Abstract
U0126 (1,4-diamino-2,3-dicyano-1,4-bis (2-aminophenylthio) butadiene), a widely used mitogen-activated protein kinase kinase (MEK) inhibitor, was found to accelerate voltage-gated K+ channel (KV) inactivation in heterologous cells expressing several types of KV. The goal of this study was to examine whether U0126 at a concentration thought to specifically inhibit MEK signaling also inhibits KV in native neurons of primary cultures or brain slices. U0126 caused a dose-dependent inhibition of both the transient (IA) and sustained (IDR) components of K+ currents in hippocampal neurons. U0126 also exhibited much higher potency on the IA and IDR than the classical KV blockers 4-aminopyridine (4-AP) and tetraethylammonium (TEA). Consistent with its inhibitory effect on KV, U0126 broadened action potential duration, profoundly affected the repolarizing phase, and dramatically reduced firing frequency in response to current pulse injections. Despite the potent and reversible action of U0126 on Kv channels, PD98059, a structurally-unrelated MEK inhibitor, did not induce such an effect, suggesting U0126 may act independently of MEK inhibition. Together, these results raise cautions for using U0126 as a specific inhibitor for studying MEK signaling in neurons; on the other hand, further studies on the blocking mechanisms of U0126 as a potent inhibitor of KV may provide useful insights into the structure-function relationship of KV in general.
Similar content being viewed by others
Introduction
The mitogen-activated protein kinase (MAPK), also known as extracellular signal-regulated kinase (ERK1/2) is activated by the dual phosphorylation catalyzed by MAPK kinase (MAPKK, also known as MEK). The MAPK cascade, one of the major intracellular signaling pathways, plays a key role in proliferation, differentiation, survival of various cell types1,2,3,4, and in several plasticity-related processes in the nervous system5. U0126 (1,4-diamino-2,3-dicyano-1,4-bis (2-aminophenylthio) butadiene) is widely used as a potent and selective non-competitive inhibitor of MEK6, therefore, the activation of its downstream target, MAPK/ERK. U0126 has been a valuable pharmacological tool for studying the ERK signaling pathway. The concentration of U0126 used to block the MAPK/ERK signaling pathway is typically 10 μM in cultured neurons7,8, and 20 μM in brain slices9,10,11. It has been demonstrated in various cell types that the ERK signaling pathway plays important roles in modulating KV12,13,14, synaptic plasticity10,15,16,17,18,19,20, and learning and memory21,22,23 (reviewed by19,24,25). On the other hand, U0126 is found to accelerate KV inactivation in heterologous cells expressing several types of KV26. Therefore, it is of interest and importance to determine whether U0126 at a concentration thought to specifically inhibit MEK-MAPK signaling can have a significant effect on KV in primary neuronal cultures and brain slices.
Voltage-gated potassium channels (KV) are key regulator of membrane excitability. Mammalian neurons express various types of KV that exhibit different voltage- and time-dependent channels kinetics. KV are multimeric proteins assembled from pore-forming α subunits and auxiliary β subunits. The α subunits of K+ channels are encoded by 12 subfamilies of genes (KV1–12)27. Previous studies have shown that the CA1 pyramidal neurons28,29, like many other types of neurons found in various brain regions30,31, express at least three major types of KV currents; the transient fast-inactivating K+ current (IA), the delayed rectifier K+ current (IDR), composed of a non-inactivating, fast delayed rectifier K+ current (ID), and the slowly inactivating delayed rectifier K+ current (IK)31. K+ channels underlying those currents possess distinct biophysical properties, pharmacology, and molecular identity32. The IA, mainly assembled from KV4.2 and KV4.333 of KV4 subfamily, are blocked by 4-AP but insensitive to TEA. IA is rapidly activated upon depolarization and quickly recovers from inactivation, and therefore can influence action potential onset time, threshold, and inter-spike intervals as well as dendritic backpropagation action potentials34. ID, likely composed of KV3.1 and KV3.2 channels, exhibit rapid activation and deactivation and are highly sensitive to both TEA and 4-AP. It plays a prominent role in promoting high firing frequency35 and is highly enriched in fast-spiking inhibitory interneurons36. IK presumably encoded by KV2 channels29, show intermediate TEA-sensitivity37 and slow activation time course. The KV2.1 are the predominant delayed rectifier KV that regulate neuronal excitability, action potential duration, and tonic spiking38.
Here, we show that bath application of U0126 resulted in a dose-dependent inhibition of both the IA and IDR on primary hippocampal cultures and acute brain slices. This inhibiting effect of U0126 appeared to be of much higher potency (100- to 1000-fold) on the IA and IDR than the classical KV blockers 4-AP or TEA.
Results
Dose-dependent blockade of K+ currents by U0126 in primary hippocampal neurons
We first used primary culture of hippocampal neurons prepared from postnatal day 0–3 rats to test U0126 effects on K+ currents (Fig. 1). In voltage-clamp mode, two kinetically distinct K+ current components, the transient fast-inactivating K+ current, IA and sustained, delayed rectifier K+ current, IDR were identified39. A prepulse voltage protocol was used to isolate the IA and IDR (Fig. 1A1,A2)40. To selectively activate the IDR (Fig. 1B1,B2), neurons were held at −80 mV and voltage was stepped to 0 mV with a prepulse to −40 mV in order to inactivate the IA. Subtracting IDR from the total K+ current elicited by a voltage step yielded the IA (Fig. 1C1,C2). Examining the dose-response relationship of IA and IDR showed a half maximal inhibitory concentration (IC50) at 9.5 ± 0.1 μM (Fig. 1D) and 19.3 + 0.4 μM (Fig. 1E), respectively. At 10 μM, a concentration commonly used in neuronal culture studies, U0126 produced a significant inhibition of the IA (65 ± 11%, n = 20; p < 0.05) (Fig. 1D) but had no significant inhibition of the IDR (6 ± 3%, n = 20; p > 0.05) (Fig. 1E). At 20 μM, U0126 significantly reduced both the IA (82 ± 4%, n = 33; p < 0.05) (Fig. 1D) and IDR (38 ± 6%, n = 33; p < 0.05) (Fig. 1E). Therefore, both IA and IDR can be inhibited by U0126, with the IA being more sensitive to the inhibition of U0126. In contrast, PD98059, a structurally-unrelated MEK inhibitor, had no effects on K+ currents (Fig. 2). These results raise a possibility that U0126 could directly affect KV at the concentration that is commonly used as a specific MEK inhibitor.
Non-selective block of voltage-dependent whole-cell K+ currents by U0126 in primary hippocampal neurons. (A1,A2) Representative traces showing superimposed currents with and without prepulse from control and U0126-treated neurons, respectively. Schematic diagram of the prepulse voltage protocol used to isolate IA (bottom) is shown at the bottom of the traces. (B1,B2) Representative traces of IDR in control and U0126-treated neurons, respectively. (C1,C2) Representative traces of IA isolated from the voltage protocol under the control and U0126 treatment condition. The IA were obtained by subtracting IDR from the whole-cell K+ currents. IA was measured at peak amplitude (indicated by a filled circle) and IDR was measured by a small, late window as indicated by a filled square. (D,E) Dose–response curves of IA and IDR in the presence of U0126. The IC50 for IA and IDR were 9.5 ± 0.1 μM and 19.3 ± 0.4 μM, respectively.
Lack of PD98059 effects on K+ currents. (A1–C1) Representative traces of the total K+ currents, IDR, and IA under the control condition. (A2–C2) Application of 50 μM PD98059 produced no changes in IA and IDR. (A3–C3) Subsequent addition of 20 μM U0126 to the same neuron resulted in dramatic reduction in peak amplitude of both IA and IDR. Similar results were obtained in three additional neurons.
U0126 blocked K+ channels with greater potency than 4-AP and TEA
We then compared the effects of U0126 on IA and IDR with classic KV inhibitors, 4-AP and TEA. As shown in Fig. 3, 3 mM 4-AP produced 33 ± 10% (n = 9, p < 0.05) inhibition of the IA (Fig. 3A) and 6 + 7% (n = 9, p > 0.05) inhibition of the IDR (Fig. 3B); whereas 30 mM TEA produced inhibition of the IDR 45 ± 8% (n = 9, p < 0.05) (Fig. 3B) and did not significantly change IA (Fig. 3A). These results are consistent with well-established observations that 4-AP preferentially blocks IA41 and low levels of TEA have no effect on IA42 but partially inhibit the residual sustained current43. These results suggest that U0126 inhibits IA and IDR in the micromolar range of concentrations, exhibiting 100- to 1000-fold higher potency than the classical KV blockers 4-AP and TEA, which work in millimolar range of concentrations.
Comparison of inhibiting effects of U0126, 4-AP and TEA on IA and IDR. (A) Representative K+ current traces recorded from a neuron before and after 4-AP application. (B) Representative K+ current traces recorded from a neuron before and after TEA application. Quantitative histograms of IA (C) and IDR (D) were constructed by normalizing current densities to that of control condition, i.e. before the application of drugs. Data are expressed as mean ± s.e.m. To determine statistical significance. One way-ANOVA was used, followed by the post hoc Scheffe’s test, *P < 0.05, ***P < 0.001.
Prolongation of action potentials by U0126
We next studied the effects of U0126 on action potentials under whole-cell current-clamp recording mode. To elicit action potentials, a series of 20 ms depolarizing current pulses in 10 pA steps from resting membrane potential were injected into cultured hippocampal neurons. To inhibit synaptic activity, 20 µM CNQX (an AMPAR antagonist) and 100 μM APV (a potent and selective antagonist for NMDA receptors) were added to the bath. Consistent with its inhibitory effects on K+ currents, bath perfusion of U0126 increased the half-width and decay time of action potential in a dose-dependent manner (Fig. 4A). With exposure to 1 and 3 μM U0126, half-width and decay time of action potentials showed no significant difference to that of controls. At 10 μM, action potential half-width was increased by 392 ± 82% (n = 16, p < 0.001) and decay time increased by 298 ± 153% (n = 16, p < 0.05); At 20 μM, U0126 had a dramatic effect, increasing the action potential half-width by 1573 ± 205% (n = 18, p < 0.001) and decay time by 2023 ± 573% (n = 18, p < 0.001) (Fig. 4B1,C1). In a comparison, 3 mM 4-AP or 30 mM TEA increased the action potential decay time by 710 ± 155% (n = 14, p < 0.001) or 296 ± 35% (n = 16, p < 0.001), respectively. Below 10 mM, TEA had no significant effect on decay time of action potentials (Fig. 4C3). These results are consistent with several previously reported effects of 4-AP and TEA on action potentials42,44. Therefore, we conclude that U0126 prolongs the action potential half-width and decay time much more efficiently than 4-AP and TEA, possibly through its high potency on K+ current inhibition.
U0126 increases the decay time and half-width of evoked action potentials in primary hippocampal neurons. (A) Under current clamp, single action potentials were evoked by injecting a 20-ms depolarizing currents at 10 pA steps from the resting membrane potential. Representatives of typical traces of control, during the application of 1 μM U0126, 3 μM U0126, 10 μM U0126, 20 μM U0126, 3 mM 4-AP and 30 mM TEA were shown. These traces were all obtained from the same cell. (B1–B3) Show quantification of the half-width of action potentials during application of various concentrations of U0126, 4-AP and TEA, respectively. (C1–C3) Show the quantification of the decay time of action potentials during application of various concentrations of U0126, 4-AP and TEA, respectively. P indicates statistical significance using One way-ANOVA followed by the post hoc Scheffe’s test, *P < 0.05 vs. control group, ***P < 0.001 vs. control group.
Suppression of action potential firing frequency by U0126
We further examined the potential effect of U0126 on the action potential firing pattern by analyzing the current-frequency (I-F) relationship. Under control conditions, the number of action potentials increased progressively with the increase of current injection steps over the course of 400 ms depolarizations from 0 to + 120 pA (Fig. 5A1,B1,C1 and E). In contrast, in the presence of 20 μM U0126, regardless the strength of current injections, only a single action potential was evoked, followed by a prolonged membrane depolarization (Fig. 5A2,B2 and C2). After washout of U0126 for 5–10 minutes, the repetitive firing pattern of action potentials was restored, similar to that of the control condition (Fig. 5A3,B3 and C3). We conclude that U0126 exerts profound influence on membrane excitability and firing pattern of cultured hippocampal neurons.
Effects of U0126 on action potential waveforms and firing patterns in primary hippocampal neurons. (A–C) Representative traces of whole-cell currents from control neurons (A), neurons treated by U0126 (20 μM) (B) and washout of U0126 (C). The voltage changes of cultured hippocampal neurons in response to a series of a 400 ms-duration depolarizing current pulse (0 pA to +120 pA in 10 pA steps) injected into neurons at rest membrane potential (RMP) were recorded and shown at 20 pA (left); 50 pA (middle) and 100 pA (right) levels. (D) shows quantitative plots of the current versus spike firing frequency relationships (I–F) curves. The number of action potentials elicited were counted and plotted against the levels of injected currents. Notice that after bath application of 20 μM U0126, only a single action potential followed by a prolonged membrane depolarization could be evoked no matter how much depolarizing currents were injected. Bars indicate standard error of the mean; N = 8 for all conditions. P indicates statistical significance using Two way-ANOVA followed by the post hoc Scheffe’s test, *P < 0.05.
Suppression of K+ currents by U0126 in acute hippocampal slices
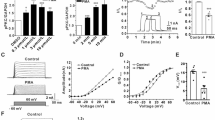
Based on the above results, we further examined the effects of bath perfusion of U0126 on K+ currents in CA1 pyramidal neurons of acute hippocampal slices. Both IA and IDR are highly expressed in CA1 pyramidal neurons and are important determinants of their membrane excitability45. Consistent with the effects of U0126 on K+ currents in primary hippocampal cultures, U0126 similarly inhibited the K+ currents of CA1 pyramidal neurons in a dose-dependent manner in acute slices. As shown in Fig. 6, bath application of 20 μM U0126 produced a small but significant reduction of the current density of the early K+ currents (measured at peak of the currents, presumably largely composed by IA: 15.4 ± 0.76 pA/pF in control; n = 9, at + 50 mV vs 13.6 ± 0.68 pA/pF in U0126; n = 7, p < 0.05), whereas the late sustained currents, presumably the IDR, were not affected (Fig. 6B). At 30 μM and 100 μM, U0126 further reduced the current density of the early K+ currents by 47.6 ± 3% and 62.7 ± 5% (at +50 mV), respectively; meanwhile, the late, sustained K+ currents (measured indicated by filled squares, Fig. 6A) showed significant reduction compared to control (Fig. 6C). These results demonstrate that U0126 also non-selectively blocks native K+ currents in brain slices.
Suppression of K+ currents by U0126 in acute hippocampal slices. (A) Representative traces of whole-cell voltage-gated K+ currents recorded from control pyramidal neurons, neurons treated by 20 μM U0126, 30 μM U0126 and100 μM U0126, as indicated. The schematic diagram of the voltage protocol used to evoke K+ currents is shown at the bottom of the traces. We recorded K+ currents in CA1 neurons in response to a voltage step from −40 mV to +50 mV with 10 mV increments. (B) Quantitative comparison of the current density of early K+ currents (measured at peak amplitude, indicated by filled circles) in CA1 neurons at different conditions as indicated. (C) Quantitative comparison of the current density of the late, sustained K+ currents (measured indicated by filled square) in CA1 neurons at different concentrations after bath application of U0126. P indicates statistical significance using Two way-ANOVA followed by the post hoc Scheffe’s test, *P < 0.05.
Effects of U0126 on action potential waveform and firing patterns in hippocampal neurons
Lastly, we examined the functional consequence of U0126 blockade of K+ channels, specifically on action potential (AP) waveform and firing patterns of hippocampal neurons in acute brain slices. To this end, we recorded two regular-spiking pyramidal neurons simultaneously from P20 mouse slices. Under control conditions, those neurons exhibit a regular firing pattern in response to depolarizing current pulses of 1 s. Bath application of 40 μM U0126 dramatically reduced the maximal firing rate from about 18–20 Hz to roughly 2–3 Hz in response to current injections of the same amplitude. Action potential waveform was also greatly broaden with a prolonged half-width and a slower decay time. Washout of U0126 for 30 minutes restored AP waveform and firing patterns to the control condition (Fig. 7A). We then compared the effects of U0126 on AP waveform and firing patterns with classic KV inhibitors, 4-AP and TEA. As shown in Fig. 7B, after adding 20 mM TEA, the two pyramidal neurons reduced their maximal firing rate from ~18–20 Hz in control to 8–9 Hz in response to current injections. After washout of TEA for 30 minutes, AP waveform and firing patterns were returned to the control condition. Subsequent addition of 3 mM 4-AP reduced the maximal firing rate reduced to 14–16 HZ. Taken together, these results suggest that U0126 is more efficacious than 4-AP and TEA in suppressing maximal firing rate of pyramidal neurons in hippocampal slices.
Effects of U0126 on action potential waveforms and firing patterns in hippocampal neurons. (A) Representative double whole-cell current clamp recordings from two regular-spiking pyramidal neurons from control, neurons treated by 40 μM U0126 and washout, as indicated. These tracings were obtained from the same neuron. (B) Representative double whole-cell current clamp recordings from two regular-spiking pyramidal neurons from control, neurons treated by 20 mM TEA, washout and treated by 3 mM 4-AP, as indicated. These tracings were obtained from the same neuron. Recordings were made from P20 mouse hippocampus. The current step is 20 pA (from −40 pA to 140 pA).
Discussion
Our major finding is that U0126, a widely-used MEK inhibitor acts to serve as a highly potent non-selective KV blocker. Consequently, it changes neuronal excitability and firing pattern at the concentrations commonly used in many previous studies. Our results thus raise cautions for using U0126 as specific inhibitor for studying MEK-MAPK signaling in neurons.
We demonstrated that bath perfusion of U0126 significantly inhibits the IA and IDR in hippocampal neurons in a dose-dependent manner in primary neuronal cultures as well as acute brain slices. Remarkably, U0126 (in micromolar range of concentrations) inhibits both the IA and IDR with much higher potency (100- to 1000-fold) than the classical KV blockers 4-AP and TEA, which preferentially inhibits the IA and IDR in the millimolar range of concentrations, respectively. Consistently, U0126 showed clear effects on action potential waveform and the ability of firing repetitive action potentials. Bath perfusion of U0126 not only increased the half-width and decay time of individual action potentials in a dose-dependent manner (Fig. 4), but also dramatically reduced firing frequency in response to long current pulse injections (Figs 5 and 7A). Interestingly, U0126’s reversible channel inhibiting effect was not mimicked by PD98059, a structurally-unrelated MEK inhibitor (Fig. 2), indicating the effect of U0126 was independent of its inhibition on MEK.
In our study, we did not differentiate IDR into ID, IK components. It would be interesting to further evaluate if U0126 exhibits similar or different inhibitory effects on ID, IK. Currently, the mechanism of U0126 blocking KV is not clear. In heterologous cells expressing the A-type K+ channels, KV4.2 and KV4.3 as well as KV1.1, a typical non-inactivating KV, U0126 exhibits its inhibition on KV by accelerating the inactivation of these different channels26. The authors further demonstrated that the action of U0126 is likely a result from a combination of open channel blocking and modulation of channel-gating but independent on its inhibitory effect on ERK or the phosphorylation of the channels. Together with our current observation that U0126 exhibits non-selective inhibitory effects on IA and IDR, we postulate that U0126 may be able to directly bind and block the channel pore of various native KV or alter channel gating by interacting with membrane lipids46.
Several studies have reported that direct phosphorylation of KV4.2 potassium channel by ERK/MAPK can inhibit IA in different subtypes of neurons including the hippocampal CA1 pyramidal neurons13,47,48,49. In particular, Watanabe49 showed that application of 20 μM U0126 to the outside of the neurons or included in the whole cell patch pipette produced a small but significant leftward shift in the activation voltage and an overall reduction in IA. In our study, we found that 20 μM U0126 produced a small but significant reduction of the current density of peak K+ currents (Fig. 6B). At higher concentrations, U0126 exhibited a greater inhibition on the late, sustained K+ currents in a concentration-dependent manner (Fig. 6C). There is no clear explanation for this discrepancy. Differences in species, age (5–8 week-old mature rats used by Watanabe et al.49 vs 2–3 week-old young mice used in our study), methodology such as perfusion speed, internal solution or different subtypes of pyramidal neurons recorded, are all plausible factors for the discrepancy.
KV blockers have been considered as potential drugs for many diseases (Tian et al., 2014) associated with low excitability or compromised motor functions such as multiple sclerosis50, spinal cord injury51 or Parkinson’s Disease52. For example, 4-AP has been used clinically in treating Lambert-Eaton myasthenic syndrome and multiple sclerosis53,54,55; and TEA was the first “ganglionic blocker” drug to be introduced into clinical practice56 but their uses have been limited due to toxicity57,58, and were soon replaced by other drugs. Because of significant adverse side effects and a narrow range for a safe clinical dose, 4-AP failed in clinical trials in treating patients with spinal cord injuries59,60. As discussed above, 4-AP or TEA typically acts on KV in the millimolar range of concentrations (Fig. 3), whereas U0126 inhibits both the transient A-type and the residual sustained K+ currents in the micromolar range of concentrations as shown in this study (Fig. 1). Such a high potency (100- to 1000-fold) and broad-spectrum action may prove to be favorable properties as a potential new class of KV blockers in treating those aforementioned diseases. Indeed, our ongoing study indicates that, beside a powerful enhancement on neuronal excitability, U0126 also greatly potentiates presynaptic release, hence evoking synchronized firing in immature cultured hippocampal neurons (unpublished data). We believe that a further understanding of the structural basis and blocking mechanisms of U0126 and its analogs as potent, non-selective inhibitors of KV and the removal of its unwanted action on MEK should not only provide useful insights into the structure-function of KV in general, but also may prove valuable in developing more efficacious and safe new KV blockers in treating various relevant diseases due to conduction deficits and low excitability in the excitable organs including brain, heart and muscle.
In conclusion, we have observed dramatic effects of U0126 on KV in primary hippocampal cultures and brain slices. The data reported here suggest that cautions should be taken when interpreting experimental results using U0126. Furthermore, our finding that U0126 exhibits much higher potency on KV than 4-AP and TEA raises the possibility that U0126 and its derivatives can be further developed as a potent, new class of KV blockers and may provide a new perspective in the development new treatments for various neuromuscular and neurodegenerative diseases.
Methods
Hippocampal primary culture
All animals were housed and maintained in accordance with procedures approved by the Ethics Committee for animal research at South China Normal University, in line with the Guidelines for Animal Care established by the National Institute of Health. All experimental procedures were approved by the Ethics Committee for animal research at South China Normal University. Hippocampal cell cultures were prepared as described previously8. Briefly, hippocampal CA1/CA3 regions were dissected from 0- to 3-days-old C57BL6/J mouse, dissociated by trypsin XI treatment followed by trituration with a siliconized Pasteur pipette, and then plated onto coverslips coated with Matrigel (Bd Biosciences, USA). Culture medium consisted of minimal essential medium (Invitrogen, CA), 0.6% glucose, 0.1 mg/l bovine transferrin (Calbiochem, CA), 0.25 mg/l insulin (Sigma-Aldrich, USA), 0.3 mg/l glutamine, 5–10% fetal bovine serum (Sigma-Aldrich, USA), 2% B-27 supplement (Invitrogen, USA), and 2 μM cytosine β-D-arabinofuranoside (Sigma-Aldrich, USA). Cultures were maintained at 37 °C in a 95% air, 5% CO2-humidified incubator. Cultures of 7–9 DIV (days in vitro) were used for whole-cell patch clamp recordings.
Patch-clamp recording
Whole-cell patch-clamp recording in hippocampal cultures
Traditional whole-cell patch-clamp recordings were performed from cultured hippocampal neurons plated on coverslips, which were placed in a recording chamber mounted on a fixed-stage inverted phase-contrast microscope (Nikon, Japan). Patch electrodes (3–5 MΩ) were made from borosilicate glass (WPI, USA). Whole-cell capacitance and series resistances were recorded and compensated to >80%, and in addition, series resistances were less than two times the tip resistance. The Tyrode’s bath solution contained (in mM): 129 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 0.01 glycine, 30 D-glucose and 25 HEPES, pH 7.2–7.4. The pipette solution contained (in mM): 110 K-gluconate, 40 HEPES, 10 EGTA, 2 Na2-ATP, 2 Mg-ATP and 0.3 GTP, pH 7.35 (adjusted with KOH). To isolate K+ currents in pyramidal neurons, 1 µM tetrodotoxin (TTX) and 2 mM MnCl2 were added to the bath to block Na+ currents, Ca2+ currents, and Ca2+-activated K+ currents40. To isolate the fast inactivating transient current (IA), cells were held at −80 mV, and the voltage was stepped to 0 mV with or without a prepulse to −40 mV40. The difference between the currents elicited with and without the prepulse was measured as IA. Leakage and capacitive currents were digitally subtracted on-line with P4 protocol40.
Whole-cell patch-clamp recording in acute hippocampal slices
Coronal brain slices (350 μm thick) were prepared from 15- to 21-days-old C57BL6/J mice in cutting solution with a vibratome (Lecia VT1000S, Germany). The cutting solution contained (in mM): 210 Sucrose, 26 NaHCO3, 3 MgSO4·7H2O, 0.75 CaCl2, 1 NaH2PO4·2H2O, 3 KCl and 10 D-glucose. Slices were incubated at room temperature for 1 hour in the artificial cerebrospinal fluid (ACSF). The ACSF contained (in mM): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4·2H2O, 1.3 MgSO4·7H2O, 26 NaHCO3, 10 D-glucose and 2.5 CaCl2. The pipette solution contained (in mM): 110 K-gluconate, 40 HEPES, 10 EGTA, 2 Na2-ATP, 2 Mg-ATP and 0.3 GTP, pH 7.35 (adjusted with KOH). At a holding potential of −60 mV, the K+ currents were evoked by voltage steps (from −40 mV to +50 mV in 10 mV increments, 400 ms) in the presence of TTX (1 μM) and CdCl2 (2 mM) to block voltage-activated Na+ and Ca2+ currents, as well as Ca2+-activated K+ currents61.
Drugs
U0126 (Sigma-Aldrich, USA and Calbiochem, CA), PD98059 (Calbiochem, CA), 4-AP and TEA (Sigma-Aldrich, USA) were dispersed in ultrapure water or DMSO as stock solution. Stock solutions were dissolved directly in the Tyrode or ACSF at the desired concentration. Neurons were exposed to different drug solutions until the steady-state effects were obtained, using a perfusion system.
Data analysis
Patch-clamp data were processed by using Clampfit 10.2 (Molecular Devices, USA) and then analyzed in Origin 8 (OriginLab, USA). Dose–response curves were fitted by non-linear curve fitting of the Boltzmann equation to the data using the Origin software. The values were presented as means ± SE. Analysis of variance (ANOVA) followed by the post hoc Scheffe’s test was used for statistical analysis. Changes were considered significant when P < 0.05.
References
Pearson, G. et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine reviews 22, 153–183, https://doi.org/10.1210/edrv.22.2.0428 (2001).
Chang, L. & Karin, M. Mammalian MAP kinase signalling cascades. Nature 410, 37–40, https://doi.org/10.1038/35065000 (2001).
Hunter, T. Signaling–2000 and beyond. Cell 100, 113–127 (2000).
Bonni, A. et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358–1362 (1999).
Sweatt, J. D. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of neurochemistry 76, 1–10 (2001).
Duncia, J. V. et al. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorganic & medicinal chemistry letters 8, 2839–2844 (1998).
Xie, Z., Smith, C. J. & Van Eldik, L. J. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia 45, 170–179, https://doi.org/10.1002/glia.10314 (2004).
Wu, G. Y., Deisseroth, K. & Tsien, R. W. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci 4, 151–158, https://doi.org/10.1038/83976 (2001).
Selcher, J. C. et al. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learning & memory 10, 26–39, https://doi.org/10.1101/lm.51103 (2003).
Winder, D. G. et al. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron 24, 715–726 (1999).
Alonso, M., Medina, J. H. & Pozzo-Miller, L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learning & memory 11, 172–178, https://doi.org/10.1101/lm.67804 (2004).
Hu, H. J., Glauner, K. S. & Gereau, R. Wt ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. Journal of neurophysiology 90, 1671–1679, https://doi.org/10.1152/jn.00340.2003 (2003).
Yuan, L. L., Adams, J. P., Swank, M., Sweatt, J. D. & Johnston, D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci 22, 4860–4868 (2002).
Schrader, L. A. et al. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. American journal of physiology. Cell physiology 290, C852–861, https://doi.org/10.1152/ajpcell.00358.2005 (2006).
Adams, J. P. et al. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. Journal of neurochemistry 75, 2277–2287 (2000).
English, J. D. & Sweatt, J. D. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. The Journal of biological chemistry 271, 24329–24332 (1996).
Martin, K. C. et al. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron 18, 899–912 (1997).
Watabe, A. M., Zaki, P. A. & O’Dell, T. J. Coactivation of beta-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci 20, 5924–5931 (2000).
Thomas, G. M. & Huganir, R. L. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5, 173–183, https://doi.org/10.1038/nrn1346 (2004).
Giachello, C. N. et al. MAPK/Erk-dependent phosphorylation of synapsin mediates formation of functional synapses and short-term homosynaptic plasticity. Journal of cell science 123, 881–893, https://doi.org/10.1242/jcs.056846 (2010).
Atkins, C. M., Selcher, J. C., Petraitis, J. J., Trzaskos, J. M. & Sweatt, J. D. The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1, 602–609, https://doi.org/10.1038/2836 (1998).
Bozon, B. et al. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos Trans R Soc Lond B Biol Sci 358, 805–814, https://doi.org/10.1098/rstb.2002.1224 (2003).
Selcher, J. C., Atkins, C. M., Trzaskos, J. M., Paylor, R. & Sweatt, J. D. A necessity for MAP kinase activation in mammalian spatial learning. Learning & memory 6, 478–490 (1999).
Adams, J. P. & Sweatt, J. D. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42, 135–163, https://doi.org/10.1146/annurev.pharmtox.42.082701.145401 (2002).
Sharma, S. K. & Carew, T. J. The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: facilitatory effects and inhibitory constraints. Learning & memory 11, 373–378, https://doi.org/10.1101/lm.81104 (2004).
Yuan, L. L., Chen, X., Kunjilwar, K., Pfaffinger, P. & Johnston, D. Acceleration of K+ channel inactivation by MEK inhibitor U0126. American journal of physiology. Cell physiology 290, C165–171, https://doi.org/10.1152/ajpcell.00206.2005 (2006).
Gonzalez, C. et al. K(+) channels: function-structural overview. Comprehensive Physiology 2, 2087–2149, https://doi.org/10.1002/cphy.c110047 (2012).
Storm, J. F. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature 336, 379–381, https://doi.org/10.1038/336379a0 (1988).
Murakoshi, H. & Trimmer, J. S. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci 19, 1728–1735 (1999).
Coetzee, W. A. et al. Molecular diversity of K+ channels. Annals of the New York Academy of Sciences 868, 233–285 (1999).
Foehring, R. C. & Surmeier, D. J. Voltage-gated potassium currents in acutely dissociated rat cortical neurons. Journal of neurophysiology 70, 51–63, https://doi.org/10.1152/jn.1993.70.1.51 (1993).
Dodson, P. D. & Forsythe, I. D. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci 27, 210–217, https://doi.org/10.1016/j.tins.2004.02.012 (2004).
Birnbaum, S. G. et al. Structure and function of Kv4-family transient potassium channels. Physiological reviews 84, 803–833, https://doi.org/10.1152/physrev.00039.2003 (2004).
Hoffman, D. A., Magee, J. C., Colbert, C. M. & Johnston, D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875, https://doi.org/10.1038/43119 (1997).
Ding, S., Matta, S. G. & Zhou, F. M. Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. Journal of neurophysiology 105, 554–570, https://doi.org/10.1152/jn.00707.2010 (2011).
Du, J., Zhang, L., Weiser, M., Rudy, B. & McBain, C. J. Developmental expression and functional characterization of the potassium-channel subunit Kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J Neurosci 16, 506–518 (1996).
Taglialatela, M. et al. Patterns of internal and external tetraethylammonium block in four homologous K+ channels. Molecular pharmacology 40, 299–307 (1991).
Malin, S. A. & Nerbonne, J. M. Delayed rectifier K+ currents, IK, are encoded by Kv2 alpha-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci 22, 10094–10105 (2002).
Klee, R., Ficker, E. & Heinemann, U. Comparison of voltage-dependent potassium currents in rat pyramidal neurons acutely isolated from hippocampal regions CA1 and CA3. Journal of neurophysiology 74, 1982–1995, https://doi.org/10.1152/jn.1995.74.5.1982 (1995).
Varga, A. W. et al. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci 24, 3643–3654, https://doi.org/10.1523/JNEUROSCI.0154-04.2004 (2004).
Bouchard, R. & Fedida, D. Closed- and open-state binding of 4-aminopyridine to the cloned human potassium channel Kv1.5. The Journal of pharmacology and experimental therapeutics 275, 864–876 (1995).
Mitterdorfer, J. & Bean, B. P. Potassium currents during the action potential of hippocampal CA3 neurons. J Neurosci 22, 10106–10115 (2002).
McGahon, M. K., Dawicki, J. M., Scholfield, C. N., McGeown, J. G. & Curtis, T. M. A-type potassium current in retinal arteriolar smooth muscle cells. Investigative ophthalmology & visual science 46, 3281–3287, https://doi.org/10.1167/iovs.04-1465 (2005).
Bean, B. P. The action potential in mammalian central neurons. Nat Rev Neurosci 8, 451–465, https://doi.org/10.1038/nrn2148 (2007).
Johnston, D. et al. Dendritic potassium channels in hippocampal pyramidal neurons. The Journal of physiology 525(Pt 1), 75–81 (2000).
Oliver, D. et al. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science 304, 265–270, https://doi.org/10.1126/science.1094113 (2004).
Schrader, L. A., Anderson, A. E., Mayne, A., Pfaffinger, P. J. & Sweatt, J. D. PKA modulation of Kv4.2-encoded A-type potassium channels requires formation of a supramolecular complex. J Neurosci 22, 10123–10133 (2002).
Schrader, L. A. et al. Kv4.2 is a locus for PKC and ERK/MAPK cross-talk. Biochem J 417, 705–715, https://doi.org/10.1042/BJ20081213 (2009).
Watanabe, S., Hoffman, D. A., Migliore, M. & Johnston, D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 99, 8366–8371, https://doi.org/10.1073/pnas.122210599 (2002).
Malerba, M., Radaeli, A., Mancuso, S. & Polosa, R. The potential therapeutic role of potassium channel modulators in asthma and chronic obstructive pulmonary disease. Journal of biological regulators and homeostatic agents 24, 123–130 (2010).
Jensen, J. M. & Shi, R. Effects of 4-aminopyridine on stretched mammalian spinal cord: the role of potassium channels in axonal conduction. Journal of neurophysiology 90, 2334–2340, https://doi.org/10.1152/jn.00868.2002 (2003).
Luca, C. C. & Singer, C. 4-aminopyridine improves freezing of gait in Parkinson’s disease. Journal of neurology 260, 2662–2664, https://doi.org/10.1007/s00415-013-7090-0 (2013).
Korenke, A. R., Rivey, M. P. & Allington, D. R. Sustained-release fampridine for symptomatic treatment of multiple sclerosis. The Annals of pharmacotherapy 42, 1458–1465, https://doi.org/10.1345/aph.1L028 (2008).
Jensen, H. B., Stenager, E. & Ravnborg, M. H. Aminopyridines for symptomatic treatment of multiple sclerosis. Ugeskrift for laeger 173, 3259–3263 (2011).
Solari, A., Uitdehaag, B., Giuliani, G., Pucci, E. & Taus, C. Aminopyridines for symptomatic treatment in multiple sclerosis. The Cochrane database of systematic reviews, CD001330, https://doi.org/10.1002/14651858.CD001330 (2002).
Moe, G. K. & Freyburger, W. A. Ganglionic blocking agents. The Journal of pharmacology and experimental therapeutics 98, 61–95 (1950).
Johnson, N. C. & Morgan, M. W. An unusual case of 4-aminopyridine toxicity. The Journal of emergency medicine 30, 175–177, https://doi.org/10.1016/j.jemermed.2005.04.020 (2006).
Pickett, T. A. & Enns, R. Atypical presentation of 4-aminopyridine overdose. Annals of emergency medicine 27, 382–385 (1996).
Hayes, K. C. et al. 4-Aminopyridine-sensitive neurologic deficits in patients with spinal cord injury. Journal of neurotrauma 11, 433–446, https://doi.org/10.1089/neu.1994.11.433 (1994).
Shi, R. & Sun, W. Potassium channel blockers as an effective treatment to restore impulse conduction in injured axons. Neuroscience bulletin 27, 36–44, https://doi.org/10.1007/s12264-011-1048-y (2011).
Locke, R. E. & Nerbonne, J. M. Role of voltage-gated K+ currents in mediating the regular-spiking phenotype of callosal-projecting rat visual cortical neurons. Journal of neurophysiology 78, 2321–2335, https://doi.org/10.1152/jn.1997.78.5.2321 (1997).
Acknowledgements
This work was supported by the National Natural Science Foundation of China Grant (31671059), a Key Research and Platform program Grant from Guangdong Provincial Department of Education (2015KGJHZ008) and the Recruitment Program of High-end Foreign Experts of the State Administration of Foreign Experts Affairs (GDT20144400031) to G.Y.W. We thank Brock Pope for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
J.Z.W., C.L., K.Y.L. and H.T.X. performed the experiments. C.L., L.L.Y. and G.Y.W. designed and supervised the study. J.Z.W., C.L., K.Y.L., H.T.X. and G.Y.W. analyzed the data. J.Z.W., C.L., L.L.Y. and G.Y.W. wrote the paper. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, JZ., Long, C., Li, KY. et al. Potent block of potassium channels by MEK inhibitor U0126 in primary cultures and brain slices. Sci Rep 8, 8808 (2018). https://doi.org/10.1038/s41598-018-27235-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27235-1
- Springer Nature Limited