of Streptococcus anginosus exhibits broad-range hemolytic activity. Med Microbiol Immunol 204, 227–237, https://doi.org/10.1007/s00430-014-0363-0 (2015).
Stulke, J. & Hillen, W. Carbon catabolite repression in bacteria. Curr Opin Microbiol 2, 195–201, https://doi.org/10.1016/S1369-5274(99)80034-4 (1999).
Gorke, B. & Stulke, J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6, 613–624, https://doi.org/10.1038/nrmicro1932 (2008).
Dahl, M. K. CcpA-independent carbon catabolite repression in Bacillus subtilis. J Mol Microbiol Biotechnol 4, 315–321 (2002).
Zeng, L. & Burne, R. A. Seryl-phosphorylated HPr regulates CcpA-independent carbon catabolite repression in conjunction with PTS permeases in Streptococcus mutans. Mol Microbiol 75, 1145–1158, https://doi.org/10.1111/j.1365-2958.2009.07029.x (2010).
Tong, H., Zeng, L. & Burne, R. A. The EIIABMan phosphotransferase system permease regulates carbohydrate catabolite repression in Streptococcus gordonii. Appl Environ Microbiol 77, 1957–1965, https://doi.org/10.1128/AEM.02385-10 (2011).
Deutscher, J., Kuster, E., Bergstedt, U., Charrier, V. & Hillen, W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol Microbiol 15, 1049–1053 (1995).
Stulke, J. & Hillen, W. Regulation of carbon catabolism in Bacillus species. Annu Rev Microbiol 54, 849–880, https://doi.org/10.1146/annurev.micro.54.1.849 (2000).
Zomer, A. L., Buist, G., Larsen, R., Kok, J. & Kuipers, O. P. Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189, 1366–1381, https://doi.org/10.1128/JB.01013-06 (2007).
Willenborg, J., de Greeff, A., Jarek, M., Valentin-Weigand, P. & Goethe, R. The CcpA regulon of Streptococcus suis reveals novel insights into the regulation of the streptococcal central carbon metabolism by binding of CcpA to two distinct binding motifs. Mol Microbiol 92, 61–83, https://doi.org/10.1111/mmi.12537 (2014).
Kinkel, T. L. & McIver, K. S. CcpA-mediated repression of streptolysin S expression and virulence in the group A streptococcus. Infect Immun 76, 3451–3463, https://doi.org/10.1128/IAI.00343-08 (2008).
Moreno, M. S., Schneider, B. L., Maile, R. R., Weyler, W. & Saier, M. H. Jr. Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol Microbiol 39, 1366–1381 (2001).
Yoshida, K. et al. Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res 29, 683–692 (2001).
Titgemeyer, F. & Hillen, W. Global control of sugar metabolism: a gram-positive solution. Antonie Van Leeuwenhoek 82, 59–71 (2002).
Iyer, R., Baliga, N. S. & Camilli, A. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J Bacteriol 187, 8340–8349, https://doi.org/10.1128/JB.187.24.8340-8349.2005 (2005).
Shelburne, S. A. 3rd et al. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci USA 105, 1698–1703, https://doi.org/10.1073/pnas.0711767105 (2008).
Abranches, J. et al. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J Bacteriol 190, 2340–2349, https://doi.org/10.1128/JB.01237-07 (2008).
Varga, J., Stirewalt, V. L. & Melville, S. B. The CcpA protein is necessary for efficient sporulation and enterotoxin gene (cpe) regulation in Clostridium perfringens. J Bacteriol 186, 5221–5229, https://doi.org/10.1128/JB.186.16.5221-5229.2004 (2004).
Almengor, A. C., Kinkel, T. L., Day, S. J. & McIver, K. S. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the Group A streptococcus. J Bacteriol 189, 8405–8416, https://doi.org/10.1128/JB.01038-07 (2007).
Tomoyasu, T. et al. Role of catabolite control protein A in the regulation of intermedilysin production by Streptococcus intermedius. Infect Immun 78, 4012–4021, https://doi.org/10.1128/IAI.00113-10 (2010).
Seidl, K. et al. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob Agents Chemother 50, 1183–1194, https://doi.org/10.1128/AAC.50.4.1183-1194.2006 (2006).
Li, C. et al. CcpA mediates proline auxotrophy and is required for Staphylococcus aureus pathogenesis. J Bacteriol 192, 3883–3892, https://doi.org/10.1128/JB.00237-10 (2010).
Tang, Y. et al. Catabolite control protein A of Streptococcus suis type 2 contributes to sugar metabolism and virulence. J Microbiol 50, 994–1002, https://doi.org/10.1007/s12275-012-2035-3 (2012).
Willenborg, J. et al. Role of glucose and CcpA in capsule expression and virulence of Streptococcus suis. Microbiology 157, 1823–1833, https://doi.org/10.1099/mic.0.046417-0 (2011).
Asam, D. & Spellerberg, B. Molecular pathogenicity of Streptococcus anginosus. Mol Oral Microbiol 29, 145–155, https://doi.org/10.1111/omi.12056 (2014).
Marciniak, B. C. et al. High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis. BMC Genomics 13, 401, https://doi.org/10.1186/1471-2164-13-401 (2012).
Gosseringer, R., Kuster, E., Galinier, A., Deutscher, J. & Hillen, W. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J Mol Biol 266, 665–676, https://doi.org/10.1006/jmbi.1996.0820 (1997).
Schumacher, M. A. et al. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell 118, 731–741, https://doi.org/10.1016/j.cell.2004.08.027 (2004).
Biswas, I., Gruss, A., Ehrlich, S. D. & Maguin, E. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol 175, 3628–3635 (1993).
Bauer, R., Mauerer, S., Grempels, A. & Spellerberg, B. The competence system of Streptococcus anginosus and its use for genetic engineering. Mol Oral Microbiol, https://doi.org/10.1111/omi.12213 (2017).
Gleich-Theurer, U. et al. Human serum induces streptococcal c5a peptidase expression. Infect Immun 77, 3817–3825, https://doi.org/10.1128/IAI.00826-08 (2009).
Aymanns, S., Mauerer, S., van Zandbergen, G., Wolz, C. & Spellerberg, B. High-level fluorescence labeling of gram-positive pathogens. PLoS One 6, e19822, https://doi.org/10.1371/journal.pone.0019822 (2011).
Law, J. et al. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol 177, 7011–7018 (1995).
Trieu-Cuot, P., Carlier, C., Poyart-Salmeron, C. & Courvalin, P. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene 102, 99–104 (1991).
Trieu-Cuot, P., Carlier, C., Poyart-Salmeron, C. & Courvalin, P. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and in gram-positive bacteria. Nucleic Acids Res 18, 4296 (1990).
Acknowledgements
The work of R.B. and B.S. were supported by the German Research Foundation DFG under grant GSC270.
Author information
Authors and Affiliations
Contributions
R.B. and B.S. conceived the experiments, R.B. and S.M. conducted the experiments, R.B. and B.S. analyzed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bauer, R., Mauerer, S. & Spellerberg, B. Regulation of the β-hemolysin gene cluster of Streptococcus anginosus by CcpA. Sci Rep 8, 9028 (2018). https://doi.org/10.1038/s41598-018-27334-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27334-z
- Springer Nature Limited