Abstract
This study was designed to investigate the prognostic value of circulating blood cell counts and subsets for patients with advanced gastric cancer (AGC) treated with neoadjuvant chemotherapy (NAC) and the factors determining pathological complete response (pCR). In 112 patients with AGC, we retrospectively examined the ratios of lymphocyte, monocyte, and neutrophil during and after NAC before surgery, and the percentages of CD3+, CD3+ CD4+, CD3+ CD8+ and CD4+/CD8+ lymphocytes as well. We also investigated their associations with the pCR rate and overall survival (OS). The ratios of lymphocyte taken before and after NAC were significantly greater in forty-four pCR cases than that in sixty-eight non-pCR cases. During NAC, the proportion of lymphocyte and the percentages of CD3+, CD3+ CD4+, and CD3+ CD8+ lymphocytes were dramatically increased in pCR group. The lymphocyte ratio showed an independent association with pCR by multivariate analysis and maintained at a relatively high level in pCR cases. By mean of 31.53% lymphocyte ratio before-NAC and 41.68% after-NAC, cases with high lymphocyte ratio showed significantly better outcome in OS. High circulating lymphocyte ratios, both before and after NAC, are positively associated with pCR and improved OS in advanced gastric cancer, which may be considered as a new prognostic biomarker.
Similar content being viewed by others
Introduction
Gastric cancer remains one of the most common malignancies around the world and causes 499,000 cancer-related deaths each year in China1,2,3,4. Moreover, a majority of gastric cancer patients (80–90%) in China are diagnosed at advanced stages with extensive regional lymph node involvement and/or invasion of adjacent structures in first medical consultation5,6. Neoadjuvant chemotherapy (NAC) with many clinical advantages is a promising strategy and currently accepted as an effective treatment for various malignant diseases, including ovarian, head and neck cancer and extremity tumors7. However, not all AGC patients benefit from NAC: studies not only suggest that the overall response rate to NAC is less than 50% but also highlight that nearly 15% of patients undergoing NAC show risks of tumor progression8,9. Additionally, the 5-year survival rate of AGC patients remains at 45–50% even after comprehensive strategies including chemotherapy and surgery10,11,12. The pathological complete response (pCR) to NAC has been reported to correlate with a favorable long-term outcome13,14,15,16. However, it turns out rarely in patients with AGC, despite the application of various combined chemotherapy regimens. Therefore, it would be advantageous to determine which factor could predict the efficacy of NAC and to identify what kind of patients might gain pCR and a better long-term outcome.
The immune response to gastric cancer is complex, involving the interaction of several cell types of the immune system, which plays a significant role in the progression of gastric cancer. Several studies have indicated that many patients with gastric cancer have various scales of immunological impairment, including decreased cellular immunity17,18. Besides, the prevalence of suppressor cells may prove to be a decisive factor for poor outcomes because regulatory T cells restrain the antitumor activity of cytotoxic T cells19,20. A recent meta-analysis study21 has shown that the elevated platelet to lymphocyte ratio could be a significant prognostic biomarker for poor overall survival (OS) in patients with gastric cancer. Since blood cell counts in peripheral blood were considered to reflect the immunological function in AGC patients, we have endeavored to determine whether the values of lymphocytes before or after NAC may serve as new parameters predicting pCR to NAC. We also examined the laboratory data of white blood cell and lymphocyte subpopulation during and after NAC period before surgery, which may reflect systemic responses against tumor cells damaged by NAC. Besides, the correlation between clinical parameters and outcomes was examined to determine the potential prognostic impact of lymphocyte on OS.
Results
Patients characteristics
From January 2005 to December 2011, 1149 patients who underwent surgical treatment for AGC were reviewed in our institution. Among them, 304 cases (26.5%) received NAC for AGC. R0 radical resection was performed in 248 (81.6%) patients, with 44 cases of them (17.7% of NAC treatment cases who underwent R0 resection) being demonstrated no residual tumor on final pathology and defined as pCR. The remaining 204 patients (82.2% of NAC treatment cases who underwent R0 resection) were identified as non-pCR after surgery, from which 68 cases (27.4%) were included, and 136 cases (54.8%) were excluded for lack of clinical records (Fig. 1).
Clinical and pathological factors
The baseline characteristics of all patients in pCR group and non-pCR group are given in Table 1. There were no differences in all clinical factors, including age, gender, Borrmann type, differentiation, histological type, clinical stage (TNM), carcinoembryonic antigen (CEA), and albumin (ALB) between pCR group and non-pCR group.
Lymphocyte ratio was associated with the pCR rate and OS
Blood cell counts recorded before and after NAC were compared between pCR group and non-pCR group, respectively (Table 2). There were no statistical differences between the two groups in some blood cell data before NAC, including the ratios of monocyte and neutrophil and the percentages of CD4+/CD8+, CD8+ CD3+, CD3+ and CD4+ CD3+ lymphocytes. And after NAC, there were no differences between the two groups in the ratios of monocyte and neutrophil and the percentages of CD4+/CD8+ and CD4+ CD3+ lymphocytes. However, both before and after NAC, the ratios of lymphocyte were significantly higher in pCR group than that in non-pCR group (31.53 ± 5.01 vs. 25.68 ± 8.93 p < 0.01, 41.68 ± 8.40 vs. 32.54 ± 11.01 p < 0.01, respectively). Also, the percentages of CD3+ and CD8+ CD3+ lymphocytes were statistically increased in pCR group after NAC (69.36 ± 13.41 vs. 61.72 ± 13.37 p < 0.05, 32.56 ± 18.85 vs. 24.38 ± 9.52 p < 0.01, respectively). Additionally, the level of platelet before NAC tended to be lower in pCR group, but marked differences weren’t noted.
As shown in Table 3, multivariate analysis revealed that lymphocyte ratios, both before and after NAC, showed independent correlation with the pCR rate (p = 0.015 and p = 0.006, respectively). In addition, after divided into high or low lymphocyte ratio groups by the mean of 31.53% before-NAC or by the mean of 41.68% after-NAC, patients in high lymphocyte ratio group showed significantly better outcomes in median OS (high group vs. low group before NAC: 36 vs. 22 months, p < 0.001; high group vs. low group after NAC: 44 vs. 24 months, p < 0.001, respectively) (Fig. 2A,B).
Leukocyte subpopulation and lymphocyte subsets during NAC
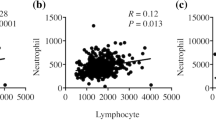
Subsequently, we examined the changes in the white blood cell subpopulation, including the ratios of monocyte and neutrophil and the count of platelet, during and after NAC until surgery. Since this was a retrospective study and the timing and frequency of blood tests were widely different from each patient, we plotted all the data of the entire patents according to the days from the initiation of NAC. The count of platelet tended to be slightly reduced during NAC (Fig. 3A) in the two groups, while the ratio of monocyte increased slightly during NAC and then gradually went down to the time of surgery (Fig. 3B). The proportion of neutrophil remained relatively stable during the treatment period (Fig. 3C) in both groups. In contrast, the ratio of circulation lymphocyte was markedly increased during NAC in the pCR group rather than non-pCR group (24.72 ± 0.56 vs. 35.51 ± 0.72, p < 0.001) (Fig. 4A,B). Consequently, the subsets of lymphocyte, including the percentages of CD3+, CD4+ CD3+, and CD8+ CD3+ lymphocytes, were examined before and after NAC among all patients in two groups (Fig. 5). Compared with the counterparts before NAC, the percentages of CD3+ (Fig. 5A) and CD4+CD3+ lymphocytes (Fig. 5B) were significantly increased after NAC in both groups (CD3+: pCR group 68.98 ± 13.32 vs. 49.89 ± 14.15, non-pCR group 61.73 ± 13.37 vs. 53.28 ± 12.39, p < 0.001 and CD4+CD3+: pCR group 37.16 ± 12.85 vs. 30.89 ± 11.56, non-pCR group 37.17 ± 9.77 vs. 32.12 ± 9.42, p < 0.001, respectively), while the percentage of CD8+CD3+ lymphocyte (Fig. 5C) was dramatically elevated only in pCR group (31.81 ± 18.38 vs. 19.04 ± 6.53, p < 0.001). Meanwhile, a higher growing of CD3+ and CD8+CD3+ lymphocyte percentages was spotted in pCR group than in non-pCR group after NAC (CD3+: 68.96 ± 13.32 vs. 61.74 ± 13.37, p < 0.05; CD8+CD3+: 31.81 ± 18.38 vs. 24.38 ± 9.52, p < 0.001, respectively).
Discussion
NAC is considered the standard treatment for patients with AGC, providing a theoretical advantage over adjuvant chemotherapy22,23. Unfortunately, the overall response rate for AGC patients to NAC is still disappointed, and the pCR event after NAC is very rare around the world10. More recently, we clarified that the pCR rate for patients with AGC underwent NAC was approximate 17.7%, which was higher than other reports (8.4–17.4%)13,14,24,25,26. However, factors predicting the efficacy of NAC, which would be essential for the optimal management of patients with AGC, have yet to be fully elucidated. Thus, we hypothesized that blood cell counts and their subsets, which presumably reflect host immune condition, may critically affect responsiveness to NAC and OS in these patients.
In the present study, we first demonstrated the positive correlation between the pCR rate and the high levels of circulating lymphocyte ratio before and after NAC in patients with AGC. It is believed that lymphocyte, which possesses potent anticancer activities that are able to inhibit growth and metastasis in several tumors including gastric cancer, plays critical roles in host immune responses and anti-tumor immunity27. Kitayama et al. indicated that peripheral blood lymphocyte has a significant impact on the complete response to radiotherapy or neoadjuvant chemoradiotherapy (NCR) in rectal cancer patients and the maintenance of circulating lymphocyte number may improve the response to NCR in rectal cancer28,29. It is thus reasonable to speculate that the lymphocyte-mediated immune response against damaged tumor cells is crucial for achieving pCR after NAC in patients with AGC.
We also showed that NAC could influence the immune responses in the host, including the increasing of the ratio of lymphocyte and its subpopulation. Several in vivo studies have suggested that cancer cells, dead or dying due to radiotherapy and/or chemotherapy, can present tumor-associated antigens to host immune cells and thereby evoke anti-tumor immune responses30,31. This could be a reasonable explanation for the results in the present study that the lymphocyte ratio was dramatically increased during NAC in pCR group rather than that in the non-pCR group, although NAC is historically regarded as detrimental to immunity because of its myelosuppressive effects. Furthermore, this change has been demonstrated in other vitro studies. A significant proliferation and differentiation of the immune cells was observed after the activation of them (initial stage), and the differentiated lymphocytes are occasionally joined as two or three cells unit to perform the unique function, searching for and then attaching to tumor cells to prevent their outgrowth. These immune cells are also actively involved in the immune surveillance to avoid the metastasis of the tumor32.
Most recently, several studies have indicated that low lymphocyte-to-white blood cell ratios33, low lymphocyte-to-monocyte ratios34, high platelet-to-lymphocyte ratios (PLR)21, and increased neutrophil-to-lymphocyte ratios (NLR)35,36 were associated with the poor prognosis in patients with AGC. And the similar results were also observed in the present study. Before NAC, patients with low NLR (<2.2) had significantly better outcome in OS (48 vs. 28 months, P < 0.001) (Supplementary Figure 1), and longer OS time was found as well among patients with low PLR (<696) (36 vs 25 months, P < 0.001) (Supplementary Figure 2). Besides, we also revealed that the ratio of lymphocyte increased gradually during NAC, and other subsets of blood cells stabilized at the same levels compared with their counterparts before NAC. Thus, the combination of the conclusions from these studies and our results may convince our hypothesis indirectly that lymphocyte should be the key point in all these changes, and the increasing of its ratio would be positively associated with pCR and improve OS in advanced gastric cancer.
Consequently, we examined the subsets of lymphocyte to illuminate which part was the primary factor for the increased lymphocyte ratio. Our results indicated that the percentages of CD8+CD3+ and CD3+ lymphocytes were dramatically increased during NAC, but the correlation between them and the pCR event was not confirmed by multivariate analysis, except the ratios of lymphocyte before and after NAC. Theoretically, chemotherapy-induced transient lymphopenia can stimulate the production of more tumor-specific T cells, thereby eradicating inhibiting regulatory T cells, which result in an increased CD8+CD3+ cells tumor homeostasis and activity37,38. Thus, the influence on the pCR rate and OS may be caused by the co-work of CD3+ and CD8+CD3+ lymphocytes, which reflects the increase in the circulating lymphocyte ratio.
Also, we revealed that OS was significantly improved in patients with high lymphocyte ratios both before and after NAC. Recently, Feng et al. determined that the low lymphocyte ratio and the high monocyte ratio were each predictive of a poor prognosis in stage II/III gastric cancer patients receiving radical D2 gastrectomy, and exhibited greater prognostic value when considered in combination33. A meta-analysis by Gu et al. showed that the elevated platelet to lymphocyte ratio could be a significant prognostic biomarker for poor OS in patients with gastric cancer21. Therefore, the improvement of OS in patients with AGC receiving NAC could be partly due to an ameliorated immune response.
We acknowledge that the retrospective evaluation of the immune cell population is a limitation of our study, and the potential calculation bias cannot be excluded. However, the significant association between the circulating lymphocyte ratio and the pCR rate supports the hypothesis that total eradication of tumor cells after NAC for AGC is dependent, at least in part, on host immune reaction. Enhancing lymphocyte-mediated immunity during chemotherapy may be a lead to the improvement of the clinical efficacy of NAC in AGC patients. Our observations warrant validation in large, independent cohorts.
In conclusion, the high ratios of circulating lymphocyte, both before and after NAC, have significant impacts on chemosensitivity and improve the OS in patients with AGC, which may be considered as new prognostic biomarkers.
Materials and Methods
Patients
This study was performed at a single institution using a retrospective design. From January 2005 to December 2011, patients with AGC who received NAC, at the department of general surgery, Jinling Hospital, were reviewed from our database. The eligibility criteria were: 1) histologically proven gastric cancer, 2) full-text clinical record, 3) surgical resection following NAC for AGC, 4) no prior anti-tumor therapy. All cases were diagnosed by endoscopic biopsy and evaluated by contrast-enhanced computed tomography (CT) scan and endoscopic ultrasound. Laparoscopy combined with peritoneal cytology was performed in patients with potentially liver and peritoneal metastases. The TNM classification of malignant tumors 7th (TNM 7th)39 is used for preoperative staging, and the number of lymph node stations was determined according to JCGC (Japanese classification of gastric carcinoma, 3rd English edition)40. pCR was defined as no tumor cells were detected at both the primary site and regional lymph nodes on pathological examination.
This study was approved by the Ethics Committee of Jinling Hospital according to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000)41, and written informed consent was obtained from the patients before both NAC and surgery. All patients were followed up by phone call or SMS and explained clearly that data collected will be intended for publication. There were no experimental animals or human participants included in the current study. All methods were performed in accordance with the relevant guidelines and regulations.
Treatment protocol and follow-up
All patients received 2 cycles of NAC with a regimen of 5-Fu/leucovorin/etoposide/oxaliplatin/epirubicin (FLEEOX) combination via intravenous and intra-arterial administration. 5-Fu (370 mg/m2) and leucovorin (200 mg/m2) were administered by intravenous infusion on day 1–5. Intra-arterial administration of etoposide (80 mg/m2), oxaliplatin (80 mg/m2) and epirubicin (30 mg/m2) was performed by Seldinger method on day 6 and 20, and the catheter was inserted through femoral artery into the celiac artery and the chemicals were injected initially at relatively high doses, followed by 14 days’ rest. The chemotherapeutic response was evaluated using contrast-enhanced CT scan by two experienced radiologists, who were blinded to any of the clinical data independently, according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines 1.142.
The patients evaluated as resectable underwent gastrectomy with D2 lymphadenectomy. D2+ lymphadenectomy would be performed if preoperative enhanced CT scan shows N3 lymph node metastases.
After surgery, all patients received 6 cycles adjuvant chemotherapy with the regimen of XELOX, oxaliplatin (130 mg/m2) on day 1 and xeloda (1000 mg/m2) on days 1 to 14 of a 28-day cycle. Patients who were subjected to recurrence would change to other regimens including adjuvant radiotherapy and/or best supportive care. For adjuvant radiotherapy, blood routine test, hepatic and renal function test and CT scan were performed before radiotherapy. Total doses ranged from 45 to 51 Gy (median 48 Gy).
All patients were followed every 3 months after adjuvant chemotherapy according to the institutional protocol. Tumor markers including CEA, CA19-9 were examined every 3 months. Chest X-ray and abdominal/pelvic enhanced CT scan were performed every 6 months. Gastroscopy was also required each year. Positron emission tomography computed tomography scan was suggested when recurrence was suspected.
Blood cell counts and lymphocyte subsets
Venous blood samples (20 ml) were drawn into heparinized tubes from patients pre- and post-chemotherapy. The absolute white blood cell and the ratios of neutrophils and lymphocyte were determined with an automated cell counter (Beckman LH750, USA). To assess the cellular immunity, peripheral blood mononuclear cells were measured by flow cytometry (BD Biosciences, San Jose, CA) and were analyzed using CellQuest software (BD Biosciences). Cells were stained with fluorescein-labeled monoclonal antibodies: CD3-allophycocyanin, CD4-fluorescein isothiocyanate, and CD8-phycoerythrin (BD Pharmingen, San Diego, CA).
Statistical analysis
Statistical analyses were performed using SPSS version 24.0 for MAC (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 7.0 for MAC (GraphPad Software Inc., San Diego, CA, USA). The associations of pCR with blood cell counts and various other clinical parameters were examined using Wilcoxon’s test and the chi-squared test, respectively. Multivariate stepwise logistic regression analysis was performed to determine the independence of all variables identified as possibly significant. Before and after NAC changes of the factors within each group were tested using a paired t-test. OS was calculated from the date of cases registered to the date of death or date was last known alive. Survival curves were calculated using the Kaplan-Meier method and compared by the log-rank (Mantel–Cox) test. The association of clinical factors with pathological response to NAC was assessed using logistic models. P values < 0.05 were considered to be significant.
Data available statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The ethics committee of the Medical School of Nanjing University (2009028). Chinese Clinical Trials Registry Number: ChiCTR-TRC-12002046. All patients were followed up by phone call or SMS and explained clearly that data collected will be intended for publication. All methods were performed in accordance with the relevant guidelines and regulations.
References
Parkin, D. M., Bray, F. I. & Devesa, S. S. Cancer burden in the year 2000. The global picture. Eur J Cancer 37(Suppl 8), S4–66 (2001).
He, J. et al. Major causes of death among men and women in China. N Engl J Med 353, 1124–1134, https://doi.org/10.1056/NEJMsa050467 (2005).
Lin, Y. et al. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol 17, 4421–4428, https://doi.org/10.3748/wjg.v17.i39.4421 (2011).
Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J Clin 66, 115–132, https://doi.org/10.3322/caac.21338 (2016).
Chen, W. et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res 25, 10–21, https://doi.org/10.3978/j.issn.1000-9604.2012.12.04 (2013).
Zhang, Q. et al. Training in early gastric cancer diagnosis improves the detection rate of early gastric cancer: an observational study in China. Medicine (Baltimore) 94, e384, https://doi.org/10.1097/MD.0000000000000384 (2015).
Yamao, T. Rationale for Neoadjuvant Chemotherapy for Advanced Gastric Cancer. (Springer Japan, 1999).
Ajani, J. A. et al. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst 85, 1839–1844 (1993).
Fujitani, K. et al. Impact of induction chemotherapy and preoperative chemoradiotherapy on operative morbidity and mortality in patients with locoregional adenocarcinoma of the stomach or gastroesophageal junction. Ann Surg Oncol 14, 2010–2017, https://doi.org/10.1245/s10434-006-9198-2 (2007).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355, 11–20, https://doi.org/10.1056/NEJMoa055531 (2006).
Fuentes, E. et al. Adjuvant Therapy Completion Rates in Patients with Gastric Cancer Undergoing Perioperative Chemotherapy Versus a Surgery-First Approach. J Gastrointest Surg 20, 172–179, https://doi.org/10.1007/s11605-015-2954-5 (2016). discussion 179.
Ychou, M. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29, 1715–1721, https://doi.org/10.1200/JCO.2010.33.0597 (2011).
Fields, R. C. et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer 104, 1840–1847, https://doi.org/10.1038/bjc.2011.175 (2011).
Lorenzen, S. et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol 24, 2068–2073, https://doi.org/10.1093/annonc/mdt141 (2013).
Becker, K. et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 253, 934–939, https://doi.org/10.1097/SLA.0b013e318216f449 (2011).
Lowy, A. M. et al. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg 229, 303–308 (1999).
Maeta, M. et al. Perioperative allogeneic blood transfusion exacerbates surgical stress-induced postoperative immunosuppression and has a negative effect on prognosis in patients with gastric cancer. J Surg Oncol 55, 149–153 (1994).
Lee, W. J., Chang, K. J., Lee, C. S. & Chen, K. M. Selective depression of T-lymphocyte subsets in gastric cancer patients: an implication of immunotherapy. J Surg Oncol 55, 165–169 (1994).
Ruffell, B., DeNardo, D. G., Affara, N. I. & Coussens, L. M. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev 21, 3–10, https://doi.org/10.1016/j.cytogfr.2009.11.002 (2010).
Shen, Z. et al. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol 136, 1585–1595, https://doi.org/10.1007/s00432-010-0816-9 (2010).
Gu, X. et al. Clinicopathological and prognostic significance of platelet to lymphocyte ratio in patients with gastric cancer. Oncotarget 7, 49878–49887, https://doi.org/10.18632/oncotarget.10490 (2016).
Urschel, J. D., Vasan, H. & Blewett, C. J. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg 183, 274–279 (2002).
Partridge, S. C. et al. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 179, 1193–1199, https://doi.org/10.2214/ajr.179.5.1791193 (2002).
Zhang, Y., Peng, Z. & Chen, L. [Survival analysis of gastric cancer cases with pathological complete response received neoadjuvant chemotherapy]. Zhonghua Yi Xue Za Zhi 96, 1582–1584, https://doi.org/10.3760/cma.j.issn.0376-2491.2016.20.008 (2016).
Stahl, M. et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27, 851–856, https://doi.org/10.1200/JCO.2008.17.0506 (2009).
Lorenzen, S. et al. Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol 18, 1673–1679, https://doi.org/10.1093/annonc/mdm269 (2007).
Biyik, M. et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 25, 435–441, https://doi.org/10.1097/MEG.0b013e32835c2af3 (2013).
Kitayama, J., Yasuda, K., Kawai, K., Sunami, E. & Nagawa, H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol 5, 47, https://doi.org/10.1186/1748-717X-5-47 (2010).
Kitayama, J., Yasuda, K., Kawai, K., Sunami, E. & Nagawa, H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer 11, 64, https://doi.org/10.1186/1471-2407-11-64 (2011).
Lorimore, S. A., Coates, P. J., Scobie, G. E., Milne, G. & Wright, E. G. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene 20, 7085–7095, https://doi.org/10.1038/sj.onc.1204903 (2001).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13, 1050–1059, https://doi.org/10.1038/nm1622 (2007).
Liu, X. Y., Pestka, S. & Shi, Y. F. Recent Advances in Cancer Research and Therapy. (Elsevier Inc. and Tsinghua Uviversity Press, 2013).
Feng, F. et al. Low lymphocyte-to-white blood cell ratio and high monocyte-to-white blood cell ratio predict poor prognosis in gastric cancer. Oncotarget, https://doi.org/10.18632/oncotarget.14136 (2016).
Cong, X., Li, S. & Xue, Y. Impact of preoperative lymphocyte to monocyte ratio on the prognosis of the elderly patients with stage II(-III)gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 19, 1144–1148 (2016).
Musri, F. Y. et al. The Neutrophil to Lymphocyte Ratio is an Independent Prognostic Factor in Patients with Metastatic Gastric Cancer. Asian Pac J Cancer Prev 17, 1309–1312 (2016).
Ock, C. Y. et al. Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer 20, 254–262, https://doi.org/10.1007/s10120-016-0613-5 (2017).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8, 59–73, https://doi.org/10.1038/nri2216 (2008).
Zitvogel, L. et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 118, 1991–2001, https://doi.org/10.1172/JCI35180 (2008).
Ainbinder, D. J., Esmaeli, B., Groo, S. C., Finger, P. T. & Brooks, J. P. Introduction of the 7th edition eyelid carcinoma classification system from the American Joint Committee on Cancer-International Union Against Cancer staging manual. Arch Pathol Lab Med 133, 1256–1261, https://doi.org/10.1043/1543-2165-133.8.1256 (2009).
Japanese Gastric Cancer, A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14, 101–112, https://doi.org/10.1007/s10120-011-0041-5 (2011).
Wells, F. The World Medical Association revises the Declaration of Helsinki. International Journal of Pharmaceutical Medicine 14, 253–253 (2000).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45, 228–247, https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Acknowledgements
The authors would like to thank Mrs. Weiming Yang, Nanjing University of Chinese Medicine, for the English language review.
Author information
Authors and Affiliations
Contributions
Yang Li, Yao Wei and Guoli Li conceived and designed this study. Yang Li and Yao Wei wrote this manuscript. Qi He and Xulin Wang searched the literatures. Qi He and Chaogang Fan inputted the data. Yang Li, Yao Wei and Chaogang Fan analyzed the data. Guoli Li revised this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Wei, Y., He, Q. et al. Clinicopathological and prognostic significance of high circulating lymphocyte ratio in patients receiving neoadjuvant chemotherapy for advanced gastric cancer. Sci Rep 8, 6223 (2018). https://doi.org/10.1038/s41598-018-24259-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24259-5
- Springer Nature Limited
This article is cited by
-
Predictive value of NLR, TILs (CD4+/CD8+) and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer
Cancer Immunology, Immunotherapy (2022)