Abstract
The association between mucosal microbiota and HIV-1 infection has garnered great attention in the field of HIV-1 research. Previously, we reported a receptor-independent HIV-1 entry into epithelial cells mediated by a Gram-negative invasive bacterium, Porphyromonas gingivalis. Here, we present evidence showing that P. gingivalis outer membrane vesicles (OMVs) promote mucosal transmission of HIV-1. We demonstrated, using the Dynabeads technology, a specific interaction between HIV-1 and P. gingivalis OMVs which led to an OMV-dependent viral entry into oral epithelial cells. HIV-1 was detected in human oral keratinocytes (HOKs) after a 20 minute exposure to the HIV-vesicle complexes. After entry, most of the complexes appeared to dissociate, HIV-1 was reverse-transcribed, and viral DNA was integrated into the genome of HOKs. Meanwhile, some of the complexes exited the original host and re-entered neighboring HOKs and permissive cells of HIV-1. Moreover, P. gingivalis vesicles enhanced HIV-1 infection of MT4 cells at low infecting doses that are not able to establish an efficient infection alone. These findings suggest that invasive bacteria and their OMVs with ability to interact with HIV-1 may serve as a vehicle to translocate HIV through the mucosa, establish mucosal transmission of HIV-1, and enhance HIV-1 infectivity.
Similar content being viewed by others
Introduction
The human immunodeficiency virus (HIV)/AIDS pandemic continues to be a major global health problem, as millions of people worldwide are currently living with HIV/AIDS1. The majority of new HIV infections originate at the genital and rectal mucosa. The mechanism of HIV transmission through the mucosal epithelium remains unclear. Generally, epithelial surfaces present the first line of defense against pathogens such as bacteria and viruses. Nevertheless, HIV-1 can penetrate a multi-layer epithelium in the absence of any apparent breach or lesion2,3. Several mechanisms have been proposed to explain how HIV-1 gains access to permissive cells across mucosal surfaces4. First, HIV-1 may cross the epithelial layer by moving through intercellular compartments. Next, mucosal dendritic cells may facilitate HIV entry through the epithelium by capturing cell-free- or cell-associated HIV-1 using their extending dendrites within the epithelial layers. Finally, epithelial cells may take-up HIV-1 particles and transfer them to target cells such as dendritic cells, T-cells, and macrophages by a process known as transcytosis5.
Recently, the role of the microbiome in HIV transmission of mucosa has drawn great attention from HIV researchers. For example, Mirmonsef and Spear showed that lower levels of Lactobacillus in the genital tract are associated with an increased risk of acquiring HIV-1 and female-to-male transmission6. Another example is that Porphyromonas gingivalis, an invasive bacterium usually found in the oral cavity may play a role in HIV-1 transmission. A series of studies by Herzberg et al. revealed an up-regulated expression of CCR5 in oral epithelial cells induced by P. gingivalis LPS and gingipains. Consequently, P. gingivalis could selectively promote R5-tropic HIV-1 infection of oral keratinocytes and viral dissemination7,8,9. Previously, we reported a specific interaction between HIV-1 and P. gingivalis that is mediated by the binding domain (HGP44) of bacterial gingipains and the viral envelope glycoprotein, gp12010. We further demonstrated that invasive P. gingivalis strains promoted both R5-tropic and X4-tropic HIV-1 entry into epithelial cells by trapping the viruses on their surfaces and carrying the virions into the cells11.
Most Gram-negative bacteria secrete outer membrane vesicles (OMVs) as either surface-bound or cell-free spherical structures. OMVs have recently gained recognition for their role in bacterial virulence and are known to play similar roles as their parent cells in mediating adherence, biofilm formation, invasion, host cell damage, and modulation of host immune responses12. We previously reported that adhesive molecules such as gingipains and FimA were enriched in P. gingivalis OMVs and that the OMVs were much more invasive than their parent cells13,14. In the study presented here, we built upon our previous work on the receptor-independent HIV-1 entry mediated by P. gingivalis cells11. We further examined the ability of P. gingivalis vesicles to promote HIV-1 entry into and spreading among human oral keratinocytes (HOKs) as well as HIV-1 permissive cells. These findings provide insights into the potential mechanisms of mucosal HIV-1 transmission.
Results
The interaction of HIV-1 and P. gingivalis vesicles
We previously reported that HIV-1 binds specifically to the surface of P. gingivalis through the interaction between the binding domains (HGP17 and HGP44) of gingipains (an important class of P. gingivalis extracellular outer membrane-associated proteases) and HIV-1 gp12010. Therefore, we speculated that P. gingivalis may serve as a vehicle for carrying HIV-1 into cells that are non-permissive to HIV, such as oral epithelial cells. Recently, we demonstrated that HGP44 was enriched in P. gingivalis OMVs that were much more invasive than their parent cells13,14. We tested if P. gingivalis OMVs also specifically interact with HIV-1. Binding experiments were performed using magnetic beads coupled with P. gingivalis vesicles. Either X4 or R5 tropic HIV-1 (NL4.3 or YU2) were incubated with the beads and visualized with immunostaining and confocal microscopy. Binding of HIV-1 to beads was found on those coupled with vesicles derived from P. gingivalis strains 33277 (the fimA I strain), FAE (the fimA mutant), and W83 (the fimA IV strain) (Fig. 1), all of which express HGP44. We also observed that the HIV-bound beads tended to cluster together when HIV bound to the beads, possibly due to HIV bridging. Conversely, we did not detect the binding of NL4.3 and YU2 virions on beads coupled with vesicles from P. gingivalis KDP128, a triple mutant that does not express gingipains15, or on those coupled with E. coli DH5α vesicles. Moreover, VSV-G pseudotyped HIV lacking HIV-1 envelope proteins did not bind to beads coupled with 33277 vesicles. These results demonstrate a specific interaction between HIV-1 and P. gingivalis vesicles.
Specific binding of HIV-1 to P. gingivalis 33277 vesicles. Vesicles derived from P. gingivalis 33277 (wild type strain expressing FimA I protein), FAE (the fimA mutant), W83 (a type strain lacking FimA), KDP128 (a gingipain triple mutant) and E. coli DH5α were coupled with Dynabeads. The beads were then incubated with HIVNL4.3 or HIVYU2. Vesicles derived from 33277 were also exposed to VSV pseudotyped HIV (VSV-HIV). Images were acquired using a confocal microscope. Bacterial vesicles (red) were stained with polyclonal antibodies specific for P. gingivalis or E.coli, and Alex Fluor 546-conjugated secondary antibodies. HIV-1 virions (NL4.3 and YU2, green) were stained with anti-p24 monoclonal antibody and Alexa Fluor 488-conjugated anti-mouse IgG.
P. gingivalis vesicle-dependent HIV-1 entry into non-permissive cells
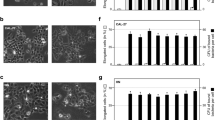
To test if P. gingivalis vesicles can carry HIV-1 into non-permissive cells such as HOKs, we co-incubated the host cells with either HIVNL4.3 alone, HIV/P. gingivalis 33277 vesicle complexes, or HIV/KDP128 vesicle complexes for 30 minutes. Cell-free complexes were removed by washing the cells with PBS, and the cells were then fixed, permeabilized, immunostained, and analyzed using confocal microscopy. As shown in Fig. 2a, intracellular vesicles were mainly observed in the perinuclear area, which is in agreement with the previous report16. HIVNL4.3 (green) was internalized only in those cells that also carried intracellular P. gingivalis 33277 vesicles (red). Some HIV-1 particles still co-localized with P. gingivalis vesicles (yellow) after a 30 min exposure, while others were independent from 33277 vesicles, suggesting that at least some HIV-1 had dissociated from the bacterial vesicles after entering the host cells. In contrast, internalization of vesicle-free HIV-1 was not observed in HOKs exposed to HIV-1 alone (data not shown). We also did not find vesicle-free and vesicle-associated HIV-1 particles in HOKs exposed to the vesicles derived from P. gingivalis KDP128 (a gingipain negative mutant)/HIV-1 complexes. The vesicles of the gingipain mutant exhibited a decreased ability to invade HOKs, which is in agreement with the previous report on the role of gingipains in P. gingivalis invasion17. As expected, the vesicles of KDP128 could not promote HIV internalization. To qualify the invasion rates, we counted HOKs with internalized vesicles or HIV in six random areas (1.34 × 1.34 mm) (Fig. 2b). We found that 59.2% of the HOKs contained the internalized vesicles and 25% of HOKs with HIV-1 after exposed to 33277 vesicle/HIV complexes. The invasion of KDP128 vesicles was much lower (4% of HOKs, P < 0.001) than that of 33277 vesicles, and few HIV-1 particle was found in the HOKs exposed to KDP128 vesicle/HIV complexes, indicating that the observed HIV-1 entry into HOKs depends on HIV and vesicle interaction as well as invasive activity of the vesicles. To further confirm HIV-1 entry of HOKs, we performed live-cell imaging using fluorescently labeled HIV-1 and P. gingivalis 33277 vesicles. We observed a rapid entry of HIV-1/vesicle complexes into the HOKs. Within 10 min of incubation with HOKs, the complexes entered the cells and reached the perinuclear region (Fig. 3, Video S1). The majority of these complexes dissociated 40 min after entry, although some HIV-vesicle complexes remained in the cells 1 h post-entry. Previously, we demonstrated that Dynasore, an inhibitor of clathrin-dependent endocytosis, blocked P. gingivalis vesicle entry into HOKs18. As expected, we did not find HIV-vesicle complexes in the cytoplasm of HOKs in the presence of Dynasore (35 µM) (Video S2). These findings reveal an endocytosis-dependent HIV-1 entry of non-permissive cells, which is mediated by P. gingivalis vesicles.
Effects of P. gingivalis vesicles on HIV-1 internalization of HOKs. HOKs were exposed to HIV-vesicle complexes for 30 min. (a) The pper panel was images of HOK exposed to HIV/P. gingivalis 33277 vesicle complexes, and the lower panel was HOK treated with HIV/the gingipain mutant KDP128 vesicle complexes. HIVNL4.3 (green) and P. gingivalis vesicles (red) were visualized in HOKs using confocal microscopy. HOKs were shown by differential interference contrast images (overlay) to show cell boundary and morphology. Scale bar represents 10 μm. (b) The numbers of HOKs with intercellular P. gingivalis vesicles were determined by counting ten random areas. Each bar represents the percentage of HOKs with intercellular vesicles or HIV-1. An asterisk indicates the statistical significance of invasive rates between 33277 vesicles and KDB128 vesicles (P < 0.05; t test).
Outcomes of co-entry of HIV and OMVs into HOKs
As shown in Fig. 3, the internalized HIV-1 could either remain associated with P. gingivalis vesicles, or free itself from the vesicles. We speculated that HIV-vesicle complexes spread from the initial host epithelial cells to neighboring cells, while vesicle-free viruses may be uncoated and have their RNA reverse-transcribed. We first determined if we could detect viral DNA in HOKs exposed to either HIVNL4.3 alone or HIV-P. gingivalis 33277 vesicle complexes for 0, 1, 4, or 24 h, respectively. Reverse-transcribed viral DNA was identified using qPCR with HIV gag and ltr specific primers. There was little viral DNA detected in HOKs exposed to HIV-1 alone even after a 24 h exposure. On the other hand, reverse-transcribed viral DNAs were detected in the HOKs exposed to the HIV/vesicle complexes after only 1 h of exposure, and a significant increase in viral DNA was found in the HOKs exposed to the complexes for 4 and 24 h (Fig. 4a,b). Approximately 30 times more viral DNA was found in HOKs treated with HIV/vesicles complexes for 1 h (30 ± 8.18, P = with gag primers; 27 ± 11.1, P = 0.041, with ltr primers) when compared to that detected in HOKs exposed to HIV alone. At least 40 times (43 ± 11.6, P = 0.025 with gag primers; 42 ± 12.3, P = 0.002 with ltr primers) and 75 times (77 ± 6.63, P = 0.0018 with gag primers; 87 ± 7.63, P = 0.002 with ltr primers) more viral DNA were detected in HOKs after 4 or 24 h exposure. Treatment of HOKs with zidovudine (AZT, 15 µM), a reverse-transcriptase inhibitor, efficiently blocked HIVNL4.3 reverse-transcription in HOKs, and the viral DNA was not detected in the HOKs exposed to the complexes in the presence of AZT.
Detection of HIV reverse-transcription in HOKs. DNA levels of HIVNL4.3 gag (a) and ltr (b) in HOKs were measured using qPCR during a 24-hr period after the initial infection. Each bar represents the relative fold increase in HIVNL4.3 DNA level detected in HOKs exposed to HIV-vesicle complexes compared to that in unexposed cells (designated as 1 unit). Error bars represent SD (n = 3). Asterisks indicate statistically significant differences between HIVNL4.3 DNA levels in exposed and unexposed host cells (P < 0.05; t test).
We further tested if the reverse-transcribed viral DNA was integrated into the genome of HOKs. Alu-LTR real-time nested PCR assays were perform using genomic DNAs isolated from HOKs treated with either HIVNL4.3 or HIV/vesicle complexes, in the presence or absence of HIV integrase inhibitor raltegravir (MK-0518, 10 µM). Integrated provirus was detected in the HOKs as early as 1 h after the cells were exposed to the HIV/vesicle complexes, its level increased by two-fold after 24 h (Fig. 5). Moreover, raltegravir treatment significantly blocked HIV integration, and provirus was also not found in the HOKs treated with AZT, apparently due to its inhibitory activity of reverse-transcriptase.
HIV-1 proviral DNA integration into the genomic DNA of HOKs. HOKs were incubated with either HIVNL4.3 alone or HIV/vesicle complexes for 1, 4, or 24 h, DNA were extracted, and levels of HIV provirus was determined using the Alu- LTR-based RT-PCR assay. As controls, HOKs also treated with either AZT or raltegravir. Data are represented as mean values, with error bars indicating SD of triplicate measurements. Asterisks indicate statistically significant differences between proviral DNA levels in HOKs exposed to HIV/vesicle complexes for 1, 4 and 24 h (P < 0.05; t test).
To examine if internalized HIV-vesicle complexes are able to exit their original host cells and enter neighboring HOKs, we first treated HOKs with P. gingivalis 33277 vesicles alone, HIVNL4.3 alone, or HIV-vesicle complexes for 1 h. Cell-free HIV or HIV-vesicle complexes were removed, and the initially treated HOKs (5 × 104 cells) were mixed with a new set of uninfected HOKs (5 × 104) that were labeled with CellTracker. The mixed cells were analyzed by confocal microscopy. As shown in Fig. 6a–e, after P. gingivalis vesicles entered the initially treated HOKs, some of the vesicles exited and re-entered to neighboring cells. Apparently, HIV-vesicle complexes were also observed in the CellTracker labeled HOKs after being mixed with HOKs initially treated with HIV-vesicle complexes, suggesting a vesicle-dependent spreading of HIV among HOKs. To exclude the possibility that the HIV and vesicles detected in the CellTracker labeled HOKs is due to extracellular HIV-1 and vesicles that were not removed after washings, we pre-treated the initially exposed HOKs with dynasore (30 µM). Previously, we showed that Dynasore efficiently prevented P. gingivalis cells and their OMVs from entering into HOKs18. As expected, HIV-vesicle complexes were not detected in the initial HOKs in the presence of Dynasore. As the result, the complexes were also not observed in the CellTracker labeled HOKs. These results demonstrate a potentially important role of P. gingivalis vesicles in facilitating mucosal transmission of HIV.
Effect of P. gingivalis vesicles on HIVNL4.3 spreading among HOKs. HOKs (unstained) were initially exposed to HIVNL4.3 alone, P. gingivalis 33277 vesicles alone, or HIV- vesicle complexes. These pretreated cells were then mixed and cultured with secondary HOKs that had not been exposed to the virus or vesicles (blue) for 24 h. Images were obtained by immunofluorescence confocal microscopy. (a) HOKs without exposure to neither HIV nor vesicles. (b) HOKs that were exposed to P. gingivalis vesicles (red) alone. (c) HOKs that were exposed to HIVNL4.3 alone. (d) HOKs that were initially exposed to HIV/vesicle complexes in the absence of Dynasore. (e) HOKs were pre-treated with Dynasore, and exposed to HIV-vesicle complexes. Scale bar, 20 μm.
We next tested if the P. gingivalis vesicle-mediated internalization of HIVNL4.3 in HOKs can be transferred to permissive cells expressing HIV receptors. A TZA assay, a reporter cell-based assay for quantifying HIV-1 infection19, was performed to determine the role of P. gingivalis vesicles in HIV translocation. We first seeded HOKs in a 96-well plate, then added HIVNL4.3 alone or HIV-P. gingivalis 33277 vesicle complexes. After 1 h incubation, extracellular HIV bacterial vesicles were removed, and the HOKs were co-cultured with TZM-bl cells for 48 h. β-gal activity was measured using a TZA assay. Some enhancement of β-gal activity was found in the wells with HOKs exposed to HIV-1 alone, which may be due to incomplete removal of extracellular HIV-1. Interestingly, a 4.5-fold higher of β-gal activity was observed in the wells with HOKs exposed to HIV-vesicle complexes compared to that in the wells with cells exposed to HIV alone, after β-gal levels were normalized with the background found in the wells with untreated cells (Table 1). HIV infectivity was significantly blocked when HOKs were treated with dynasore (30 µM), which was consistent with our data on HIV-vesicle spreading among HOKs. These data indicate that a P. gingivalis vesicle-induced and endocytosis-dependent pathway is involved in this HIV-1 transmission between epithelial cells and HIV-1 permissive cells.
To test if P. gingivalis vesicles can also enhance HIV-1infectivity of permissive cells, HIVNL4.3-SecNluc-Sec infection was performed in the presence or absence of vesicles derived from P. gingivalis 33277. HIVNL4.3-Nluc-Sec NL4.3-SecNluc is a reporter virus with the secretory nanoluc gene inserted into the nef gene of the HIVNL4.3 genome 22. MT4 cells (human CD4 + T lymphoblastoma cells) were infected with a series of low infecting doses of NL4-3-SecNluc ranging from 1/8 to 2 TCID50. As expected, the virus infected MT4 cells at 2 TCID50 with or without the vesicles (Fig. 7). In the absence of vesicles, we did not observe NL4-3-SecNluc replication in MT4 cells infected with lower multiplicities of infection (1/2 or 1/8 TCID50/well of the virus) under the experimental conditions (Fig. 7). In contrast, viruses at the same low infecting doses successfully infected MT4 cells in the presence of the vesicles. These results indicate that P. gingivalis vesicles enhanced the infectivity of NL4-3-SecNluc by at least 10-fold. This enhancement was likely due to the ability of P. gingivalis vesicles to carry the virions into MT4 cells.
P. gingivalis vesicles enhanced HIV-1 infection of MT4 cells. MT4 cells (1 × 105 cells) were seeded in 96-well plates and infected with NL4-3-SecNluc at TCID50 = 2, 1/2, and 1/8 per well in the presence (dashed lines) or absence (solid lines) of P. gingivalis vesicles (V) at 1/2 µg/ml. After infection, the culture supernatants were collected every day for four days. The luciferase activity (expressed as RFU × 1,000), used as a surrogate for virus replication, was measured with a PerkinElmer Victor II luminometer. Asterisks indicate significant differences between the infectivity levels detected in MT4 cells infected with HIV alone and HIV-vesicle complexes, respectively (P < 0.05, t test).
Discussion
The association of HIV infection and mucosal microbiome, especially at the intestinal and genital surfaces, has been intensely investigated in recent years20. Studies have shown that decreased levels of Lactobacillus, associated with vaginosis, correlates with a higher susceptibility to HIV infection as well as with a decreased effectiveness of HIV treatment in women in the KwaZulu-Natal province of South Africa21,22. A mounting body of evidence suggests that the relationship between the microbiome and HIV infection may be a mutually-beneficial one for the pathogens23. Moreover, the microbiome, including microbes and their products, can induce local and systemic inflammation and immune activation. The persistent immune activation appears to be associated with enhanced morbidity and mortality of HIV/AIDS patients20. On the other hand, HIV-1 infection may lead to alterations in the composition of the gut microbiota, such as a decrease in Lactobacillus levels, which may cause structural damage to the gastrointestinal tract24. Previously, we reported a novel mechanism of HIV entry into non-permissive cells, in which P. gingivalis, an invasive oral bacterium, interacts specifically with HIV-1 and promotes a CD4-independent HIV entry into epithelial cells. Here, we further demonstrated that OMVs derived from P. gingivalis also play an important role in HIV mucosal infection and transmission. These data may explain how HIV-1 breaches the epithelial barrier and reaches HIV permissive cells, such as T-cells and dendritic cells. Based on our findings, we propose that invasive microbes and their outer membrane vesicles capable of specifically interacting with HIV-1 may serve as a “vehicle/carrier” that translocates HIV across the mucosal epithelia. Findings from previous studies suggest that intracellular P. gingivalis exits the gingival epithelial cells via a recycling pathway, and that knockdown of Rab11, RalA and exocyst complex subunits significantly prevents P. gingivalis from exiting the infected cells25,26. Therefore, it is possible that the translocation of HIV-vesicle complexes also exploits a similar mechanism.
Based on our previous observations that P. gingivalis is able to enter, exit and reenter HOKs18, and that P. gingivalis vesicles can translocate through monolayered HOKs13, we hypothesized that P. gingivalis vesicles are capable of carrying HIV-1 from cells to cells and spreading among HOKs. The current study provides solid evidence to support P. gingivalis vesicles-mediated HIV-1 transcytosis. Several studies have reported cell-free HIV transcytosis through intestinal, vaginal, genital, and oral epithelial cells, although the mechanism has not been elucidated27,28,29. A recent report showed that HIV-1 entered oral and genital epithelial cells and became sequestered in the endosomes for as long as 9 days30. The authors of this study also revealed that the release of the sequestered HIV-1 from endosomes was induced by an increase in the intracellular calcium level and the disruption of cortical actin in the epithelial cells. Here, we discovered a rapid HIV entry into and translocation among HOKs, which is mediated the bacterial outer membrane vesicles. Intracellular HIV-vesicle complexes were observed within 20 minutes after initial exposing the HOKs to the HIV-vesicle complexes. Translocation of HIV-1 among HOKs was found within 24 h of exposure in the presence of P. ginigvalis vesicles, and spreading HIV-1 from HOKs to CD4+ cells within 48 h. These data present a novel mechanism of efficient HIV-1 entry into and spread among non-permissive cells. Moreover, we also showed that some of intracellular HIV-1 could be reverse-transcribed and integrated into the genome of HOKs within 1 h. It is likely that the reverse-transcription occurred when HIV-1 had freed from P. gingivalis vesicles, although the mechanism(s) of HIV-1 dissociating from P. gingivalis vesicles and releasing to cytoplasm of HOKs is currently not known. A number of intracellular pathogens such as Listeria, Rickettsia, Shigella, and Burkholderia are internalized in a vacuole31,32. The bacteria rapidly escape from the vacuole after invasion, and free bacteria in the cytosol can be detected within 30 minutes after invasion. The mechanism of Listeria monocytogenes escape involves in the bacterial enzyme listeriolysin O that inserts into the vacuolar membrane by binding cholesterol, which leads to pore formation and membrane interruption33,34. It is found that vacuole acidification is a trigger for bacterial escape from the vacuole, as listeriolysin O is optimally active only in an acidic environment (pH 5.5)35. Conditions requiring for escape of HIV-1 and/or HIV/vesicle complexes from endosomes are now under investigation in our laboratories. In general, HIV-1 entry into the permissive cells occurs 1–3 h after infection. Reverse transcription initiates over the 6 to 48 hours, and viral genome integration takes place a few hours after reverse transcription36,37. Compared to conventional routes of HIV-1 infection, the vesicle-mediated viral entry, reverse transcription, and integration appear to occur at a much faster pace. We previously reported that P. gingivalis vesicles enter host cells in less than 15 minutes13, which may contribute to more rapid reverse-transcription and integration observed in the HOKs. However, the mechanism(s) responsible for the fast kinetic of viral life cycle mediated by the vesicle remains to be determined.
In addition to their ability to promote receptor-independent HIV-1 entry into non-permissive cells, P. gingivalis vesicles also enhanced HIV infection of T-cells at low doses that otherwise could not initiate a productive infection in the absence of the vesicles. Presumably, by binding to P. gingivalis vesicles, HIV-1 particles, even at non-infectious doses, can now enter permissive cells using the vesicles as a vehicle. It was previously estimated that only 0.01–1% of HIV-1 particles in a culture sample are infectious38,39,40,41,42,43,44,45. In other words, the majority of virions cannot infect HIV-1 susceptible cells due to defects in their viral proteins or genes. Some of the non-infectious viral particles may have aged or are abnormally assembled HIV-1 Env trimers that are compromised in viral entry. A specific interaction between P. gingivalis vesicles and HIV-1 may enable such non-infectious virions to enter CD4+ cells through an endocytic pathway46. Once inside the cells, HIV-1 with non-lethal mutations can start a new life cycle and produce infectious progenies. Therefore, our findings suggest that P. gingivalis vesicles not only promote HIV translocation from mucosal surfaces to the subcutaneous tissues, but also enhance HIV infection of permissive cells.
The mechanism by which OMVs facilitate HIV entry into human oral epithelial cells may also be relevant to HIV transmission across the genital, rectal, and vaginal mucosal epithelia. This is because a wealth of microbial flora is also present in these areas, including Gram-negative invasive bacteria such as the Prevotella and Porphyromonas species. Hence, manipulating the mucosal microbiome and disrupting bacterial invasion may have an impact on preventing mucosal transmission of HIV-1.
Methods
Bacterial strains and vesicle preparation and quantification
P. gingivalis 33277 was grown from frozen stocks in trypticase soy broth (TSB) or on TSB blood agar plates supplemented with yeast extract (1 mg/mL), hemin (5 μg/mL) and menadione (1 μg/mL), and incubated at 37 °C in an anaerobic chamber (85% N2, 10% H2, 5% CO2). P. gingivalis vesicles were prepared as previously described46. Briefly, P. gingivalis was grown to the late exponential phase, and growth media were collected by centrifugation at 10,000 × g for 15 minutes at 4 °C and filtered through 0.22-μm-pore-size filters (Cell Treat, MA) to remove residual bacteria. Vesicles were collected by ultracentrifugation at 126,000 × g for 2 h at 4 °C and resuspended in phosphate-buffered saline (PBS) containing 10% glycerol. OMVs were quantitated using both protein and lipid assays. Proteins and lipids were extracted from vesicles in PBS using a BugBuster® Protein Extraction Reagent (Novagen, MA).
Cell culture and HIV production
Human oral keratinocytes (HOKs) were obtained from ScienCell Research Laboratories (Carlsbad, CA) and cultured in the oral keratinocyte medium recommended by ScienCell. TZM-bl cells were obtained from the NIH AIDS Reagent Program and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 µg/mL) at 37 °C in 5% CO2. X4-tropic HIV-1NL4–3 and R5-tropic HIV-1YU2 were produced by transfecting 293 T cells with pNL4-3 or pYU2 (AIDS Research and Reference Reagent Program, NIAID) using the FuGENE 6 transfection reagent (Promega, Madison, WI). The viral supernatants were collected at 48 h post-transfection, and stored in aliquots at −80 °C. The viral stocks were quantified by measuring p24 antigens using an ELISA kit (PerkinElmer Life Sciences, MA)10.
Examination of HIV-1 and P. gingivalis vesicle interactions
P. gingivalis vesicles were coupled with Dynabeads (Life Technologies, CA) according to the manufacturer’s instructions. Briefly, 20 µl of Dynabeads (8 × 106) were washed with 1 ml of Buffer 1 (0.1 M sodium phosphate buffer, pH 7.4–8.0) and resuspended in 500 µl of Buffer 1. The Dynabeads were then coupled with 500 ng of the vesicles for 30 minutes. Bovine serum albumin (BSA, 1% w/v) was added to block the free epoxy groups on the beads. The beads were then washed three times with 1 ml of Buffer-2 (PBS supplemented with 0.1% BSA and 2 mM EDTA, pH 7.4) and resuspended in 500 µl of Buffer-2. HIV-1 (NL 4.3 or YU2, 10 ng of p24) was then incubated with bead for 1 h. The beads were then washed three times with PBST (PBS supplemented with 0.02% Tween-20) to remove unbound viruses. The beads were then fixed with 3.8% formaldehyde, permeabilized with 0.1% Triton-X 100, and blocked with 3% BSA in PBS for 1 h. The beads were then stained with a mouse anti-p24 monoclonal antibody (NIH AIDS Reagent Program) and pan-specific rabbit anti P. gingivalis, followed by Alexa Fluor 488-conjugated chicken anti-mouse IgG and Alexa Fluor 546-conjugated donkey anti-rabbit IgG (Life Technologies). The beads were mounted on a glass slide with ProLong® Diamond Antifade Mountant (Thermo Fisher Scientific, MA). Confocal images were acquired using a Nikon A1R confocal microscope.
P. gingivalis vesicles mediated HIV-1 entry
P. gingivalis vesicles were incubated with HIVNL4.3 for 15 minutes to facilitate the formation of HIV-vesicles complexes. HOKs (4 × 105 cells) were grown overnight on glass-bottom microwell dishes (MatTek, MA) and were exposed to P. gingivalis 33277 vesicles, HIVNL4.3 or HIV (10 ng of p24)/vesicle (50 ng) complexes for 0.5 or 1 h. The infected cells were fixed with 3.8% formaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 5% bovine serum albumin. P. gingivalis vesicles were stained with rabbit polyclonal FimA antisera followed by goat anti-rabbit Alexa Flour 546-conjugated antibodies (Life Technologies). Confocal images were acquired using a Nikon A1R confocal microscope.
Live-cell imaging of HIV entry was performed using an A1R confocal microscope equipped with a heated cell chamber supplemented with 5% CO2. Briefly, P. gingivalis 33277 vesicles were labeled using the Alexa Fluor 594 Microscale Protein Labeling Kit (Thermo Fisher Scientific). The labeled vesicles were collected and washed twice with PBS by centrifugation at 28,000 rpm for 2 h. The labeled vesicles were then incubated with green fluorescent protein (GFP)-tagged HIV (Vpr-GFP-tagged NL4.3, 10 ng of p24) for 15 minutes. HOKs were first cultured on 35 mm glass bottom dishes for 16 h and then exposed to HIVNL4.3 alone or HIV/vesicle complexes and mounted on the 37 °C microscope stage. Images were collected using a 40×/1.3-numerical aperture (NA) oil platform objective at 10-sec intervals using Nikon Advanced Research Imaging Software (Nikon Instruments).
Quantitative real-time PCR (qPCR)
DNA was extracted using an Easy DNATM Kit (Life Technologies). Real-time qRT-PCR was conducted using a QuantiTect SYBR Green RT-PCR kit (Qiagen, Germany) on an iCycler MyiQTM Real-Time PCR detection system (Bio-Rad Laboratories, Inc, CA) according to manufacturer’s instructions. Viral DNA was quantified with HIV gag- and ltr- specific primers (Table S1)11. Human glyceraldehyde 3-phosphate dehydrogenase gene (gapdh) was used as a normalizing gene. The melting curve profile was analyzed to verify a single peak for each sample. The levels of gag and ltr genes for the test sample were determined relative to the untreated calibrator sample by using the comparative cycle threshold (ΔΔC T ) method.
Alu-LTR real-time nested PCR assay
Integration of viral DNA into the genomic DNA of HOKs was determined using an Alu-long terminal repeat (LTR)-based real-time nested-PCR assay47. Two human genomic Alu forward primers (Alu1 and Alu2) and an HIV-1 LTR reverse primer extended with a lambda phage-specific heel sequence at the 5′ end of the oligonucleotide (M667–L) were used to enrich host genomic DNA with downstream proviral DNA using PCR. The integrated HIV-1 provirus was then quantified using an iQ Supermix kit (Bio-Rad) with a reaction solution containing a lambda-specific primer (Lambda T) and an ltr primer (AA55M) (Table S1), and the PCR products from the first-round of PCR. Amplification of human gapdh gene was used for normalizing levels of HIV-1 provirus.
Examination of HIV spreading among HOKs
HOKs were exposed to either HIVNL4.3 alone or HIV/vesicle complexes for 30 minutes. The exposed HOKs were collected by trypsinization, washed two times with PBS, and mixed with a new set of uninfected HOKs labeled with CellTracker Blue CMAC (Invitrogen). The mixed HOKs were seeded in glass-bottom microwell dishes and cultured for 24 h. These cells were then immunostained with anti-P. gingivalis polyclonal antibodies to detect P. gingivalis vesicles, and anti-viral p24 to detect HIV. Confocal microscopy images were obtained using a Nikon A1R confocal microscope.
TZA assay
TZA assay was performed as previously described with some modifications19. HOKs (2 × 104 cells) were seeded in four replicates in a 96-well plate and first cultured in oral keratinocyte medium to 70% confluence. HOKs were then exposed to P. gingivalis vesicles, HIVNL4.3 alone, or HIV-vesicle complexes for 30 minutes. After five washes with PBS, TZM-bl cells (1.5 × 104 cells) were added to each well, and both cells types were co-cultured in DMEM containing 10% FBS for 48 h. The wells were washed, and Beta-Glo reagent (Promega, Madison, WI) was added and incubated with the cells for 1 h. Chemiluminescence was measured using a FLUOstar Microplate Reader (BMG Labtech, Cary, NC).
Multi-cycle viral replication in MT4 cell assay
HIV-1 NL4-3 Nanoluc-sec at various low infecting doses (ranging from 1/8 to 2 TCID50/well) was used to infect MT4 cells (1 × 105 cells/mL) in the presence or absence of HIVNL4.3 or HIV-vesicle complexes in 96-well plates. Supernatant samples were harvested on day 3 post-infection and assayed for luciferase activity using the Nano-Glo® Luciferase Assay System (Promega). A relative Luminescence level 10-time higher than background (cell only) was scored as a productive viral infection.
Statistical analyses
A student’s t-test was used to determine the statistical significance of the differences in HOK response to exposure to HIV-1 alone or HIV-P. gingivalis vesicle complexes. P < 0.05 was considered significant. Values shown are ± SD unless stated otherwise.
References
Kawwass, J. F. et al. Strategies for Preventing HIV Infection Among HIV-Uninfected Women Attempting Conception with HIV-Infected Men - United States. MMWR. Morbidity and mortality weekly report 66, 554–557, https://doi.org/10.15585/mmwr.mm6621a2 (2017).
Acheampong, E. A. et al. Molecular interactions of human immunodeficiency virus type 1 with primary human oral keratinocytes. Journal of virology 79, 8440–8453, https://doi.org/10.1128/JVI.79.13.8440-8453.2005 (2005).
Maher, D., Wu, X., Schacker, T., Horbul, J. & Southern, P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proceedings of the National Academy of Sciences of the United States of America 102, 11504–11509, https://doi.org/10.1073/pnas.0500848102 (2005).
Morrow, G., Vachot, L., Vagenas, P. & Robbiani, M. Current concepts of HIV transmission. Current HIV/AIDS reports 4, 29–35 (2007).
Kohli, A. et al. Oral and vaginal epithelial cell lines bind and transfer cell-free infectious HIV-1 to permissive cells but are not productively infected. Plos One 9, e98077, https://doi.org/10.1371/journal.pone.0098077 (2014).
Mirmonsef, P. & Spear, G. T. The barrier to HIV transmission provided by genital tract Lactobacillus colonization. American journal of reproductive immunology 71, 531–536, https://doi.org/10.1111/aji.12232 (2014).
Giacaman, R. A., Nobbs, A. H., Ross, K. F. & Herzberg, M. C. Porphyromonas gingivalis selectively up-regulates the HIV-1 coreceptor CCR5 in oral keratinocytes. Journal of immunology 179, 2542–2550 (2007).
Giacaman, R. A. et al. Porphyromonas gingivalis induces CCR5-dependent transfer of infectious HIV-1 from oral keratinocytes to permissive cells. Retrovirology 5, 29, https://doi.org/10.1186/1742-4690-5-29 (2008).
Vacharaksa, A. et al. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology 5, 66, https://doi.org/10.1186/1742-4690-5-66 (2008).
Xie, H., Belogortseva, N. I., Wu, J., Lai, W. H. & Chen, C. H. Inhibition of human immunodeficiency virus type 1 entry by a binding domain of Porphyromonas gingivalis gingipain. Antimicrobial agents and chemotherapy 50, 3070–3074, https://doi.org/10.1128/AAC.01578-05 (2006).
Mantri, C. K., Chen, C., Dong, X., Goodwin, J. S. & Xie, H. Porphyromonas gingivalis-mediated Epithelial Cell Entry of HIV-1. Journal of dental research 93, 794–800, https://doi.org/10.1177/0022034514537647 (2014).
Bonnington, K. E. & Kuehn, M. J. Protein selection and export via outer membrane vesicles. Biochimica et biophysica acta https://doi.org/10.1016/j.bbamcr.2013.12.011 (2013).
Mantri, C. K. et al. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. MicrobiologyOpen 4, 53–65, https://doi.org/10.1002/mbo3.221 (2015).
Ho, M. H., Chen, C. H., Goodwin, J. S., Wang, B. Y. & Xie, H. Functional Advantages of Porphyromonas gingivalis Vesicles. Plos One 10, e0123448, https://doi.org/10.1371/journal.pone.0123448 (2015).
Grenier, D. et al. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infection and immunity 71, 4742–4748 (2003).
Belton, C. M., Izutsu, K. T., Goodwin, P. C., Park, Y. & Lamont, R. J. Fluorescence image analysis of the association between Porphyromonas gingivalis and gingival epithelial cells. Cellular microbiology 1, 215–223 (1999).
Inaba, H. et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cellular microbiology 16, 131–145, https://doi.org/10.1111/cmi.12211 (2014).
Ho, M. H. et al. Two Small Molecules Block Oral Epithelial Cell Invasion by Porphyromons gingivalis. Plos One 11, e0149618, https://doi.org/10.1371/journal.pone.0149618 (2016).
Sanyal, A. et al. Novel assay reveals a large, inducible, replication-competent HIV-1 reservoir in resting CD4+T cells. Nature medicine 23, 885–889, https://doi.org/10.1038/nm.4347 (2017).
Williams, B. et al. A Summary of the First HIV Microbiome Workshop 2015. AIDS research and human retroviruses 32, 935–941, https://doi.org/10.1089/AID.2016.0034 (2016).
Cohen, J. INFECTIOUS DISEASE. Vaginal microbiome affects HIV risk. Science 353, 331, https://doi.org/10.1126/science.353.6297.331 (2016).
Klatt, N. R. et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 356, 938–945, https://doi.org/10.1126/science.aai9383 (2017).
Dillon, S. M., Frank, D. N. & Wilson, C. C. The gut microbiome and HIV-1 pathogenesis: a two-way street. Aids 30, 2737–2751, https://doi.org/10.1097/QAD.0000000000001289 (2016).
Mudd, J. C. & Brenchley, J. M. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. The Journal of infectious diseases 214(Suppl 2), S58–66, https://doi.org/10.1093/infdis/jiw258 (2016).
Takeuchi, H., Takada, A., Kuboniwa, M. & Amano, A. Intracellular periodontal pathogen exploits recycling pathway to exit from infected cells. Cellular microbiology 18, 928–948, https://doi.org/10.1111/cmi.12551 (2016).
Takeuchi, H., Furuta, N. & Amano, A. Cell entry and exit by periodontal pathogen via recycling pathway. Communicative & integrative biology 4, 587–589, https://doi.org/10.4161/cib.4.5.16549 (2011).
Tugizov, S. M. et al. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 409, 211–222, https://doi.org/10.1016/j.virol.2010.10.004 (2011).
Bomsel, M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature medicine 3, 42–47 (1997).
Meng, G. et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+cells. Nature medicine 8, 150–156, https://doi.org/10.1038/nm0202-150 (2002).
Yasen, A., Herrera, R., Rosbe, K., Lien, K. & Tugizov, S. M. Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLoS pathogens 13, e1006247, https://doi.org/10.1371/journal.ppat.1006247 (2017).
Gouin, E., Welch, M. D. & Cossart, P. Actin-based motility of intracellular pathogens. Current opinion in microbiology 8, 35–45, https://doi.org/10.1016/j.mib.2004.12.013 (2005).
Ray, K., Marteyn, B., Sansonetti, P. J. & Tang, C. M. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nature reviews. Microbiology 7, 333–340, https://doi.org/10.1038/nrmicro2112 (2009).
Gedde, M. M., Higgins, D. E., Tilney, L. G. & Portnoy, D. A. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infection and immunity 68, 999–1003 (2000).
Beauregard, K. E., Lee, K. D., Collier, R. J. & Swanson, J. A. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. The Journal of experimental medicine 186, 1159–1163 (1997).
Shaughnessy, L. M., Hoppe, A. D., Christensen, K. A. & Swanson, J. A. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cellular microbiology 8, 781–792, https://doi.org/10.1111/j.1462-5822.2005.00665.x (2006).
Holmes, M., Zhang, F. & Bieniasz, P. D. Single-Cell and Single-Cycle Analysis of HIV-1 Replication. PLoS pathogens 11, e1004961, https://doi.org/10.1371/journal.ppat.1004961 (2015).
Hulme, A. E. & Hope, T. J. Live Cell Imaging of Retroviral Entry. Annual review of virology 1, 501–515, https://doi.org/10.1146/annurev-virology-031413-085502 (2014).
Kim, J. H., Song, H., Austin, J. L. & Cheng, W. Optimized Infectivity of the Cell-Free Single-Cycle Human Immunodeficiency Viruses Type 1 (HIV-1) and Its Restriction by Host Cells. Plos One 8, e67170, https://doi.org/10.1371/journal.pone.0067170 (2013).
Finzi, D., Plaeger, S. F. & Dieffenbach, C. W. Defective virus drives human immunodeficiency virus infection, persistence, and pathogenesis. Clinical and vaccine immunology: CVI 13, 715–721, https://doi.org/10.1128/CVI.00052-06 (2006).
Layne, S. P. et al. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189, 695–714 (1992).
Bourinbaiar, A. S. The ratio of defective HIV-1 particles to replication-competent infectious virions. Acta virologica 38, 59–61 (1994).
Kwon, Y. J., Hung, G., Anderson, W. F., Peng, C. A. & Yu, H. Determination of infectious retrovirus concentration from colony-forming assay with quantitative analysis. Journal of virology 77, 5712–5720 (2003).
Rusert, P. et al. Quantification of infectious HIV-1 plasma viral load using a boosted in vitro infection protocol. Virology 326, 113–129, https://doi.org/10.1016/j.virol.2004.05.022 (2004).
Thomas, J. A., Ott, D. E. & Gorelick, R. J. Efficiency of human immunodeficiency virus type 1 postentry infection processes: evidence against disproportionate numbers of defective virions. Journal of virology 81, 4367–4370, https://doi.org/10.1128/JVI.02357-06 (2007).
Platt, E. J., Kozak, S. L., Durnin, J. P., Hope, T. J. & Kabat, D. Rapid dissociation of HIV-1 from cultured cells severely limits infectivity assays, causes the inactivation ascribed to entry inhibitors, and masks the inherently high level of infectivity of virions. Journal of virology 84, 3106–3110, https://doi.org/10.1128/JVI.01958-09 (2010).
Furuta, N. et al. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infection and immunity 77, 4187–4196, https://doi.org/10.1128/IAI.00009-09 (2009).
Brussel, A. & Sonigo, P. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. Journal of virology 77, 10119–10124 (2003).
Acknowledgements
This work was supported by NIDCR grants DE022428 and 025332 (HX). The project described was also supported by NIAID P30 AI110527. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank the Meharry Office for Scientific Editing and Publications for scientific editing support (S21MD000104).
Author information
Authors and Affiliations
Contributions
H.X. and C.C. designed the project. M.H.H., C.C. and H.X. performed the experiments. X.D., B.L., C.D., J.H., C.C. and H.X. analyzed the data. H.X. and C.C. wrote the main manuscript text. All the authors contributed to the discussion and provided comments on the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, XH., Ho, MH., Liu, B. et al. Role of Porphyromonas gingivalis outer membrane vesicles in oral mucosal transmission of HIV. Sci Rep 8, 8812 (2018). https://doi.org/10.1038/s41598-018-27284-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27284-6
- Springer Nature Limited