Abstract
Understanding the rhizodeposited carbon (C) dynamics of winter wheat (Triticum aestivum L.), is crucial for soil fertility and C sequestration. Pot-grown winter wheat was pulse labelled with 14CO2 at the key growth stages. 14C in the shoots, roots and soil was measured at 5 or 2 days after 14C-labelling (DAL 5/2) at each growth stage and at harvest. The 14C in the shoots increased from 4% of the net 14C recovered (shoots + roots + soil) during tillering to 53% at harvest. Approximately 14–34% of the net 14C recovered was incorporated into the soil. Allocation of photosynthesized C was extrapolated from the pot experiment to field condition, assuming a planting density of 1.8 million plants ha−1. The estimated C input to the soil was 1.7 t C ha−1, and 0.7 t C ha−1 of root residues was retained after wheat harvest; both values were higher than those previously reported (0.6 and 0.4 t C ha−1, respectively). Our findings highlight that C tracing during the entire crop season is necessary to quantify the temporal allocation of photosynthesized C, especially the contribution to soil carbon in intensified farming system.
Similar content being viewed by others
Introduction
Wheat covers approximately 220 million hectares of farmland worldwide1 and plays an important role in food supply and soil organic carbon (SOC) regulation2. SOC is primarily derived from plants3, such as deposition of both crop straw4 and root litter5,6, as well as rhizodeposits3. Rhizodeposits include root exudates and other root-borne organic substances released into the rhizosphere during plant growth as well as sloughed root hairs and decaying root cells3,7; and it also affects SOC and nutrient cycling8,9,10,11. Accurate investigation of rhizodeposits during the entire wheat season is crucial important for the achievement of high crop yield and understanding of its contribution to farmland C sequestration, especially in intensified agricultural region such as northern China12,13.
Types and developmental stage of crop growth significantly influences the distribution of photosynthesized C. Mathew et al.14 found that grasses can store up to 45% of their C stocks in the roots, while cereals show much lower rates with 16% for maize and 23% for wheat. The belowground allocation of photosynthesized C to the soil decreases from approximately 10% at tillering to 5% at grain-filling in wheat and barley (Table 1). This decrease might be attributed to differences in photosynthetic capacity and C requirements for root growth at different growth stages3,11. Extrapolating the values from short periods to the whole lifetime of wheat plants (approximately 240 days after emergence in northern China) might overestimate the amount of C allocated to the belowground pool, as young plants exhibit faster root growth and greater sink strength of C allocated to the roots and soil than do older plants15,16.
Compared with conventional non-tracing methods, C tracers (13C or 14C) labelling method can distinguish between soil-derived and root-derived C in the soil, determine the C allocation in different crop stages and completely quantify the whole seasonal C rhizodeposition3,17. Continuous labelling18,19 is particularly appropriate for the estimation of total C transferred to the soil and belowground pools. However, highly sophisticated instrumentation required for continuous isotopic 14C-labelling and sampling makes field studies difficult17, especially for long-lived crops such as winter wheat (up to eight months). As an alternative, 13CO2 or 14CO2 pulse labelling3,8,9 at different growth stages provides discrete information about the temporal C dynamics associated with specific crop stages9,10,11. Pulse labelling also has the advantage of being simple and applicable under field conditions and provides seasonal dynamics of assimilate partitioning. A series of 14C-labelling pulses applied at regular intervals during crop growth has been proven to accurately estimate cumulative belowground C inputs3,11, especially in calcareous soils11. It was commonly considered that in conventional pulse-chase labeling studies, the distribution of assimilated C was completed at the time when the losses of labeled CO2 by respiration could no longer be detected11,16. As the plant tissues and soil microorganisms prefer to utilize the labile organic substrates, the decline or disappearance of the labeled CO2 respiration may not necessarily indicate that the transfer of the less labile components (such as root debris and sloughed-off cells) into the soil is also completed20. Therefore, due to the short duration of labelling and chasing periods, most previous conventional pulse–chase labelling studies provides information only on the newly assimilated C3,11.
In current study, we undertook the pulse labelling at different growth stages of the whole winter wheat season and harvested the winter wheat at 5 days after 14C-labelling (DAL) at each growth stage and at the end of the wheat growth season10,15,20,21. The 14C distribution in the wheat biomass and soil pools at the end of the growing season represents the net contribution of photosynthates formed at various stages of wheat15,20,21. We aimed to quantify the temporal 14C distribution in the winter wheat biomass and soil pools throughout the whole wheat season. We hypothesized that the C allocated into belowground (roots and soil) by pulse labelling quantified for the whole wheat season was much lower than that C quantified by extrapolating the C amount from the young stage to the whole wheat season. To validate this hypothesis, we labelled the winter wheat with 14C at key growth stages, i.e., tillering, elongation, anthesis and grain filling stages, and measured the photosynthesized C allocated into shoot, roots and soil pools and lost by respiration. The results from the pot labelling were also estimated to field level in discussion Section, to evaluate the contribution of wheat production to farmland SOC changes under agricultural intensification process.
Results
Biomass C of shoots, roots and whole wheat plants
As the winter wheat plants grew, the biomass C of the shoots, roots and whole plants increased and peaked at the anthesis stage, after which it remained stable, but the biomass C of the roots decreased after the anthesis stage (Fig. 1). The ratios of roots/shoots and shoots/whole wheat plants decreased and increased, respectively, from emergence until the grain-filling stage. The growth rate of the whole wheat plants was quite low (8.8 mg C d−1 pot−1) at the tillering stage but increased to 189.0 and 269.4 mg C d−1 pot−1 at the elongation and anthesis stages, respectively; at the grain-filling stage, the growth rates were negligible.
Biomass C of (a) shoots, (b) roots, (c) whole plants, and (d) the root/shoot and shoot/whole plant ratios as well as (e) winter wheat growth rates at different growth stages. Vertical bars represent the standard errors of the means (n = 3). Letters (a–c) indicate significant differences (p < 0.05, LSD) for comparison among different stages.
Specific 14C activity of the shoots, roots and soil
Enrichment of labelled 14C was highest in the shoots, followed by the roots and soil for all four labelling events (Fig. 2a–d). The specific 14C activity of the shoots, roots and soil at 5 DAL (DAL 5; DAL 2 at the tillering stage) was in the order of tillering> elongation> anthesis ≈ grain-filling stages. With the prolonged wheat growth duration and the dilution of plant biomass and respiratory release, the specific 14C activities of shoots and roots decreased. For the labelling at the tillering stage, the specific 14C activity of the soil remained stable between DAL 2 and DAL 175 but declined thereafter (Fig. 2a); at the other three labelling stages (Fig. 2b,c,d), the specific 14C activity of the soil was stable after labelling.
Turnover of specific 14C activity for shoot, root and soil components at different DAL at the (a) tillering, (b) elongation, (c) anthesis and (d) grain-filling stages of winter wheat. Vertical bars represent the standard errors of the means (n = 3). LSD values (p < 0.05) are presented as whisked segments for comparison among different components at the same stage.
14C recovery and allocation within the wheat–soil system
The 14C recovered in the soil was highest for the labelling at the tillering stage, followed by that in the roots and shoots (with the exception of DAL 2 in the roots; Fig. 3a). For the labelling at the other three stages, the 14C recovered always followed the order of shoots> roots> soil (Fig. 3b,c,d).
We quantified the proportion of 14C recovered in the shoots, roots and soil at DAL5/2 and at harvest, and that in the respired losses from DAL 5/2 to harvest. Of the total net 14C recovered (shoots + roots + soil), 36.4%, 67.8%, 58.8% and 74.7% were allocated in the shoots at DAL 5/2 at the tillering, elongation, anthesis and grain-filling stages, respectively; these proportions decreased to 4.0% (tillering), 34.3% (elongation), 49.9% (anthesis) and 52.9% (grain-filling) at harvest (Fig. 4a). The proportion of 14C in the total net 14C recovered in the soil (Fig. 4c) was lower than that in the shoots but higher than that in the roots (Fig. 4b; except at the tillering stage), and this proportion decreased from DAL 5/2 to harvest (Fig. 4b,c). Between DAL 5/2 and harvest, the proportion of 14C via respiration (aboveground and belowground respiration) was highest (63.9%) at the tillering stage but decreased to 46.7%, 13.8% and 22.1% at the elongation, anthesis and grain-filling stages, respectively (Fig. 4d). In absolute values, the amount of respired C was 0.21, 2.47, 0.51 and 0.07 C pot−1 at the tillering, elongation, anthesis and grain-filling stages, respectively.
Proportion of 14C allocated at DAL 5/2 and at the end of the growing season (harvest) for (a) shoots, (b) roots, (c) soil, and (d) respiration at harvest for the different labelling events. Vertical bars represent the standard errors of the means (n = 3). Letters (a–c) indicate significant differences (p < 0.05, LSD) for comparison among different stages. Asterisks (*) denote significant differences (p < 0.05, t-test) between DAL 2/5 and harvest.
Contribution of photosynthesized C to SOC
At the end of wheat growing season, the contribution of photosynthesized C to SOC at the four growth stages was calculated by multiplying the relative distribution of assimilated 14C in the soil to the increment of wheat biomass C (Eq. (3)). Approximately 3.3 g C pot−1 was respired (i.e., 20% of the total photosynthate C recovered), 9.1 g C pot−1 (55%) remained in the shoots, 1.1 g C pot−1 (7%) was in the roots, and 2.9 g C pot−1 (18%) was translocated to the soil (Fig. 5). Regarding the 2.9 g C pot−1 allocated to the soil, contributions of 42.1% and 48.5% occurred during the elongation and anthesis stages; these percentages were significantly higher than those at the tillering (6.4%) and the grain-filling (3.0%) stages.
Discussion
Assimilated C is translocated to plant–soil compartments such as shoots and roots, exuded into the soil and lost due to respiration, and this allocation pattern changes with crop growth15,20,21,22. In the present study, the assimilated 14C in the winter wheat shoots at the labelling event of tillering stage was only 36.4%, but increased at the later labelling events: i.e., the shoots retained the majority (60–74.7%) of their assimilated 14C at DAL 5 during the elongation, anthesis and grain-filling stages (Fig. 4). Similar results were also reported for rice20,23, wheat, barley22,24,25 and maize16 but not for pasture grasses26. Several reasons could explain the higher proportion of assimilated C allocated belowground in pasture plants than in cereal plants: (i) approximately 80% of pasture plants are perennial and have well-developed roots that are used as for C storage for newly grown plant tissues; (ii) a long history of selective cultivation of cereals has led to the preferential allocation of assimilates to aboveground parts14; and (iii) intensive fertilization of crops significantly decreases the assimilates in roots needed for the uptake of soil nutrients3,27. These findings are consistent with those of other studies (Table 1), which have reported that wheat and barley allocate more than 90% of photosynthates to their shoots during the mature stage in comparison with approximately 60% during the tillering stage. In a broader context, this indicates that different types of crop have various potentialities of atmospheric C sequestration14.
Photosynthates are actively translocated to belowground parts more during the early establishment of wheat and barley crops than at the mature stage28,29,30 (Table 1). The summary for previous labelling studies (Table 1) showed that the C sink in the roots was greater in young plants (27%) than in mature plants (3%) (Table 1). In our study, the percentage of 14C incorporated into the roots also decreased from the tillering to the grain-filling stages (Fig. 4). However, as the wheat growth rate at the anthesis and elongation stages (189−269 mg C d−1 pot−1) was much greater than that at the grain-filling and tillering stages (8.8−15.4 mg C d−1 pot−1), C translocation to the soil also occurred mainly at the anthesis and elongation stages, i.e., 48.5% and 42.1% of the total C was translocated to the soil, respectively. The majority of labelling studies have been conducted at the early growth stages of wheat, e.g., 60 days after emergence by continuous labelling31,32 and <150 days after emergence by pulse labelling33,34. Hence, extrapolating the values from early wheat stages to the whole lifetime of wheat plants (approximately 240 days after emergence in northern China) substantially overestimated the amount of C allocated to the belowground pool, as young plants exhibit faster root growth and greater sink strength of C allocated to the roots and soil than do older plants15,16.
Using a field planting density of 1.8 million plants ha−1 in northern China35, we found that the assimilated C input to the soil, excluding the C allocated to the roots (690 kg C ha−1), was approximately 1730 kg C ha−1 throughout the whole winter wheat season. This estimate is higher than reported 300 kg C ha−1 for maize16, 460–822 kg C ha−1 for rice23, and 710–1020 kg C ha−1 for wheat36. If the C retained as root residue after harvest (690 kg C ha−1) was also included in the total C input to the soil, the total C input belowground by winter wheat was 2420 kg C ha−1, which is significantly higher than both the values (992 kg C ha−1) summarized in Table 2 and the rough estimates (1500 kg C ha−1) by Kuzyakov and Domanski3 based on C tracer studies. The differences between our study and other studies might be related to the higher root biomass C resulting from agricultural intensification in our study than the quantity defined using tracer techniques (690 in our study vs. 402 kg C ha−1 in Table 2). In addition, the ratio of net rhizodeposited C into the soil to root was reported to be 25% by Kuzyakov and Domanski3 and was therefore 2.5-fold lower than that in our study. This result is because most of the estimates of Kuzyakov and Domanski3 for wheat labelling have been carried out at young plant stages, during which translocation is relatively higher than that during subsequent stages of growth. Again, this finding validates our hypothesis and highlights that labelling and sampling throughout the entire growing season of winter wheat (for instance> 200 days in our study) are necessary to accurately quantify the C budget within the crop–soil system.
Agricultural intensification has not only increased crop productivity but also contributed to increased SOC in northern China12. Smith et al.37 reported that inputs of crop residues lead to higher rates of C sequestration (0.7 Mg C ha yr−1) than does mineral nitrogen (N) fertilizer (0.2 Mg C ha yr−1). The estimated wheat C inputs in our study were 2420 kg C ha−1 (soil + root) and 1730 kg C ha−1 (soil only), which were two-fold greater than those of comparable studies (Table 2). The high amount of C allocated to belowground plant parts in this calcareous soil might explain the rapid increase in SOC in northern China12.
Conclusions
During a growing season, the estimated photosynthesized C input from wheat to the soil was 1.7 t C ha−1 as rhizodeposits. Wheat plant also produced 0.7 t C ha−1 of root residues that was retained in the soil after harvest. Therefore, approximately 2.4 t C ha−1 of atmospheric CO2 was fixed as a relatively stable form in the soil after the wheat season; this value is approximately twice that of other comparable studies. Of the wheat C input to the soil, 90.6% was photoassimilated between the elongation and anthesis stages. This information will be critical in the construction of predictive models of C dynamics in wheat–soil systems if the amount of plant C transferred to the soil requires estimation.
Materials and Methods
Experimental setup
Soil samples were collected from the ploughing layer (0–30 cm) of crop fields at the Quzhou Experimental Station of China Agricultural University in Hebei Province, China (36˚52′N; 115˚01′E). The main soil properties were as follows: 17.1 g kg−1 SOC, 7.8 g kg−1 soil inorganic C, 1.6 g kg−1 total N, pH 8.0 (soil/water = 1/2.5), 148 mg kg−1 available potassium (K), and 9.9 mg kg−1 Olsen phosphorus (P). The soil was sieved (5 mm), after which each polyvinylchloride (PVC) plastic pot was filled (height: 50 cm, inner diameter: 10 cm; 5.6 kg soil pot–1 with calculated bulk density of 1.42 g cm−3) and rewetted to 65% of the water-holding capacity (0.21 g water g−1 dry soil).
Winter wheat (Triticum aestivum L. cv. Jingdong 8) seeds were surface-sterilized in 30% hydrogen peroxide for 30 min, after which they were soaked for 6 h in saturated copper sulphate solution and rinsed with deionized water. This pretreatment effectively sterilized seeds and improved germination rates38. After disinfection, the seeds were placed into glass Petri dishes lined with wet filter paper and germinated for 2 days at 22 °C in darkness. Six healthy germinated winter wheat seeds were transplanted to an individual pot (2-cm depth below the soil). One week after wheat germination, three vigorous seedlings were kept in each pot, i.e., equivalent to a field density of 1.8 million plants per ha, which was estimated from local wheat fields. In accordance with local farming practices, the soil was premixed with urea, diammonium phosphate and potassium chloride at rates of 0.15 g N, 0.09 g P and 0.25 g K kg−1 soil as a base fertilizer, respectively. Urea was top-dressed at the elongation stage (mid-March of the following year) at a rate of 0.15 g N kg−1 soil.
The pots with transplanted winter wheat were placed inside a greenhouse to provide light and temperature conditions similar to those in the field. The soil water content of each pot was controlled gravimetrically to simulate local wheat production and was adjusted daily to 65% (during the seedling stage), 70% (tillering), 80% (elongation), 80% (anthesis) and 70–75% (grain-filling) of field water-holding capacity. When the daily average air temperature dropped to 4–5 °C during the winter season, the pots with wheat plants were placed underground (50-cm depth) to prevent cold damage. The total wheat growing period was 230 days, and six different growth stages were recognized in terms of days after sowing (DAS): (i) seeding (1–17 DAS); (ii) tillering (18–38 DAS); (iii) wintering (39–150 DAS); (iv) elongation (151–179 DAS); (v) anthesis (180–193 DAS); (vi) grain-filling (194–214 DAS); and (vii) harvest (215–230 DAS).
14C pulse labelling
14CO2 labelling was performed at the tillering, elongation, anthesis, and grain-filling stages (i.e., 21, 168, 189 and 203 DAS, respectively). In total, 33 pots of wheat were labelled: 12 pots were labelled at the tillering stage (21 DAS) for destructive sampling at days 2, 154, 175 and 203 (harvest) after labelling (3 pots per sampling); nine pots were labelled at the elongation stage (168 DAS) for destructive sampling at days 5, 28 and 56 (harvest) after labelling; six pots were labelled at the anthesis stage (189 DAS) for destructive sampling at days 5 and 35 (harvest) after labelling; and six pots were labelled at the grain-filling stage (203 DAS) for destructive sampling at days 5 and 21 (harvest) after labelling.
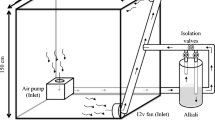
The labelling system adapted from Cheng39 consisted of a labelling chamber and a pot containing transplanted wheat. Each pot was closed at the bottom with a rubber stopper and had an air inlet and air outlet. The soil surface was covered with a PVC board and sealed with silicon, including around the winter wheat stems. A flask containing Ba14CO3 with a 14C activity of 713 kBq was placed inside the labelling chamber. The chamber was then closed, and 14CO2 was released into the chamber by carefully adding an excess volume of 4 M HClO4 to the Ba14CO3 solution with a syringe to ensure complete evolution of the 14CO2 into the labelling chamber atmosphere. To help guarantee a uniform distribution of 14CO2, an electric fan was used to homogenize the gases inside the chamber.
CO2 depletion under identical conditions in another chamber supplied with unlabelled CO2 was monitored using an infrared gas analyser (GXH305, Beijing Analytical Equipment Co., China)40,41. This system was used because 14CO2 could not be directly monitored, as the infrared range was set for 12CO2 and only slightly overlapped with the range for 14CO240,41. If the speed of CO2 concentration decrease slowed considerably (less than 200 μL L−1), 12CO2 was released into the chamber until the 14CO2 + 12CO2 concentrations increased to approximately 400 μL L−1 by adding with a syringe an aliquot of 4 M HClO4 to an unlabelled NaHCO3 solution. The wheat plants were labelled for 1.5 h to facilitate the assimilation of 14CO2. After Ba14CO3 labelling, the air within the labelling chamber was pumped for 0.5 h through 50 mL of 1 M NaOH solution to remove unassimilated 14CO2 before the labelling chamber was opened.
Plant and soil sampling
Destructive sampling of wheat and soil samples was carried out for the C and 14C analyses. The shoots were cut at the base of the wheat plants, and all the soil was removed from the pots. The roots were separated from the soil manually and then washed with 125 mL of deionized water to remove the soil adhering to the roots42. The soil samples were placed onto thick paper (0.5 cm) and divided into 100 groups. Approximately 1 g per group was sampled with a spoon and mixed to obtain a representative soil sample38. The shoots, roots and soil samples were oven-dried at 65 °C to a constant weight.
Sample analysis
The plant and soil samples were ground (<500 µm) using a ball mill (Restol MM2000, Retsch, Haan, Germany) prior to analysing 14C content and determining total organic C. To determine the 14C content of SOC, carbonates were removed from the soil samples using a 0.5 M HCl solution for 6 h4. The soil was then washed using deionized water and centrifuged three to four times to remove the HCl. The 14C content of the plant (approximately 0.2 g) and soil (approximately 1 g) samples was measured after combustion with an oxidizer unit. The evolved 14CO2 was directly trapped in a scintillation cocktail, followed by liquid scintillation counting (FJ-2101, Xi’an Analytical Equipment Co., Xi’an, China). The 14C counting efficiency was approximately 94%.
Calculations and statistical analysis
At 2 DAL (DAL 2) at the tillering stage or 5 DAL (DAL 5) at the elongation, anthesis and grain-filling stages, we assumed that the allocation of 14C photosynthesized within the wheat–soil system represented the initial photosynthate distribution in the shoot, root and soil compartments15,20,21. At each sampling, the amount of net 14C recovered (Fig. 3) was calculated by multiplying the specific 14C activity (Fig. 2) by the corresponding C amount in the shoots, roots or soil (Fig. 1). The net 14C recovered at DAL 5 or 2 (DAL 5/2) served as the basis for the calculation and comparison of photosynthesized C distributed in different wheat–soil compartments during the later days of wheat growth and at the harvest period.
The (Net 14C recovered)DAL5/2 of winter wheat was estimated by summing the 14C amount in the shoots (14Cshoot), roots (14Croot) and soil (14Csoil) measured at DAL 5/2 at each stage16,17,18:
The per cent distribution of 14C recovered (Distribution%) either at DAL 5/2 or at the end of the wheat growing season was calculated as:
where 14Csample is the 14C amount in the measured pools, i.e., the shoots, roots, and soil and the respired CO2 (aboveground and belowground) at DAL 5/2 or at the end of the growing season.
The whole wheat seasonal contribution (g C pot−1 soil) of photosynthesized C to the soil formed during the four growth stages of winter wheat was calculated by the relative distribution of 14C recovered in the soil to the accumulation of plant biomass C as follows:
where (14Csoil)end is the amount of 14C in the soil at the end of wheat growing season; (net 14C recovered)DAL 5/2 is calculated by Eq. (1); WPGR is the wheat plant growth rate (mg C d−1 pot−1; Fig. 1e); and D represents the number of days in each growth stage. Seventeen days of the seeding stage was considered to encompass tillering, i.e., a total of 38 days at the tillering stage. Within 111 days of the wintering period, wheat stopped growing, and its growth rate was considered 0 mg C d−1 pot−1. The duration of the elongation, anthesis and grain-filling stages was 29, 14 and 21 days, respectively.
The respired C assimilation (from the shoots, roots and soil) was estimated from the accumulation of plant biomass C and from the 14C distribution by respiratory losses at the end of the wheat growing season. The amount of respired C (aboveground and belowground) was calculated as follows:
where (14Ctotal respiration)end indicates 14C-CO2 losses from total respiration calculated by the difference between the net 14C recovered at DAL 5/2 and the amount of 14C remaining in the wheat–soil system at the end of the growing season.
Statistical analysis
Winter wheat growth stage was the experimental factor in this study. The experiment was carried out involving three replicates and was arranged in a completely randomized design. The data were subjected to one-way analysis of variance (ANOVA) using SPSS (Version 11.0, 2002, SPSS Inc., USA). Fisher’s least significant difference (LSD; p < 0.05) was used to test differences in the measured variables among the different labelling events.
References
FAO. FAO statistics. http://faostat.fao.org (2016).
Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627 (2004).
Kuzyakov, Y. & Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sc. 163, 421–431 (2000).
Meng, F. et al. Coupled incorporation of maize (Zea mays L.) straw with nitrogen fertilizer increased soil organic carbon in Fluvic Cambisol. Geoderma. 304, 19–27 (2016).
Jones, D. L., Nguyen, C. & Finlay, R. D. Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil. 321, 5–33 (2009).
Rasse, D. P., Rumpel, C. & Dignac, M. F. Is soil carbon mostly root carbon? Plant Soil. 269, 341–356 (2005).
Hütsch, B. W., Augustin, J. & Merbach, W. Plant rhizodeposition- an important source for carbon turnover in soils. J. Plant Nutr. Soil Sc. 165, 397–407 (2002).
Nguyen, C. Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie. 23, 375–396 (2003).
Mwafulirwa, L. et al. Barley genotype influences stabilization of rhizodeposition-derived C and soil organic matter mineralization. Soil Biol. Biochem. 95, 60–69 (2016).
Qiao, Y., Miao, S., Han, X., Yue, S. & Tang, C. Improving soil nutrient availability increases carbon rhizodeposition under maize and soybean in mollisols. Sci. Total Environ. 603, 416–424 (2017).
Swinnen, J., Van Veen, J. A. & Merckx, R. 14C pulse-labelling of field-grown spring wheat: an evaluation of its use in rhizosphere carbon budget estimations. Soil Biol. Biochem. 26, 161–170 (1994).
Liao, Y., Wu, W. L., Meng, F. Q., Smith, P. & Lal, R. Increase in soil organic carbon by agricultural intensification in northern china. Biogeosciences. 12, 1403–1413 (2015).
Zhang, X. et al. Agricultural sustainable intensification improved nitrogen use efficiency and maintained high crop yield during 1980-2014 in Northern China. Sci. Total Environ. 61, 596–597 (2017).
Mathew, I., Shimelis, H., Mutema, M. & Chaplot, V. What crop type for atmospheric carbon sequestration: Results from a global data analysis. Agriculture, Ecosystems & Environment. 243, 34–46 (2017).
Jin, J. et al. Seasonal allocation of photosynthetically fixed carbon to the soybean-grown Mollisols in Northeast China. Crop Pasture Sci. 62, 563 (2011).
Meng, F. et al. Investigation of photosynthate-C allocation 27 days after 13C-pulse labeling of Zea mays L. at different growth stages. Plant Soil. 373, 755–764 (2013).
Paterson, E., Midwood, A. J. & Millard, P. Through the eye of the needle: a review of isotope approaches to quantify microbial processes mediating soil carbon balance. New Phytol. 184, 19 (2009).
Ge, T. et al. Tracking the photosynthesized carbon input into soil organic carbon pools in a rice soil fertilized with nitrogen. Plant Soil. 392, 17–25 (2015).
Tan, L. M. et al. Input and distribution of rice photosynthesized carbon in the tillering stage under different nitrogen application following continuous 13C labeling. Environmental Science. 35, 1933–1938 (2014).
Lu, Y., Watanabe, A. & Kimura, M. Contribution of plant-derived carbon to soil microbial biomass dynamics in a paddy rice microcosm. Biol. Fert. Soils. 36, 136–142 (2002).
Fernandez, J., Curt, M. D., Aguado, P. & Magro, E. Carbon allocation in a sweet sorghum-soil system using 14C as a tracer. J. Plant Nutr. Soil Sc. 166, 23–30 (2003).
Chowdhury, S., Farrell, M. & Bolan, N. Photoassimilated carbon allocation in a wheat plant-soil system as affected by soil fertility and land-use history. Plant Soil. 383, 173–189 (2014).
Tian, J. et al. Allocation and dynamics of assimilated carbon in rice-soil system depending on water management. Plant Soil. 363, 273–285 (2013).
Gregory, P. J. & Atwell, B. J. The fate of carbon in pulse-labelled crops of barley and wheat. Plant Soil. 136, 205–213 (1991).
Keith, H., Oades, J. & Martin, J. Input of carbon to soil from wheat plants. Soil Biol. Biochem. 18, 445–449 (1986).
Kuzyakov, Y., Kretzschmar, A. & Stahr, K. Contribution of Lolium perenne rhizodeposition to carbon turnover of pasture soil. Plant Soil. 213, 127–136 (1999).
Hafner, S. et al. Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling. Global Change Biol. 18, 528–538 (2012).
Atwell, B. J., Fillery, I. R. P., McInnes, K. J. & Smucker, A. J. M. The fate of carbon and fertiliser nitrogen when dryland wheat is grown in monoliths of duplex soil. Plant Soil. 241, 259–269 (2002).
Swinnen, J., Van Veen, J. A. & Merckx, R. Carbon fluxes in the rhizosphere of winter wheat and spring barley with conventional vs integrated farming. Soil Biol. Biochem. 27, 811–820 (1995).
Swinnen, J., Van Veen, J. A. & Merckx, R. Root decay and turnover of rhizodeposits in field-grown winter wheat and spring barley estimated by 14C pulse-labelling. Soil Biol. Biochem. 27, 211–217 (1995).
Liljeroth, E., Kuikman, P. & Van Veen, J. A. Carbon translocation to the rhizosphere of maize and wheat and influence on the turnover of native soil organic matter at different soil nitrogen levels. Plant Soil. 161, 233–240 (1994).
Marx, M. et al. Determination of the fate of 13C labelled maize and wheat rhizodeposit-C in two agricultural soils in a greenhouse experiment under 13C-CO2-enriched atmosphere. Soil Biol. Biochem. 39, 3043–3055 (2007).
Butterly, C. R., Armstrong, R., Chen, D. & Tang, C. Carbon and nitrogen partitioning of wheat and field pea grown with two nitrogen levels under elevated CO2. Plant Soil. 391, 367–382 (2015).
Martens, R., Heiduk, K., Pacholski, A. & Weigel, H. Repeated 14CO2 pulse-labelling reveals an additional net gain of soil carbon during growth of spring wheat under free air carbon dioxide enrichment (FACE). Soil Biol. Biochem. 41, 2422–2429 (2009).
Dai, Z., Fan, Y. Q., Wu, R. Q., Cao, L. & Wang, Z. L. Effects of planting density on content of HMW-GS and size distribution of glutenin macropolymer in wheat grain. Journal of Triticeae Crops. 33, 952–956 (2013).
Swinnen, J., Van Veen, J. A. & Merckx, R. Rhizosphere carbon fluxes in field-grown spring wheat: model calculations based on 14C partitioning after pulse-labelling. Soil Biol. Biochem. 26, 171–182 (1994).
Smith, P. et al. Carbon sequestration potential in european croplands has been overestimated. Global Change Biol. 11, 2153–2163 (2005).
Qu, B. et al. Growth and carbon sequestration of winter wheat at its tillering stage in high yield farm ecosystem of North China. Chinese Journal of Ecology. 26, 25–30 (2007).
Cheng, W. Measurement of rhizosphere respiration and organic matter decomposition using natural 13C. Plant Soil. 183, 263–268 (1996).
Roper, M. M. et al. Allocation into soil organic matter fractions of 14C captured via photosynthesis by two perennial grass pastures. Soil Res. 51, 748 (2013).
Yu, Z., Li, Y., Jin, J., Liu, X. & Wang, G. Carbon flow in the plant-soil-microbe continuum at different growth stages of maize grown in a Mollisol. Arch. Agron. Soil Sci. 63, 362–374 (2017).
Cheng, W. X., Coleman, D. C., Carroll, C. R. & Hoffman, C. A. Investigating short-term carbon flows in the rhizospheres of different plant-species, using isotopic trapping. Agron. J. 86, 782–788 (1994).
Palta, J. A. & Gregory, P. J. Drought affects the fluxes of carbon to roots and soil in 13C pulse-labelled plants of wheat. Soil Biol. Biochem. 29, 1395–1403 (1997).
Martin, J. K. & Kemp, J. R. The measurement of C transfers within the rhizosphere of wheat grown in field plots. Soil Biol. Biochem. 18, 103–107 (1986).
Lodhi, A., Sajjad, M. H., Mahmood, A. & Tahir, S. & FarooQ-E-Azam. Photosynthate partitioning in wheat (Triticum aestivum L.) as affected by root-zone salinity and form of N. Pak. J. Bot. 41, 1363–1372 (2009).
Jensen, B. Rhizodeposition by field-grown winter barley exposed to 14CO2 pulse-labelling. Appl. Soil Ecol. 1, 65–74 (1994).
Qi, X. & Wang, J. G. Distribution and translocation of assimilated C pulse-labeled with 13C for winter wheat (Trticum aestivums L.) as affected by nitrogen supply. Journal of Agro-Environment Science. 27, 2524–2530 (2008).
Jensen, B. Rhizodeposition by 14CO2-pulse-labelled spring barley grown in small field plots on sandy loam. Soil Biol. Biochem. 25, 1553–1559 (1993).
Zagal, E. Influence of light intensity on the distribution of carbon and consequent effects on mineralization of soil nitrogen in a barley (Hordeum vulgare L.)-soil system. Plant Soil. 160, 21–31 (1994).
Martin, J. K. & Merckx, R. The partitioning of photosynthetically fixed carbon within the rhizosphere of mature wheat. Soil Biol. Biochem. 24, 1147–1156 (1992).
Acknowledgements
This work received financial support from the National Key R&D Project (No. 2017YFD0800605) and the National Natural Science Foundation (Nos 31370527 and 30870414).
Author information
Authors and Affiliations
Contributions
F.Q.M. conceived and designed the experiments, and B.Q. performed the experiments. Z.A.S., Q.C., X.H., R.B., B.Q. and F.Q.M. analysed the data, and Z.A.S. and F.Q.M. wrote the paper. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, Z., Chen, Q., Han, X. et al. Allocation of photosynthesized carbon in an intensively farmed winter wheat–soil system as revealed by 14CO2 pulse labelling. Sci Rep 8, 3160 (2018). https://doi.org/10.1038/s41598-018-21547-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21547-y
- Springer Nature Limited
This article is cited by
-
Reduced belowground allocation of freshly assimilated C contributes to negative plant-soil feedback in successive winter wheat rotations
Plant and Soil (2024)
-
Impact of root-associated strains of three Paraburkholderia species on primary and secondary metabolism of Brassica oleracea
Scientific Reports (2021)
-
Phenological Stages and Aluminum Presence Influences Arbuscular Mycorrhizal Fungi Communities in Roots of Plant Cereals
Journal of Soil Science and Plant Nutrition (2021)