Abstract
Fc receptors are known to have a pivotal role in the initiation and regulation of many immunological and inflammatory processes. This study aimed to investigate the association of Fc receptor family gene polymorphisms with ocular Behçet’s disease (BD) in Han Chinese. A two stage case–control study was performed in 1022 BD cases and 1803 healthy controls. Twenty-three SNPs were genotyped using the MassARRAY system (Sequenom), TaqMan SNP Genotyping Assay and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The expression of FCGR3A was examined by real-time PCR and cytokine production was measured by enzyme linked immunosorbent assay (ELISA). A significantly higher frequency of the FCGR3A/rs428888 CT genotype (Pc = 1.96 × 10−7, OR = 1.897) and a lower frequencies of CC genotype and C allele (Pc = 1.96 × 10−7, OR = 0.527; Pc = 7.22 × 10−7, OR = 0.554 respectively) were found in ocular BD as compared with controls. Functional experiments showed an increased FCGR3A expression (P = 0.005) and increased cytokine protein expressions of MCP-1, IL-1β and TNF-α by LPS stimulated PBMCs in CT carriers of FCGR3A rs428888 compared to CC carriers (P = 0.034; P = 0.025; P = 0.04; respectively). Our findings demonstrate that FCGR3A/rs428888 confers genetic susceptibility for ocular BD in Han Chinese.
Similar content being viewed by others
Introduction
Behçet’s disease (BD) is a systemic autoinflammatory disease featuring recurrent oral aphthae, relapsing bilateral uveitis, typical skin lesions and genital ulcers. However, many other organs including the vascular, neurological and musculoskeletal systems can also be affected1,2,3. It is primarily prevalent in populations from Asia and the Middle East4. To date, the exact pathogenesis of BD remains unknown, although genetic factors coupled with a triggering event may play a crucial role in its development5,6,7,8.
BD is generally regarded as a T cell mediated disorder9,10,11,12. Recent studies have however suggested an additive role for B cells in its pathogenesis13,14,15. As receptors for the Fc domain of immunoglobulins (Ig), Fc receptors (FcRs) are extensively distributed on various types of immune cells, and are involved in a variety of innate and adaptive immune responses. They promote the activation of B cells and mediate the specific recognition of antigens by leucocytes16. The Fc receptor family has a large number of members with different structures and functions, which are composed of six main subfamilies, according to their binding of specific immunoglobulin isotypes: FcA receptors (IgA), FcG receptors (IgG), FcD receptors (IgD), FcE receptors (IgE), FcM receptors (IgM) and FcR-like receptors (FcRLs)17,18.
Fc receptors are known to have a pivotal role in the initiation and regulation of many immunological and inflammatory processes, and gene polymorphisms identified so far, contribute to the development of a range of chronic inflammatory and autoimmune diseases19,20,21,22,23,24,25,26,27. Previous studies showed that FcAR was associated with the genetic predisposition to granulomatosis with polyangiitis (GPA), and susceptibility to a serious renal disorder19. FCGRs polymorphisms have been shown to play a role in the development of SLE20 and FCGR2 was reported to be associated with immune mediated diseases like type I diabetes, celiac disease (CD) and Kawasaki disease21,22. FCRL3 variants can confer risk to multiple sclerosis (MS) and RA23,24, and FCRL4 is associated with ankylosing spondylitis (AS) in Chinese Han25. The association between genetic polymorphisms of Fc receptors with BD has been tested in relatively small cohorts of BD patients and did not encompass the complete family of Fc receptor genes26,27. We therefore decided to expand these studies using a complete set of currently known Fc receptor gene polymorphisms in a large cohort of Chinese Han ocular BD patients.
Results
Features of recruited cases
The demographic characteristics and clinical features of the 1022 recruited ocular BD patients and 1803 controls are shown in Table 1. All cases had uveitis. The mean age of the BD patients was 34.1 ± 8.4 years (range, 9–61 years), and included 861 males (84.2%) and 161 females (15.8%). Twenty-three SNPs of various Fc receptor genes were genotyped successfully and did not deviate from the Hardy-Weinberg equilibrium in healthy controls.
Genotyping results in the first phase study
Polymorphisms of Fc receptor genes were investigated in 449 BD cases and 658 healthy controls for the first-phase study. A decreased frequency of the FCGR3A/rs428888 CC genotype was found in BD (Pc = 2.02 × 10−2, OR = 0.551) (Table 2). The frequency of the FCGR3A/rs428888 CT genotype in cases was significantly higher compared to controls (Pc = 2.02 × 10−2, OR = 1.814) (Table 2). No statistically significant association was found between the other 22 SNPs tested and ocular BD (Supplementary Table S1). Linkage disequilibrium(LD) was estimated for the five SNPs of FCGR3A using our own data, and showed that there was not a strong LD between the tested SNPs (Table S2, Fig. S1).
Genotyping results in the second phase study and combined study
To validate the significant association of the FCGR3A gene polymorphism with BD found in the first phase study, another set of 573 BD cases and 1145 healthy controls were evaluated in a second phase test. The results confirmed the lower frequencies of the FCGR3A/rs428888 CC genotype and C allele in BD (Pc = 1.63 × 10−4, OR = 0.516; Pc = 3.60 × 10−4, OR = 0.543 respectively) and the higher frequency of the CT genotype (Pc = 1.63 × 10−4, OR = 1.939) (Table 2). The combined data of the two studies showed a stronger significant association of rs428888 in FCGR3A with BD. (CC genotype: Pc = 1.96 × 10−7, OR = 0.527; C allele: Pc = 7.22 × 10−7, OR = 0.554; CT genotype: Pc = 1.96 × 10−7; OR = 1.897 respectively). (Table 2)
mRNA expression and downstream inflammatory factors
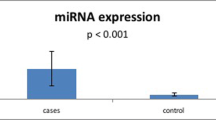
In view of the results shown above, we tested the biological function of the various genetic variants of FCGR3A. This was done by measuring the expression of FCGR3A in PBMCs obtained from healthy genotyped controls since the presence of systemic inflammation as well as immunosuppressive drug treatment of the BD patients might affect gene expression. CT carriers of FCGR3A rs428888 showed a significantly higher mRNA expression of FCGR3A than CC carriers when testing unstimulated PBMCs (P = 0.005) (Fig. 1). Male individuals also showed a higher mRNA expression of FCGR3A than females when testing unstimulated PBMCs (P = 0.042) (Fig. 2).
The effect of different genotypes of rs428888 on cytokine protein production was tested in LPS stimulated PBMCs. We tested the PBMC expression of a number of cytokines, that have been shown to play a role in BD pathogenesis including TNF-α, IFN-γ, IL-1β, IL-8, IL-10 and MCP-1. A higher cytokine protein expression of MCP-1, IL-1β and TNF-α by stimulated PBMCs was found in CT carriers as compared to CC carriers (P = 0.034, P = 0.025, P = 0.04; respectively Fig. 3a–c). The other cytokines tested were not affected by the rs428888 genotype (Fig. 3d–f).
The influence of rs428888 on cytokine production. The production of MCP-1 (a), IL-1beta (b), TNF-α (c), IFN-γ (d), IL-8 (e), IL-10 (f) by LPS stimulated PBMCs from healthy controls carrying different genotypes of rs428888. (male: n = 16 (CT: n = 6; CC: n = 10), female: n = 5 (CT: n = 3; CC: n = 2)).
Discussion
The present study shows that ocular BD in Chinese Han is associated with FCGR3A rs428888. Individuals carrying the CT genotype of FCGR3A rs428888 had a higher risk of developing BD, whereas the CC genotype of this locus provided protection against BD. Further functional studies indicated that mRNA expression of FCGR3A was higher in carriers with the CT genotype of rs428888. Additionally, the protein expression of MCP-1, TNF-α and IL-1β by LPS stimulated PBMCs was significantly increased in carriers with the CT genotype of rs428888 as compared to those carrying the CC genotype.
An earlier study on the association of gene variants of the Fc receptor family in BD patients from a department of Rheumatology, showed a significant association with rs396991 of FCGR3A26 Our data showed no significant association between rs396991 of FCGR3A and BD in Chinese Han. This may be due to ethnic differences in BD risk or due the fact that all our patients came from a department of Ophthalmology. A Korean study tested a number of gene variants including FCGR3A in a cohort of 61 BD patients with severe uveitis and also found a significant association (p = 0.047) with FCGR3A/rs39699127. The association was weak and the authors did not correct for multiple comparisons.
It has been shown that Fc receptors play a major role in orchestrating cytokine production and maintaining immune homeostasis28. Moreover, multiple genetic variations have been identified for the various Fc receptors, in particular the FCGRs, which affect receptor function and were shown to be associated with several autoimmune diseases16. A meta-analysis showed that an FCGR3A polymorphism was found to be associated with RA in Europeans but not in Asians29. Another family-based study was performed in 119 SLE cases and 316 family members of these cases and the results showed that FCGR3A/rs428888 was involved in genetic susceptibility to SLE in Chinese30.
Fc receptor-like genes (FCRLs) which are homologous to the FCGRs in structure, contain six Ig superfamily members that are known as FCRL1–FCRL6 according to their chromosomal order31. Previous studies showed that polymorphisms of FCRLs were involved in the development of many autoimmune disorders such as rheumatoid arthritis (RA), autoimmune thyroid disease (AITD) and Graves’ disease32,33. Earlier studies from our research group showed a significant association with SNPs of FCRL3 in BD34. This gene was not repeated in the current study, but no significant associations were found for the other FCRLs tested.
The FCGR3A gene is located on chromosome 1q23, and interacts with the Fc domain of immunoglobulin G. It plays an important role in the clearance of immune complexes and in antibody dependent cellular cytotoxicity (ADCC)35. It is shown to be constitutively expressed as a transmembrane protein on NK cells, tissue specific macrophages, monocytes, dendritic cells and subsets of T cells36. It was reported that the number of NK cells in aqueous humor and peripheral blood of BD patients increases37,38 and NK cells have been considered to play an important role in controlling BD inflammation39,40. Whether FCGR3A variants affect NK cell function is not yet known and deserves further study. We performed functional tests using PBMCs and showed that the CT risk genotype of FCGR3A/rs428888 is associated with an increased FCGR3A gene expression. A higher expression of this receptor might play a role in the exaggerated inflammatory response to microbial antigens, which is currently one of the mechanisms thought to cause BD. We additionally showed that healthy carriers of the CT genotype had an increased production of TNF-α, IL-1beta and MCP-1 by LPS stimulated PBMCs. LPS can activate many cells related to inflammation, induce the release of proinflammatory cytokines and may eventually lead to systemic inflammation41,42.TNF-α and IL-1beta have been regarded as inflammatory markers and important regulators of Th17 cell differentiation. Th17 cells are defined by their ability to produce IL-17 besides other proinflammatory cytokines43, which all play important roles in the pathogenesis of BD. Our findings are in agreement with previous findings showing that the MCP-1 level in active BD patients is much higher than in normal controls44.
Our study has a number of limitations. First of all, the FCGR3A gene expression was only examined in healthy genotyped individuals, since our patients show a variable degree of systemic inflammation and are often under treatment with immunosuppressive agents, which might affect the response of PBMCs. Further functional experiments concerning FCGR3A expression in genotyped BD patients with varying degrees of disease severity are required to address this subject. We tested PBMCs and future functional analysis using isolated cell populations may shed more light on which cell types are especially relevant concerning Fc receptor genes expression. It should be noted that most of our patients are male and that the control group was not exactly matched for gender. As yet we did observe an effect of gender on FCGR3A expression. As mentioned above, all our patients had uveitis and came from a department of ophthalmology and further multidisciplinary study should be performed to investigate whether the observed association is dependent on possible subtypes of BD. It should also be noted that we tested all currently known SNPs as reported in earlier studies concerning the association of FcR family genes with autoimmune or autoinflammatory diseases and it is possible that we might have missed unknown SNPs. Furthermore, only common variants were examined and the potential correlation between rare genetic variants and BD will necessitate detailed sequence analysis.
In conclusion, our results show that FCGR3A/rs428888 confers genetic susceptibility for ocular BD in Chinese Han. The risk genotype of FCGR3A/rs428888 regulates FCGR3A expression and production of the proinflammatory cytokines IL-1β, TNF-α and MCP-1.
Materials and Methods
Case-control cohorts
In the present study, all participants including 1022 BD cases and 1803 controls came from the Department of Ophthalmology of the First Affiliated Hospital of Chongqing Medical University and were recruited between April 2008 to October 2015. BD cases were diagnosed in accordance with the International Study Group criteria45. In parallel, random control groups were matched geographically and ethnically with the cases. A two stage case–control study was performed. There were 449 BD cases and 658 normal controls in the first stage. For the validation of data obtained in the first study we used a different set of 573 cases and 1145 controls for the second stage. Written informed consent was obtained from all participants and the study was approved by the First Affiliated Hospital of Chongqing Medical University Clinical Ethics Research Committee. All experimental procedures were conducted in line with guidelines and regulations, and abided by the tenets of the Declaration of Helsinki.
Single nucleotide polymorphisms (SNPs) selection
SNPs were selected on the basis of previously published reports concerning the association between FcR gene polymorphisms with autoimmune or autoinflammatory disorders16,21,22,23,24,25,26,27,28,29,30. Haploview 4.2 software was used to analyze Minor Allele Frequency (MAF) and Linkage disequilibrium (LD) (the MAF was required to be greater than 0.05 in Chinese Han Beijing data, and an r2 threshold of 0.8 in LD). By following these above principles, we chose twenty-three SNPs of FC receptor family genes including FCGR2A/(rs1801274, rs6658353, rs10800309), FCGR2B/(rs1050501, rs10917661, rs12118043, rs1249347), FCGR3A/(rs396991, rs10919543, rs403016, rs428888, rs486062), FCRL1/(rs4971154), FCRL4/(rs2777963, rs10489674, rs14335), FCRL5/(rs6427384, rs12036228, rs6679793, rs2012199, rs3811035, rs6692977), FCRLB/ (rs4657093). There are no data concerning rs428888 in a Chinese Han Beijing population in the HapMap database and 1000genomes. FCGR3A/rs428888 was shown to be associated with SLE in Chinese Han30. Since there were no literature reports concerning the association of autoinflammatory disorders with gene polymorphisms of FCDR, FCER and FCMR, the study did not cover these genes. SNPs of FCRL3 were also not included in this study because these data have been reported previously by our group32.
SNP genotyping
Genomic DNA from peripheral blood leukocytes of all the participants was isolated with the QIAamp DNA Blood Mini Kit (QIAGEN Valencia,CA,USA) according to the manufacturer’s instructions. In the first phase, the majority of SNPs were genotyped using the MassARRAY system platform (Sequenom Inc, CA, USA) and iPLEX Gold Assay. Only rs6658353 was genotyped by TaqMan® SNP Genotyping Assay in the Real-Time PCR system (Applied Biosystems, USA). The genotyping results were respectively analyzed using TYPER Software and TaqMan Genotyper Software. For the second phase study, genotyping were performed by PCR-restriction fragment length polymorphism (PCR-RFLP) method.
Real-time PCR
Peripheral blood mononuclear cells (PBMCs) of healthy controls were separated by Ficoll-Hypaque density-gradient centrifugation, and cultured with lipopolysaccharide (LPS,100ng/ml, Sigma, Missouri, USA) for 24 hours at a density of 1 × 106 cells/ml. The whole RNA was extracted from LPS-stimulated and non-stimulated PBMCs using TRIzol reagent (Invitrogen, San Diego, California, USA) and the Prime Script RT reagent Kit (TaKaRa, Dalian, China) was used for reverse transcription. FCGR3A gene mRNA expression(primer:5′-GCAGCTAGAAGTCCATATCGG-3′ and 5′-CTTCCTGCCTTTGCCATTCTG-3′) and β-actin gene expression(primer: forward 5′-GGATGCAGAAGGAGATCACTG-3′and reverse5′-CGATCCACACGGAGTACTTG-3′) were conducted on an ABI 7500 real-time system. Relative gene expression levels were measured using the 2−ΔΔCt method after data were normalized to mRNA β-actin.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of TNF-α, IL-8, IFN-γ, IL-10, IL-1β and MCP-1 in culture supernatants of PBMCs were examined using human Duoset ELISA development kits (R&D Systems, Minneapolis, USA).
Statistical analysis
Hardy-Weinberg equilibrium (HWE) was measured through the SHEsis website. Differences of genotype and allele frequencies between cases and controls were analyzed using the χ2 test with the SPSS 17.0 software. The p values were corrected to Pc with the method of Bonferroni for correction of multiple comparisons, and it was considered to be significant when Pc was less than 0.05. FCGR3A expression levels and various cytokines between the two genotype groups were analyzed by the independent samples T-test or nonparametric Mann-Whitney U test.
References
Yang, P. et al. Clinical features of Chinese patients with Behcet’s disease. Ophthalmology. 115, 312–318 (2008).
Commodaro, A. G., Bueno, V., Belfort, R. Jr. & Rizzo, L. V. Autoimmune uveitis: the associated proinflammatory molecules and the search for immunoregulation. Autoimmun Rev. 10, 205–209 (2011).
Gul, A. Behcet’s disease: an update on the pathogenesis. Clinical and experimental rheumatology. 19, S6–12 (2001).
Khairallah, M., Accorinti, M., Muccioli, C., Kahloun, R. & Kempen, J. H. Epidemiology of Behcet disease. Ocul Immunol Inflamm. 20, 324–335 (2012).
Pineton, de et al. New insights into the pathogenesis of Behcet’s disease. Autoimmun Rev. 11, 687–98 (2012).
Hou, S. et al. Two-stage association study in Chinese Han identifies two independent associations in CCR1/CCR3 locus as candidate for Behçet’s disease susceptibility. Hum Genet. 131, 1841–50 (2012).
Remmers, E. F. et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R, IL12RB2 regions associated with Behcet’s disease. Nature genetics. 42, 698–702 (2010).
Meguro, A. et al. Genetics of Behcet disease inside and outside the MHC. Ann.Rheum. Dis. 69, 747–754 (2010).
Melikoglu, M., Kural-Seyahi, E., Tascilar, K. & Yazici, H. The unique features of vasculitis in Behcet’s syndrome. Clin Rev Allerg Immu. 35, 40–46 (2008).
Kim, J. et al. Imbalance of Th17 to Th1 cells in Behcet’s disease. Clin. Exp Rheumatol. 28, S16–19 (2010).
Amadi-Obi, A. et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 13, 711–718 (2007).
Mantas, C., Direskeneli, H., Eksioglu-Demiralp, E. & Akoglu, T. Serum levels of Th2 cytokines IL-4 and IL-10 in Behcet’s disease. J. Rheumatol. 26, 510–512 (1999).
Belguendouz, H. et al. B cell activation during Behçet disease: Possible BAFF involvement in pathogenesis. Cytokine. 63, 247 (2013).
van der Houwen, T. B. et al. Chronic signs of memory B cell activation in patients with Behçet’s disease are partially restored by anti-tumour necrosis factor treatment. Rheumatology. 56, 134–144 (2017).
Sadreddini, S., Noshad, H., Molaeefard, M. & Noshad, R. Treatment of retinal vasculitis in Behçet’s disease with rituximab. Mod Rheumatol. 18, 306–8 (2008).
Nimmerjahn, F. & Ravetch, J. V. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 8, 34–47 (2008).
Hulett, M. D. & Hogarth, P. M. Molecular basis of Fc receptor function. Adv Immunol. 57, 1–127 (1994).
Ehrhardt, G. R. et al. Fc receptor-like proteins (FCRL): immunomodulators of B cell function. Adv exp med boil. 596, 155–162 (2007).
Kelley, J. M. et al. IgA and IgG antineutrophil cytoplasmic antibody engagement of Fc receptor genetic variants influences granulomatosis with polyangiitis. Proc Natl Acad Sci USA 108, 20736–20741 (2011).
Harley, I. T. et al. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 10, 285–290 (2009).
Behrooz, Z. A. et al. Association analysis of functional variants of the FcgRIIa and FcgRIIIa genes with type 1 diabetes,celiac disease and rheumatoid arthritis. Human Molecular Genetics. 16, 2552–2559 (2007).
Onouchi, Y. et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 44, 517–521 (2012).
Matesanz, F. et al. The high producer variant of the Fc-receptor like-3 (FCRL3) gene is involved in protection against multiple sclerosis. J Neuroimmunol. 195, 146–150 (2008).
Raychaudhuri, S. et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 41, 1313–1318 (2009).
Zeng, Z. et al. Association of FCRL4 polymorphisms on disease susceptibility and severity of ankylosing spondylitis in Chinese Han population. Clin Rheumatol. 31, 1449–1454 (2012).
Aksu., K. et al. FcgammaRIIa, IIIa and IIIb gene polymorphisms in Behçet’s disease: do they have any clinical implications? Clin Exp Rheumatol. 26, S77–83 (2008).
Kim, S. J. et al. Targeted resequencing of candidate genes reveals novel variants associated with severe Behçet’s uveitis. Exp Mol Med. 45, e49 (2013).
Guilliams, M., Bruhns, P., Saeys, Y., Hammad, H. & Lambrecht, B. N. The function of Fcγ receptors in dendritic cells and macrophages. Nature Reviews Immunology. 14, 94–108 (2014).
Lee, Y. H., Ji, J. D. & Song, G. G. Associations between FCGR3A polymorphisms and susceptibility to rheumatoid arthritis: a meta analysis. J Rheumatol. 35, 2129–35 (2008).
Pan, F. et al. Genetic susceptibility and haplotype analysis between Fcgamma receptor IIB and IIIA gene with systemic lupus erythematosus in Chinese population. Lupus. 17, 733–8 (2008).
Davis, R. S. et al. Fc receptor homologs: newest members of a remarkably diverse Fc receptor gene family. Immunol Rev. 190, 123–36 (2002).
Newman, W. G. et al. Rheumatoid Arthritis Association With the FCRL3 –169 C Polymorphism Is Restricted to PTPN22 1858T–Homozygous Individuals in a Canadian Population. Arthritis Rheum. 54, 12 (2006).
Wellcome Trust Case Control Consortium et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nature Genetics. 39, 1329–1337(2007).
Ke, L. et al. Association between polymorphisms of FCRL3, a non-HLA gene, and Behçet’s disease in a Chinese population with ophthalmic manifestations. Molecular Vision. 14, 2136–2142 (2008).
Takai, T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol. 25, 1–18 (2005).
Nimmerjahn, F. & Ravetch, J. V. Fc-receptors as regulators of immunity. Adv Immunol. 96, 179–204 (2007).
Suzuki, Y., Hoshi, K., Matsuda, T. & Mizushima, Y. Increased peripheral blood gamma deltaþ T cells and natural killer cells in Behcet’s disease. J Rheumatol. 19, 588–592 (1992).
Yu, H. G. et al. The number of CD8þ T cells and NKT cells increases in the aqueous humor of patients with Behcet’s uveitis. Clin Exp Immunol. 137, 437–443 (2004).
Ahn, J. K., Chung, H., Lee, D. S., Yu, Y. S. & Yu, H. G. CD8bright CD56þ T cells are cytotoxic effectors in patients with active Behcet’s uveitis. J Immunol. 175, 6133–6142 (2005).
Yamaguchi, Y. et al. Natural killer cells control a T-helper 1 response in patients with Behcet’s disease. Arthritis Res Ther. 12, R80 (2010).
Beutler, B. & Rietschel, E. T. Innate immune sensing andits roots:the story of endotoxin. Nat Rev Immunol. 3, 169–76 (2003).
Lu, Y. C., Yeh, W. C. & Ohashi, P. S. LPS/TLR4 signal transduction pathway. Cytokine. 42, 145–51 (2008).
Tesmer, L. A., Lundy, S. K., Sarkar, S. & Fox, D. A. Th17 cells in human disease. Immunol. Rev. 223, 87–113 (2008).
Hamzaoui, K. et al. Cytokine profile in Behcet’s diseas epatients.Relationship with disease activity. Scand.J. Rheumatol. 31, 205–210 (2002).
Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 335, 1078–80 (1990).
Acknowledgements
Thanks to all donors enrolled in the present study. This work was supported by National Key R&D Program of China(2016YFC0904000), Natural Science Foundation Major International (Regional) Joint Research Project (81720108009), Natural Science Foundation Major International (Regional) Joint Research Project (81320108009), Chongqing Key Laboratory of Ophthalmology (CSTC, 2008CA5003), National Key Clinical Specialties Construction Program of China, Chongqing Science & Technology Platform and Base Construction Program(cstc2014pt-sy10002)and Research fund for Traditional Chinese Medicine of Chongqing Health and Family Planning Commission (ZY201401013).
Author information
Authors and Affiliations
Contributions
Peizeng Yang and Donglei Zhang conceived the idea and designed the experiments. Donglei Zhang, Jieying, Lin Li, Guo Huang and Qingfeng Cao performed the experiments and analyzed the data. Donglei Zhang wrote the paper. Aize Kijlstra, Guannan Su and Peizeng Yang reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, D., Qin, J., Li, L. et al. Analysis of the association between Fc receptor family gene polymorphisms and ocular Behçet’s disease in Han Chinese. Sci Rep 8, 4850 (2018). https://doi.org/10.1038/s41598-018-23222-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23222-8
- Springer Nature Limited
This article is cited by
-
Alterations in immune cell phenotype and cytotoxic capacity in HER2+ breast cancer patients receiving HER2-targeted neo-adjuvant therapy
British Journal of Cancer (2023)
-
Identification of novel genes in Behcet’s disease using integrated bioinformatic analysis
Immunologic Research (2022)