Abstract
Macrophage infiltrations (inflammation) are associated with prostate disorders such as prostatitis, prostatic hyperplasia and prostate cancer. All prostate disorders have elevated cell proliferation, and are initiated from normal prostate epithelial cells. To date, the mechanism of how macrophages regulate normal prostate epithelial cell proliferation remains largely unknown. Using a 3D co-culture system, we here show that Raw 264.7 macrophages increased cell proliferation of normal prostate epithelial PZ-HPV-7 cells. In addition, these Raw 264.7 macrophages expressed higher levels of Ym1 and CD206. We further identify macrophage-secreted cytokines including CCL3, IL-1ra, osteopontin, M-CSF1 and GDNF as mediators for potentiating PZ-HPV-7 cell proliferation in 3D. All these cytokines differentially activated ERK and Akt. Blockade of both kinases through their inhibitors hindered macrophage-induced cell proliferation of PZ-HPV-7 cells. Hence, our data provide mechanistic insight of how inflammation may contribute to development of prostatic diseases at a very early stage through augment of cell proliferation of normal prostate epithelial cells.
Similar content being viewed by others
Introduction
Prostate disorders including prostatitis, prostatic hyperplasia, and prostate cancer are the most commonly encountered health issue among men older than 50 years. The etiology and pathogenesis of the prostate disorders, so far, remains unclear. All these disorders are associated with an increase of inflammation1,2,3,4,5,6 and elevated cell proliferation of prostate cells7,8,9,10,11,12. A sustained inflammatory cell environment and uncontrolled cell proliferation, both of which can lead to tumorigenesis. In despite of a large body of evidence that inflammation promotes cancer initiation and development in many types of cancers13,14, how inflammation positively contributes to prostate disorders, particularly prostate cancer, is still an ongoing debate. This is because of certain conflicting results from clinical studies15,16,17,18,19,20.
In response to infections of bacteria or pathogens, and injury, immune cells are rapidly activated to defend the body from further damage, known as inflammation. During inflammation, macrophages are the major type of immune cells activated to execute their tasks including pathogen killing and wound healing21,22. In addition, genetic mutations, epigenetic alterations, age, obesity and environmental stimuli such as diet have been demonstrated to generate a more inflammatory environment by upregulating reactive oxygen species (ROS)23,24,25,26,27,28. Depending on their activators, macrophages are classified into either classically-activated/M1 or alternatively-activated/M2 subtypes. M1 macrophages activated by lipopolysaccharide and interferon γ destroy pathogens through producing nitric oxide and inflammatory cytokines29,30. Meanwhile, M2 macrophages activated by interleukin 4, interleukin 13 and other can repair wounds, synthesize extracellular matrix and promote cell growth through their secreted anti-inflammatory cytokines31,32.
We, herein, show that macrophage-secreted cytokines are mediators to increase cell proliferation of normal prostate epithelial cells in a 3D cell culture system. Moreover, these macrophage cytokines activate ERK and Akt, and inhibition of both protein kinases abolish macrophage-medicated cell proliferation. Therefore, we provide evidence for mechanistic insight into how inflammation leads to a set-up for initiating prostate diseases through induction of a higher cell proliferation rate of normal prostate epithelial cells.
Results
Macrophages promote cell proliferation of normal prostate epithelial cells
To decipher the effect of macrophage-mediated process on cell proliferation of normal prostate epithelial cells, we co-cultured Raw 264.7 macrophages with immortalized normal prostate PZ-HPV-7 epithelial cells on matrigel in a three dimensional setting. These two types of cells were seeded in separated compartments of a co-cultivation system (see scheme in Fig. 1A), which only allows cells to share soluble substances released in the media instead of physical contacts. As reported previously33, PZ-HPV-7 cells when cultured in 3D formed acinar clusters (Fig. 1B). In order to directly evaluate the cell proliferation under a 3D environment without any extra artificial inputs, we fixed cells in 3D and then used nuclear cyclin D1 as a readout for cell proliferation34,35,36 by immunostaining cells with a cyclin D1 specific antibody. As shown in Fig. 1B, when co-cultured with Raw 264.7 macrophages in 3D, more PZ-HPV-7 cells expressed nuclear cyclin D1. Results from quantification of PZ-HPV-7 cell clusters demonstrated a statistically significant increase of cell proliferation of PZ-HPV-7 cells in the presence of Raw 264.7 macrophages (Fig. 1C). Given that a physical interaction is not required for inducing PZ-HPV-7 cell proliferation by macrophages, we next used Raw 264.7-conditioned media to treat PZ-HPV-7 cells. As shown in Fig. 1D,E, Raw 264.7-conditioned media increased numbers of nuclear cyclin D1 positive cells of PZ-HPV-7. Moreover, Raw 264.7-conditioned media had a better effect on PZ-HPV-7 cell proliferation as compared to co-cultivation of Raw 264.7 macrophages. Altogether, these data indicated a promoting role of macrophages in proliferation of normal prostate epithelial cells.
Macrophages promote cell proliferation of normal prostate epithelial cells. (A) A diagram for demonstrating cell co-cultivation of normal prostate epithelial PZ-HPV-7 cells (blue area) and Raw 264.7 cells (purple area). Cells were plated on matrigel in µ-Slides (Ibidi) and overlaid with KSF media. (B) PZ-HPV-7 cells grown on matrigel in 3D with or without Raw264.7 cells in a co-culture system (described in A) were fixed and immuno-stained with cyclin D1 (green). DAPI (blue) was used to visualize all cell nuclei. Scale bar: 20 µm. (C) Cell proliferation index of PZ-HPV-7 cells that were cultured with or without Raw 264.7 cells in 3D was quantified. *p < 0.05. (D) PZ-HPV-7 cells were cultured on matrigel in 3D in Raw 264.7-conditioned media or control media. Cells were fixed and immune-stained with cyclin D1 (green). DAPI (blue) was used to visualize all cell nuclei. Scale bar: 20 µm. (E) Cell proliferation index of PZ-HPV-7 cells that were cultured in 3D with control media or Raw 264.7-conditioned media was quantified. *p < 0.05.
Macrophage-secreted CCL3 and IL-1ra mediate cell proliferation of normal prostate epithelial cells
To identify the macrophage-secreted factors that promoted PZ-HPV-7 cell proliferation in 3D matrigel culture, we performed cytokine profiler arrays for analyzing Raw 264.7-conditioned media. Among 40 tested cytokines (Fig. S1), only four cytokines including CCL3, CCL4, CXCL2 and IL-1ra were present in Raw 264.7-conditioned media (Fig. 2A). We next tested the capability of these identified cytokines on PZ-HPV-7 cell proliferation in 3D matrigel culture. Upon CCL3 stimulation, cell proliferation of PZ-HPV-7, as judged by the cyclin D1 nuclear localization, was increased in a dose dependent manner (Fig. 2B). Similarly, IL-1ra was also capable of inducing proliferation of PZ-HPV-7 cells (Fig. 2E). Neither CCL4 nor CXCL2 affected cell proliferation of normal prostate PZ-HPV-7 epithelial cells in a 3D culture setting (Fig. 2C and D). A dual treatment of CCL3 and IL-1ra in PZ-HPV-7 cells additionally elevated cell proliferation as compared to either CCL3 or IL-1ra alone (Fig. 2F). This suggests that these two cytokines may activate different downstream signaling pathways that led to cell proliferation.
CCL3 and IL-1ra secreted by macrophages mediate cell proliferation of normal prostate epithelial cells. (A) Raw 264.7-conditioned media and control media were subjected to a mouse cytokine array for identifying the cytokines present in Raw 264.7-conditioned media. A1/2, A23/24, and F1/2 contain positive controls, and F23/F24 contains negative controls. (B–E) PZ-HPV-7 cells grown on matrigel in 3D were treated with the cytokines identified from Raw 264.7-conditioned media including CCL3 (B), CCL4 (C), CXCL2 (D) and IL-1ra (E) at the indicated concentrations. Cell proliferation index of PZ-HPV-7 cells under these treatments was quantified. *p < 0.05. (F) PZ-HPV-7 cells grown on matrigel in 3D were treated control/ddH2O, CCL3 (50 ng/mL), IL-1ra (10 ng/mL) or both for 48 h. Cell proliferation index under these conditions was quantified. *p < 0.05.
Osteopontin, M-CSF1 and GDNF upregulated by Ym1+/CD206+ macrophages promote cell proliferation of normal prostate epithelial cells
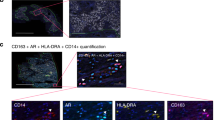
In response to stimulations under different cell environments, macrophages are either M1 pro-inflammatory or M2 immune-suppressive for executing their distinct tasks, e.g. killing the infected cells by foreign pathogens for M1 macrophages vs repairing wounds for M2 macrophages21,22. To investigate which subtype of macrophages was present in our 3D matrigel co-culture system that resulted in PZ-HPV-7 cell proliferation, we immune-stained the macrophages cultured in either control media or co-culture media with several well-established markers including iNOS, CD38 for M1 macrophages29,37, and Ym1, CD206 for M2 macrophages30,31 respectively (Fig. 3A,B). Indeed, all Raw 264.7 macrophages expressed F4/80, a pan-macrophage marker (Fig. 3A,B). In addition, higher levels of Ym1 and lower levels of iNOS were expressed in Raw 264.7 macrophages that generated Raw 264.7-conditioned media to promote PZ-HPV-7 cell proliferation in 3D matrigel culture. Consistent result was observed using CD38 as alternative M1 macrophage marker, and CD206 as additional M2 macrophage marker (Fig. 3B), suggesting that the macrophages resulted in PZ-HPV-7 cell proliferation were Ym1+/CD206+ M2 subtype.
Osteopontin, M-CSF1 and GDNF upregulated by Ym1+/CD206+ macrophages promote cell proliferation of normal prostate epithelial cells. (A) Raw 264.7 cells were cultured in control media or KSF complete media for 24 h. Cells were immuno-stained with F4/80 (orange, pan-macrophage marker), iNOS (green, M1 macrophage marker) and Ym1 (pink, M2 macrophage marker). Cell nuclei were visualized by DAPI staining. Scale bar: 20 µm. (B) Similar to A, Raw 264.7 cells were cultured in control media or KSF complete media and immuno-stained with F4/80 (orange, pan-macrophage marker), CD38 (green, M1 macrophage marker) and CD206 (pink, M2 macrophage marker). Cell nuclei were visualized by DAPI staining. Scale bar: 20 µm. (C) RNA samples from Raw 264.7 cells cultured in control media or KSF complete media were subjected to growth factor PCR arrays for identifying the growth factors upregulated in Raw264.7 macrophages grown in KSF complete media. A scatter plot was shown for upregulated growth factors (red), unaltered growth factors (black) and downregulated growth factors (green). (D,E) PZ-HPV-7 cells grown on matrigel in 3D were treated either control/ddH2O, osteopontin (250 ng/mL), M-CSF1 (100 ng/mL), GDNF (50 ng/mL) or combination of these cytokines as indicated. Cell proliferation index under these conditions was quantified. *p < 0.05 as compared to the control/ddH2O.
To screen more potential cytokines that were upregulated in these macrophages and actually functioned as growth factors to modulate cell proliferation of PZ-HPV-7, we utilized real time qPCR arrays. Among 84 examined genes expressed in the macrophages cultured in the control or co-culture media, 6 genes were upregulated at least 2-fold higher and 2 genes were downregulated at least 2-fold lower in the macrophages grown in the co-culture media than these in the control media. (Fig. 3C). In addition, 76 genes remained at similar levels (within a 2-fold difference). The 6-upregulated genes included Spp1 (known as osteopontin), M-CSF-1, GDNF, B2M, IL-18 and MDK; and the 2-downregulated genes were IGF-1 and FGF13. Out of the 8 genes that showed different expressions in macrophages, Spp1, M-CSF1 and GDNF held the strongest change. Therefore, we next focused on effects of Spp1, M-CSF1 and GDNF on PZ-HPV-7 cell proliferation in a 3D matrigel culture setting. As shown in Fig. 3D, additions of either recombinant osteopontin, M-CSF1 or GDNF significantly increased cell proliferation of PZ-HPV-7 cells as judged by the percentage of cells expressing nuclear cyclin D1. However, combined treatments of these three cytokines did not result in any synergistic growth as compared to each single treatment (Fig. 3E), suggesting that osteopontin, M-CSF1 and GDNF may activate similar signaling pathways to mediate cell proliferation.
Macrophages potentiate cell proliferation of normal prostate epithelial cells through activation of ERK and Akt
In response to growth factor and cytokine stimulations, activation of mitogen-activated protein kinase (MAPK, also known as ERK) and Akt leads to cell proliferation, differentiation and survival under physiological conditions38,39,40. To test the activation status of these two protein kinases in our 3D matrigel culture of PZ-HPV-7 cells that are stimulated with the identified macrophage-regulated cytokines as described previously, we subjected cell lysates collected from the 3D culture system to immunoblots analysis. Addition of cytokines CCL3, IL-1ra or both to PZ-HPV-7 cells for 1 h activated ERK, but not Akt (Fig. S2A). Treatment of IL-1ra for 48 h slightly activated Akt signaling in PZ-HPV-7 cells (Fig. 4A). In addition, either CCL3 or IL-1ra treatment for 48 h upregulated ERK activation. Treatment of either osteopontin, M-CSF1 or GDNF in the PZ-HPV-7 cells that are grown on matrigel in a 3D setting for 1 h slightly increased phosphorylated Akt and ERK (Fig. S2B). 48 h treatment of either these cytokines strongly elevated both Akt and ERK activation (Fig. 4B). Notably, a higher-fold increase in levels of the phosphorylated Akt than that of the phosphorylated ERK was detected. These results indicated a differential activation between Akt and ERK in response to various macrophage-regulated cytokines. To examine if ERK and Akt activated by macrophage-secreted factors indeed mediate cell proliferation, we used inhibitors U0126 and Wortmannin to target ERK and Akt respectively in our 3D culture of PZ-HPV-7 cells in the presence or absence of Raw 264.7 macrophage-conditioned media. Although a disruption of big clusters of PZ-HPV-7 cells formed in 3D cultures was observed when either inhibitor was added, it had minimal (not statistically significant) effects on cell proliferation (Fig. S3, and Fig. 4C). Treatment of U0126 or Wortmannin decreased cell proliferation that was induced by Raw 264.7-conditioned media (Fig. 4C). Moreover, a dual inhibition of both kinases had a synergistic effect and further lowered the cell proliferation rate of PZ-HPV-7 cells.
Macrophages potentiate cell proliferation of normal prostate epithelial cells through activation of ERK and Akt. (A) PZ-HPV-7 cells grown on matrigel in 3D were treated with CCL3, IL-1ra or both for 48 h. Cell lysates were collected and subjected to immunoblotting for examining the protein of interests as indicated. (B) Similar to A, PZ-HPV-7 cells cultured on matrigel in 3D were treated with control/ddH2O, osteopontin (OPN), M-CSF1 or GDNF for 48 h. Cell lysates were collected from 3D culture and subjected to immunoblotting for examining the protein of interests as indicated. (C) U0126 or wortmannin (WT) was added to 3D cultures of PZ-HPV-7 cells in the presence or absence of Raw 264.7-conditioned media for 48 h. Vehicle (DMSO) was used as control. Cell proliferation index under these conditions was quantified. *p < 0.05 as compared to the control/vehicle. **p < 0.05 as compared to Raw 264.7-conditioned media. (D) A scheme to summarize how macrophages may promote cell proliferation of normal prostate epithelial cells.
Altogether, these data suggested that when co-cultured with normal prostate epithelial cells in a 3D environment, macrophages were polarized to Ym1+/CD206+ subtype. These Ym1+/CD206+ macrophages promoted cell proliferation of normal prostate epithelial cells through their secreted cytokines, including CCL3, IL-1ra, osteopontin, M-CSF1 and GDNF, which activate ERK and Akt signaling (Fig. 4D).
Discussion
Increased cell proliferation is one of the major characteristics of prostate disorders including benign prostatic hyperplasia (BPH) and prostate cancer. These disorders typically progress with age advancement especially in senior males. Other risk factors for these prostatic disorders include obesity and African ancestry. All these described contributing factors are associated with elevated inflammation41,42,43,44,45. Macrophages are the major type of immune cells activated during inflammation. How macrophages affect normal prostate epithelial cells to initiate prostate diseases remains majorly unclear.
Our results indicate that macrophage-upregulated cytokines including CCL3, IL-1ra, osteopontin, M-CSF1 and GDNF are mediators for inducing cell proliferation of normal prostate epithelial PZ-HPV-7 cells in 3D cultures (Figs 2B,E and 3D,E). It is the first time to link these cytokines to cell proliferation of the immortalized and normal prostate epithelial cells. In pathological conditions, these cytokines have been reported to regulate cell proliferation, migration, invasion and metastasis46,47,48,49,50,51,52,53,54. In prostate disease BPH, macrophage-secreted CCL3 was downstream target of androgen receptor (AR), which is also secreted by macrophages, to promote prostate stromal cell proliferation6. In a mouse model of experimental autoimmune prostatitis, CCL3 was shown to induce chronic pelvic pain55. IL-1ra from CD11b+Gr1+ myeloid cells has been demonstrated to overcome cell senescence of PTEN null prostate tumors in mice56. Transplanting IL-1ra knockout myeloid cells to the PTEN-null mice accelerated cell senescence. In CD133+ stem/progenitor cells of prostate cancer and CD133+ C4–2 prostate cancer cells, IL-1ra expressed by CD133+ cells mediates testicular nuclear receptor 4 (TR4)-induced drug resistance57.
In addition to CCL3 and IL-1ra, we also showed that macrophage-upregulated cytokines osteopontin, M-CSF1 and GDNF in the co-culture media were capable of inducing cell proliferation of normal prostate epithelial cells (Fig. 3D,E). Notably, among these identified cytokines, osteopontin has been reported to promote cell proliferation and cell survival in several types of cells including smooth muscle cells, quiescent prostate epithelial cells, erythroblasts and neural stem cells58,59,60,61. It also regulates cell adhesion, migration and bone remodeling, which associate with bone metastasis in breast cancer and prostate cancer62,63. In prostate cancer, higher expressions of osteopontin were detected in the patient tissue samples of prostate cancer as compared to these of normal and BPH64. Osteopontin also elevates the anchorage-independent growth, intravasation potential, and EGF-dependent cell proliferation in cultured human prostate cancer cell lines65,66,67. Our data demonstrated that the macrophages that upregulated the cytokines to increase cell proliferation of the immortalized normal prostate epithelial cells in the co-culture system were similar to alternatively activated/M2 macrophages (Fig. 3A,B), which express Ym1 and CD20630,31. This result is in support of the notion that M2-like macrophages participate in cell proliferation and tissue repair21,68,69.
Our data showed that CCL3 and IL-1ra activated ERK rather than Akt kinase in the normal prostate epithelial cell line (Fig. 4A). Interesting, cytokines osteopontin, M-CSF1 and GDNF that were also upregulated by macrophages in the 3D culture system strongly activated Akt than ERK (Fig. 4B). This suggests that macrophages ensure to activate both ERK and Akt signaling through secreting various cytokines. When stimulating PZ-HPV-7 cells with CCL3 or IL-1ra for 1 h, increased phospho-ERK at similar levels to that of 48 h treatment was observed (Fig. 4A and Fig. S2A). It suggested a direct activation of ERK pathway by CCL3 and IL-1ra. Activation of Akt and ERK by other macrophage cytokines including osteopontin, M-CSF1 and GDNF may be indirect since both protein kinases required a longer time to gain much higher activities (Fig. 4B and Fig. S2B). When treating cells with Akt or ERK inhibitor, each inhibitor did not significantly reduce the basal level cell proliferation in a 3D environment (Fig. 4C, first 3 lanes). Notably, a disruption of PZ-HPV-7 cell cluster formation was observed (Fig. S3). However, these smaller cell clusters of PZ-HPV-7 were viable and proliferative. Altogether, these results indicated that the used dose of U0126 and wortmannin was not toxic to cells, and that both kinases may participate in cell-cell and/or cell-matrix interactions.
In summary, we provided evidence to demonstrate that macrophages potentiate cell proliferation of immortalized normal prostate epithelial cells in a 3D environment. We also delineated the mechanism of how macrophages achieve this task by activation of ERK and Akt kinases through macrophage-secreted cytokines including CCL3, IL-1ra, osteopontin, M-CSF1 and GDNF. Given that these immortalized normal prostate epithelial cells have increased cell proliferation in response to inflammatory/macrophage stimulations and that elevated cell proliferation is reported in certain prostatic diseases, our findings may provide plausible explanations on how inflammation contributes to giving rise on these prostatic diseases.
Materials and Methods
Cell lines, antibodies and reagents
Raw 264.7 macrophage cells (ATCC) were cultured in RPMI 1640 containing 10% FBS and 100 U/ml penicillin/streptomycin. PZ-HPV-7 cell (ATCC) were cultured in Keratinocyte Serum Free media containing 0.05 mg/mL bovine pituitary extract and 5 ng/mL human recombinant EGF. Cells were maintained in a 37 °C incubator supplemented with 5% CO2. To obtain Raw 264.7-conditioned media, 2.5 × 105 cells per 6-well dish were grown in Keratinocyte Serum Free complete media, and supernatant was collected. Conditioned media were freshly prepared for each experiment. Both anti-F4/80 and anti-cyclin D1 antibodies were from Thermo Fisher Scientific; anti-iNOS and anti-CD206 antibodies were obtained from Abcam. The anti-CD38 antibody was from Novus Biologicals; anti-Akt, anti-phospho-Akt (ser473) and anti-ERK antibodies were purchased from Cell Signal Technology. The anti-phospho-ERK1/2 antibody was from Santa Cruz Biotechnology, and the anti-Ym1 antibody was obtained from STEMCELL Technologies. Human recombinant CCL3, CCL4, CXCL2, IL-1ra, M-CSF1 and GDNF were purchased from PeproTech. Human recombinant osteopontin was obtained from BioLegend. Matrigel was from Corning. U0126 and wortmannin (WT) were obtained from Cell Signaling Technology. Other reagents used are described in the specific experiment sections.
3D culture of PZ-HPV-7 cells and Treatment
PZ-HPV-7 single cells were plated on top of 50% matrigel and stimulated with the indicated recombinant proteins with the indicated concentrations or vehicle control. For U0126 or wortmannin (WT) treatment, 10 µM U0126 or 0.5 µM WT were added to the cells simultaneously with Raw 264.7-conditioned media or control media after cells were seeded on matrigel.
Cell proliferation assay
PZ-HPV-7 cells grown in 3D matrigel culture with the indicated stimulation/treatment for 48 h were rinsed twice with PBS (140 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4 and 1.5 mM KH2PO4 [pH 7.2]), fixed with 4% paraformaldehyde-PBS for 20 min, permeabilized with 1% TritonX-100 in PBS for 10 min, 0.5% TritonX-100 in PBS for 20 min, 0.1% SDS in PBS for 1 min, and blocked in 10% goat serum 1 h at room temperature. Samples were incubated with anti-cyclin D1 antibody (1:250) overnight at 4 °C. Samples were washed three times with PBS containing 0.05% Tween-20. Secondary antibodies Alexa Fluor 488 goat-anti-rabbit (1:500) from Thermo Fisher Scientific were added for 30 min at room temperature. After another three washes of PBST, DAPI was used to label cell nuclei. Images were visualized in ibidi mounting media. Serial sections of images (Z-stack) were captured by a Zeiss Axiovert 200 M inverted fluorescent microscope with a 20x objective lens. Each 3D image were reconstructed from Z-stack images. Numbers of nuclear cyclin D1 were counted in randomly five 3D images per condition. Cell proliferation index was calculated using the ratio: numbers of nuclear cyclin D1/numbers of all cells (DAPI). For the experiment using U0126 and wortmannin, cell proliferation index was used solely based on DAPI counts due to the interference of these two compounds on the formation of cell clusters of PZ-HPV-7.
Cellular lysates and Immunoblotting
Cells were washed twice with PBS, lysed in buffer A (50 mM Tris/HCl [pH 7.4], 1% TritonX-100, 150 mM NaCl, and 5 mM EDTA [pH 7.4]) supplemented with protease inhibitor cocktail (Thermo Fisher Scientific), incubated on ice for 30 min and centrifuged at 4 °C, 14000 rpm for 10 min. The supernatants were were subjected to SDS-PAGE, and resolved proteins were transferred onto nitrocellulose membranes. The membranes were blocked in 5% BSA in TBST (50 mM Tris.HCl [pH 7.6], 150 mM NaCl, 0.05% Tween 20) and incubated with the antibodies of interest in 5% BSA in TBST overnight at 4 °C. The appropriate horseradish peroxidase-conjugated 2° antibodies were applied for 30 min at room temperature. Samples were visualized with ECL and X-ray film.
Immunocytochemistry and Confocal microscope imaging
Cells grown in the indicated conditions were rinsed twice with PBS, fixed in 4% paraformaldehyde-PBS for 20 min, permeabilized with 0.1% TritonX-100 in PBS for 10 min, and blocked in 10% goat serum 1 h at room temperature. Samples were incubated with primary antibodies (anti-F4/80 (1:200); anti-iNOS (1:250); anti-Ym1 (1:200); anti-CD38 (1:200); anti-CD206 (1:1000)) overnight at 4 °C. Samples were washed three times with PBS containing 0.05% Tween-20. Secondary antibodies (Alexa Fluor 488, 594 and 647 obtained from Thermo Fisher Scientific at 1:500) were added for 30 min at room temperature. Samples were washed three times with PBST. Images were captured using a LSM 700 confocal laser scanning microscope (Zeiss) with a 10x objective lens.
Cytokine array
The cytokines secreted by Raw 264.7 macrophages were examined using the Proteome Profiler Mouse Cytokine Array Kit (R&D Systems) according to the manufacturer’s instructions.
Growth factor PCR array
The growth factors expressed by Raw 264.7 macrophages were determined using the RT2 Profiler PCR Array PAMM-041Z (Qiagen) according to the manufacturer’s instructions. Genes of the examined growth factors changed greater than 2-fold were plotted in a scatter plot.
Statistical analysis
Data are presented as means ± SE. P-values were acquired with the Student’s t test using Prism (GraphPad Software), and P < 0.05 is considered statistically significant.
References
Ellem, S. J., Wang, H., Poutanen, M. & Risbridger, G. P. Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic pre-malignancy. Am J Pathol 175, 1187–1199 (2009).
Nickel, J. C., Downey, J., Young, I. & Boag, S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int 84, 976–981 (1999).
Sfanos, K. S. & De Marzo, A. M. Prostate cancer and inflammation: the evidence. Histopathology 60, 199–215 (2012).
Sfanos, K. S., Isaacs, W. B. & De Marzo, A. M. Infections and inflammation in prostate cancer. Am J Clin Exp Urol 1, 3–11 (2013).
Simons, B. W. et al. A human prostatic bacterial isolate alters the prostatic microenvironment and accelerates prostate cancer progression. J Pathol 235, 478–489 (2015).
Wang, X. et al. Increased infiltrated macrophages in benign prostatic hyperplasia (BPH): role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J Biol Chem 287, 18376–18385 (2012).
Dermer, G. B. Basal cell proliferation in benign prostatic hyperplasia. Cancer 41, 1857–1862 (1978).
Johnson, M. I. et al. Expression of Bcl-2, Bax, and p53 in high-grade prostatic intraepithelial neoplasia and localized prostate cancer: relationship with apoptosis and proliferation. Prostate 37, 223–229 (1998).
Kim, J. H. et al. Proliferation of Prostate Stromal Cell Induced by Benign Prostatic Hyperplasia Epithelial Cell Stimulated With Trichomonas vaginalis via Crosstalk With Mast Cell. Prostate 76, 1431–1444 (2016).
Majumder, P. K. et al. A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell 14, 146–155 (2008).
Preston-Martin, S., Pike, M. C., Ross, R. K., Jones, P. A. & Henderson, B. E. Increased cell division as a cause of human cancer. Cancer Res 50, 7415–7421 (1990).
Xu, Y., Chen, S. Y., Ross, K. N. & Balk, S. P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res 66, 7783–7792 (2006).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
De Nunzio, C. et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol 60, 106–117 (2011).
Kryvenko, O. N. et al. Inflammation and preneoplastic lesions in benign prostate as risk factors for prostate cancer. Mod Pathol 25, 1023–1032 (2012).
Platz, E. A. & De Marzo, A. M. Epidemiology of inflammation and prostate cancer. J Urol 171, S36–40 (2004).
Schatteman, P. H., Hoekx, L., Wyndaele, J. J., Jeuris, W. & Van Marck, E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur Urol 37, 404–412 (2000).
Terakawa, T. et al. Inverse association between histologic inflammation in needle biopsy specimens and prostate cancer in men with serum PSA of 10-50 ng/mL. Urology 72, 1194–1197 (2008).
Yli-Hemminki, T. H. et al. Histological inflammation and risk of subsequent prostate cancer among men with initially elevated serum prostate-specific antigen (PSA) concentration in the Finnish prostate cancer screening trial. BJU Int 112, 735–741 (2013).
Mills, C. D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol 32, 463–488 (2012).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122, 787–795 (2012).
Afanas’ev, I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis 5, 52–62 (2014).
Fernandez-Sanchez, A. et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci 12, 3117–3132 (2011).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114, 1752–1761 (2004).
Lim, S.O. et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology 135, 2128-2140, 2140 e2121-2128 (2008).
Liou, G. Y. & Storz, P. Reactive oxygen species in cancer. Free Radic Res 44, 479–496 (2010).
Vial, G. et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol 54, 348–356 (2011).
MacMicking, J., Xie, Q. W. & Nathan, C. Nitric oxide and macrophage function. Annu Rev Immunol 15, 323–350 (1997).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677–686 (2004).
Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm 2015, 816460 (2015).
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A. & Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229, 176–185 (2013).
Harma, V. et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One 5, e10431 (2010).
Radu, A., Neubauer, V., Akagi, T., Hanafusa, H. & Georgescu, M. M. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol 23, 6139–6149 (2003).
Tamamori-Adachi, M. et al. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res 92, e12–19 (2003).
Tong, W. & Pollard, J.W. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol 19, 2251-2264 (1999).
Jablonski, K. A. et al. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS One 10, e0145342 (2015).
Conte, E. et al. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One 6, e24663 (2011).
Sun, Y. et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35, 600–604 (2015).
Zhang, W. & Liu, H. T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12, 9–18 (2002).
Emanuela, F. et al. Inflammation as a Link between Obesity and Metabolic Syndrome. J Nutr Metab 2012, 476380 (2012).
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu Rev Immunol 29, 415–445 (2011).
Nedelec, Y. et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell 167, 657–669 e621 (2016).
Quach, H. et al. Genetic Adaptation and Neandertal Admixture Shaped the Immune System of Human Populations. Cell 167, 643–656 e617 (2016).
Woods, J. A., Wilund, K. R., Martin, S. A. & Kistler, B. M. Exercise, inflammation and aging. Aging Dis 3, 130–140 (2012).
Hsu, C. J. et al. AMP-activated protein kinase activation mediates CCL3-induced cell migration and matrix metalloproteinase-2 expression in human chondrosarcoma. Cell Commun Signal 11, 68 (2013).
Liao, Y. Y. et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/ VEGF-A axis in human osteosarcoma cells. Oncotarget 7, 4310–4325 (2016).
Liu, H., Ma, Q. & Li, J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Mol Cell Biochem 347, 95–101 (2011).
Okazaki, T. et al. Macrophage colony-stimulating factor induces vascular endothelial growth factor production in skeletal muscle and promotes tumor angiogenesis. J Immunol 174, 7531–7538 (2005).
Shevde, L. A. & Samant, R. S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol 37, 131–141 (2014).
Song, H. & Moon, A. Glial cell-derived neurotrophic factor (GDNF) promotes low-grade Hs683 glioma cell migration through JNK, ERK-1/2 and p38 MAPK signaling pathways. Neurosci Res 56, 29–38 (2006).
Wai, P. Y. & Kuo, P. C. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev 27, 103–118 (2008).
Wang, J. et al. Granulocyte-colony stimulating factor promotes proliferation, migration and invasion in glioma cells. Cancer Biol Ther 13, 389–400 (2012).
Liou, G. Y. et al. The Presence of Interleukin-13 at Pancreatic ADM/PanIN Lesions Alters Macrophage Populations and Mediates Pancreatic Tumorigenesis. Cell Rep 19, 1322–1333 (2017).
Quick, M. L. et al. CCL2 and CCL3 are essential mediators of pelvic pain in experimental autoimmune prostatitis. Am J Physiol Regul Integr Comp Physiol 303, R580–589 (2012).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Yang, D. R. et al. Increased chemosensitivity via targeting testicular nuclear receptor 4 (TR4)-Oct4-interleukin 1 receptor antagonist (IL1Ra) axis in prostate cancer CD133+stem/progenitor cells to battle prostate cancer. J Biol Chem 288, 16476–16483 (2013).
Elgavish, A. et al. Osteopontin stimulates a subpopulation of quiescent human prostate epithelial cells with high proliferative potential to divide in vitro. Prostate 35, 83–94 (1998).
Gadeau, A. P., Campan, M., Millet, D., Candresse, T. & Desgranges, C. Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler Thromb 13, 120–125 (1993).
Kang, J. A. et al. Osteopontin regulates actin cytoskeleton and contributes to cell proliferation in primary erythroblasts. J Biol Chem 283, 6997–7006 (2008).
Rabenstein, M. et al. Osteopontin mediates survival, proliferation and migration of neural stem cells through the chemokine receptor CXCR4. Stem Cell Res Ther 6, 99 (2015).
Carlinfante, G. et al. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis 20, 437–444 (2003).
Koeneman, K. S., Yeung, F. & Chung, L. W. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 39, 246–261 (1999).
Forootan, S. S. et al. Prognostic significance of osteopontin expression in human prostate cancer. Int J Cancer 118, 2255–2261 (2006).
Angelucci, A. et al. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate 59, 157–166 (2004).
Khodavirdi, A. C. et al. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res 66, 883–888 (2006).
Thalmann, G. N. et al. Osteopontin: possible role in prostate cancer progression. Clin Cancer Res 5, 2271–2277 (1999).
Xiao, X. et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci USA 111, E1211–1220 (2014).
Zhang, Q.Z. et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 28, 1856-1868 (2010).
Acknowledgements
We thank Paris Grady for helping with analyzing cell proliferation in 3D systems. We are grateful for Dr. Shafiq Khan for sharing PZ-HPV-7 cells. This work was supported by a grant from the Center for Cancer Research and Therapeutic Development (CCRTD) at Clark Atlanta University funded by NIH G12MD007590 to G.Y.L. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
G.Y. L. conceived the project and designed the experiments. T.D. and G.Y.L. performed experiments and data analysis. G.Y. L. wrote and edited the manuscript. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dang, T., Liou, GY. Macrophage Cytokines Enhance Cell Proliferation of Normal Prostate Epithelial Cells through Activation of ERK and Akt. Sci Rep 8, 7718 (2018). https://doi.org/10.1038/s41598-018-26143-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26143-8
- Springer Nature Limited
This article is cited by
-
CCR2+ monocytes/macrophages drive steroid hormone imbalance-related prostatic fibrosis
Scientific Reports (2024)
-
Tumor-derived interleukin-1 receptor antagonist exhibits immunosuppressive functions and promotes pancreatic cancer
Cell & Bioscience (2023)
-
Obesity and prostate cancer — microenvironmental roles of adipose tissue
Nature Reviews Urology (2023)
-
Cancer-inducing niche: the force of chronic inflammation
British Journal of Cancer (2022)
-
TNF is a potential therapeutic target to suppress prostatic inflammation and hyperplasia in autoimmune disease
Nature Communications (2022)