Abstract
Earthquakes are important natural events, yet their impacts on animal communities are poorly known. Understanding earthquake impacts on groundwater communities is essential to assess their resilience and hence to perform conservation actions. We investigated how a 6.3 Mw earthquake that occurred in 2009 altered the community structure (diversity, evenness, dominance, species abundance distributions and beta-diversity) of microcrustaceans (Crustacea Copepoda) inhabiting springs fed by the Gran Sasso Aquifer (Central Italy). Sampling was done in low-discharge (1997), high-discharge (2005), and post-seismic (2012) hydrological years. Stygobites (obligate groundwater species) and non-stygobites (non-obligate groundwater species) showed different patterns. A high-water discharge in 2005 altered abundance patterns of non-stygobites. The earthquake re-established former abundance patterns. Stygobites were less affected by high-water discharge in 2005, and showed strong increases in diversity and evenness after the earthquake. This effect was due to the fact that the earthquake induced a strong population decline of previously dominant stygobites (especially of Nitocrella pescei) in the aquifer, and subsequently at the main spring outlets, thus allowing a more equitable species-abundance distribution. These results highlight the importance of considering species ecology to understand the effects of a significant earthquake event on animal communities.
Similar content being viewed by others
Introduction
Earth is a dynamic planet, whose surface is continuously re-shaped by extreme, sudden events, such as fires, floods, storms, volcanic eruptions, earthquakes, and tsunamis. These phenomena are considered “natural disasters” from the human perspective, because they injure people and produce economic damages. From the ecosystem’s perspective, they are forms of disturbance, defined as any discrete event in time and space that disrupts ecosystem, community, or population structure, and changes resources, substrate availability, or the physical environment1,2. As severe phenomena of disturbance, natural disasters may affect biodiversity by increasing mortality and altering habitat quality3,4.
Human activities have recently increased the severity and frequency of some types of extreme events (such as storms and wildfires)5,6,7,8, which may therefore represent sources of threats for biodiversity conservation. Even extreme events that are not influenced by human activities, such as volcanic eruptions and seismic events (with the exception of earthquakes on a small scale caused partially or completely by human activities9,10,11) are areas of concern, because they impact on an already threatened biodiversity12. In general, the study of ecosystem responses to major disturbance events may produce important ecological and resource management insights13 and there is increasing literature on the effects of fires14,15, floods16,17, hurricanes18,19,20, tornadoes21,22, volcanic eruptions23,24,25, and tsunamis26,27 on biodiversity. However, information on how animal communities respond to the disturbance of seismic events is still very limited12,28. In particular, the consequences of earthquakes on invertebrate biodiversity have been rarely addressed and remain therefore largely unknown29,30,31,32.
Although earthquakes can happen in any part of the world, the frequency of earthquakes is higher in the areas of boundaries between lithospheric plates. One of these seismically active areas is the Mediterranean-Alpine-Himalayas region, which extends from the Azores to the eastern coast of Asia33. Placed in the centre of the Mediterranean Basin, Italy is frequently hit by strong (6.0–6.9 Mw) and sometimes major (7.0–7.9 Mw) earthquakes (https://en.wikipedia.org/wiki/List_of_earthquakes_in_Italy). In the last 20 years, for example, seven strong earthquakes have occurred in Italy, among which the 6.3 Mw that struck the city of L’Aquila on 6 April 2009. This earthquake had a profound impact on the hydrogeological setting of the Gran Sasso Aquifer (GSA) by inducing an increase in the bulk hydraulic conductivity of the recharge area, near the ruptured fault zone together with fracture clearing and/or microcrack formations, and led to an anomalous rising of the water table (up to one metre) and flow rate (≥30% of the previous 15 years) in discharge zones34. As a result, the groundwater flow of the Tirino River valley, where ~65% of the aquifer discharge is located35, was altered, with important changes in the discharge of the Tirino Springs (TS), which are the main outlets of GSA, and which changed from rheo-limnocrene to predominantly limnocrene36,37. Previous research demonstrated that these changes altered the community organisation of subsurface (i.e. below the spring bed) microcrustaceans at TS, by reducing the abundance of obligate groundwater species (i.e. stygobites)30 and their spatial niche overlap32.
Understanding the impacts of earthquakes on groundwater communities is crucial to assess the resilience and sustainability of subterranean ecosystems and hence to perform conservation actions, such as a strict regulation of water extraction. In the present paper, we investigate if the earthquake altered the microcrustacean community structure in terms of diversity, evenness, dominance, species abundance distributions and beta-diversity. In general, disturbance events are expected to reduce diversity and evenness1, leading species abundance distributions to shift towards patterns characterized by a higher dominance of a few species14,38,39,40.
In two previous papers30,32, we demonstrated that L’Aquila earthquake has had profound impacts on microcrustacean population density, species spatial segregation and niche overlap. Using the same data set, in the present, complementary paper, we test if the earthquake decreased diversity and changed species abundance distributions by comparing microcrustacean communities in low-discharge (1997), high-discharge (2005), and post-seismic, very high-discharge (2012) hydrological years. As in the previous papers30,32, we used copepods (Crustacea Copepoda) because they comprise ~80% of the total abundance of meiofauna community at TS30,36,37, being therefore the best suited model organisms for this type of study.
Results
Comparison of pre-seismic communities
A total of 22 copepod species (9 stygobites and 13 non-stygobites) were found in both pre-seismic sampling years; and 18 copepod species in the post-seismic sampling year. The two pre-seismic communities showed similar values in most diversity indices (Table 1). When stygobites and non-stygobites were considered together, significant differences were only found for Simpson dominance (P = 0.022) and Berger–Parker dominance (P < 0.001) (Table 1). Simpson dominance was lower in 2005 than in 1997, which is a reflection of the increased relative abundance of certain species. In particular, the non-stygobiotic Pesceus schmeili (Mrázek, 1893), which accounted for 9.9% of the total copepod abundance in 1997, represented 24.4% of total copepod abundance in 2005; the non-stygobiotic Moraria poppei meridionalis Chappuis, 1929, which accounted for 0.6% of the total copepod abundance in 1997, was 16.8% in 2005; the non-stygobiotic Bryocamptus typhlops (Mrázek, 1893), which accounted for 4.8% of the total copepod abundance in 1997, represented 10.3% of the individuals in the 2005 community. Decrease in the Berger–Parker dominance is explained by the fact that this index is simply the proportion of the most abundant species. The most dominant species in the 1997 community was the stygobiotic Nitocrella pescei Galassi & De Laurentiis, 1997 with 33.0% of the individuals, followed by the non-stygobiotic Bryocamptus echinatus (Mrázek, 1893) with 10.4% of the individuals; no other species had a relative abundance >10%. By contrast, in the 2005 community, four species had abundance values >10%: P. schmeili (24.4%), M. poppei meridionalis (16.8%), B. typhlops (10.3%) and N. pescei (21.7%).
Comparison of pre- and post-seismic communities
The post-seismic community showed lower values of dominance and higher values of diversity and evenness in comparison with both pre-seismic communities. Namely, the post-seismic community differed significantly (P < 0.001) from the two pre-seismic communities for all indices reported in Table 1 except the Margalef and Menhinick indices (which only consider total richness and total abundance) and for the Berger–Parker index related to 2005–2012. The post-seismic community had a slightly lower richness (18 species) compared with that of the two pre-seismic communities (which had the same number of species: 21 species in both cases). Regarding total abundance (expressed as the number of individuals found in the total volume of sampled water, i.e. 1920 L each year), the post-seismic community had a total abundance (910 individuals, i.e. 0.47 individuals L−1) very similar to the 1997 pre-seismic community (992 individuals, i.e. 0.52 individuals L−1). The 2005 pre-seismic community included a larger number of sampled individuals (2750 individuals, i.e. 1.43 individuals L−1), i.e. the sampled total abundance was about three times higher, but this difference is strongly reduced in the index calculation, because the total abundance is logarithmised in the Margalef index and square-rooted in the Menhinick index. In 2012, three of the four most dominant species in 2005 returned to lower abundances (P. schmeili: 7.9%, M. poppei meridionalis: 4.1%, and Nitocrella pescei: 9.5%; B. typhlops increased to 20.1%).
Comparison of pre- and post-seismic communities for non-stygobites
When stygobites and non-stygobites are analysed separately, different patterns emerge (Table 1). The non-stygobiotic species showed a significant (P < 0.001) reduction in diversity and evenness and an increase in dominance in the 2005 community compared with the 1997 community (Table 1). The post-seismic community differed from the 1997 community only for an increase in Berger-Parker dominance (P = 0.028), whereas it differed from the 2005 community for significant (P < 0.001) increases in diversity and evenness and reduction in dominance indices (Table 1).
Comparison of pre- and post-seismic communities for stygobites
For the stygobites, the two pre-seismic years differed only for the Berger-Parker dominance (higher in 2005, P = 0.019), whereas the post-seismic community showed higher diversity (0.001 < P < 0.004) and evenness (0.001 < P < 0.023), and lower dominance (P < 0.001), in comparison with the two pre-seismic communities (Table 1).
Species-abundance analysis
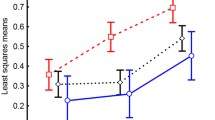
The copepod community before the earthquake was best fitted by a lognormal model in 1997 and by a geometric series in 2005; in the post-seismic year (2012) the geometric series and the lognormal series fitted the copepod species abundance distribution equally well (Table 2). Thus, the copepod community shifted from the 1997 rather equitable species abundance distribution (lognormal) to a distribution characterised by lower evenness (geometric series) in 2005, and finally returned to a more balanced distribution of species abundances in 2012, after the earthquake. Because the 2005 and the 2012 communities are adequately fitted by the geometric series, rank-abundance distributions were modelled using a regression approach (Fig. 1a). The slope of the 2012 line was significantly lower than the slope of the 2005 line (equality of slopes: F = 9.881, P = 0.003), which indicates that the post-seismic community was less influenced by the most dominant species.
Rank-abundance distribution of the copepods of Tirino Springs (Central Italy) in pre-seismic (1997, grey diamonds; 2005, black squares) and post-seismic (2012, red dots) years. Panel a: comparison for all species. Regression statistics for the 1997 pre-seismic community: log(abundance) = (−0.122 ± 0.004) × rank + (2.479 ± 0.044), R² = 0.984, F1,19 = 1190.221, P < 0.0001. Regression statistics for the 2005 pre-seismic community: log(abundance) = (−0.144 ± 0.004) × rank + (3.026 ± 0.045), R² = 0.988, F1,19 = 1593.945, P < 0.0001. Regression statistics for the post-seismic community: log(abundance) = (−0.119 ± 0.007) × rank + (2.478 ± 0.081), R² = 0.941, F1,17 = 255.883, P < 0.0001. The 1997 community followed the lognormal series series but was modelled here for comparative purposes. Panel b: comparison for non-stygobites. Regression statistics for the 1997 pre-seismic community: log(abundance) = (−0.201 ± 0.013) × rank + (2.433 ± 0.094), R² = 0.961, F1,10 = 245.442, P < 0.0001. Regression statistics for the 2005 pre-seismic community: log(abundance) = (−0.205 ± 0.010) × rank + (2.998 ± 0.076), R² = 0.977, F1,11 = 461.579, P < 0.0001. Regression statistics for the post-seismic community: log(abundance) = (−0.175 ± 0.015) × rank + (2.573 ± 0.100), R² = 0.940, F1,9 = 141.585, P < 0.0001. Panel c: comparison for stygobites. Regression statistics for the 1997 pre-seismic community: log(abundance) = (−0.299 ± 0.023) × rank + (2.654 ± 0.127), R² = 0.961, F1,8 = 174.495, P < 0.0001. Regression statistics for the 2005 pre-seismic community: log(abundance) = (−0.422 ± 0.033) × rank + (3.161 ± 0.165), R² = 0.965, F1,6 = 166.370, P < 0.0001. Regression statistics for the post-seismic community: log(abundance) = (−0.307 ± 0.039) × rank + (2.292 ± 0.173), R² = 0.926, F1,5 = 62.997, P = 0.0005. In all cases, errors refer to standard errors.
When stygobites and non-stygobites are analysed separately, the geometric series gave the best fit in all cases (Table 2). When rank-abundance distributions were modelled using a regression approach, no significant differences between slopes were found (1997 vs. 2005: F = 9.081, P = 0.779; 1997 vs. 2012: F = 3.146, P = 0.091; 2005 vs. 2012: F = 1.736, P = 0.203), which indicates that non-stygobiotic species showed no changes in the dominance pattern (Fig. 1b). For the stygobiotic species (Fig. 1c), slopes were significantly different between the two pre-seismic communities (F = 10.010, P = 0.007) and marginally different between the 2005 and the post-seismic community (F = 5.149, P = 0.044), but not between the 1997 and the post-seismic community (F = 0.030, P = 0.865), which indicates that the dominance of the stygobiotic species after the earthquake returned to be similar to the 1997 situation after the increase in 2005.
Beta-diversity analysis
Average beta-diversity values were similar among years for the whole community (arithmetic mean ± SE: 0.419 ± 0.059 for 1997; 0.467 ± 0.070 for 2005; 0.444 ± 0.043 for 2012) and for the stygobiotic species (0.526 ± 0.065 for 1997; 0.632 ± 0.072 for 2005; 0.633 ± 0.049 for 2012; difference between 1997 and 2012 is in fact marginally significant), whereas increased significantly after the earthquake for the non-stygobites (0.340 ± 0.048 for 1997; 0.323 ± 0.044 for 2005; 0.535 ± 0.043 for 2012) (Table 3). Beta-diversity values observed in 1997 were significantly correlated with those recorded in 2005 for the entire community and for the non-stygobiotic species, but not for the stygobites (Table 3). Beta-diversity values observed in 1997 were significantly correlated with those recorded in 2012 only for the stygobites and no correlation was detected between 2005 and 2012 beta-diversity patterns (Table 3).
Discussion
Data about groundwater communities of unconsolidated (porous) or fractured aquifers are usually gathered by sampling drilling wells because they are directly connected with the aquifer41,42,43,44. However, groundwater-fed springs may represent a more interesting, albeit more complex, source of information in the case of karstic aquifers, because the structure of their animal communities is influenced by the full variety of habitats that occur across the entire aquifer and transport history45. Groundwater-fed springs host species belonging to different ecological categories, including crenic species (which dwell exclusively in the spring46,47,48 but that are rare among copepods), stygobiotic species (which colonize the spring from the aquifer49) and non-stygobiotic species (species that live in spring habitats being cold stenothermic or generalists, species coming from downstream, and species flushed out from surface waters of the recharge area of the aquifer30,50,51). The same disturbance event may affect non-stygobiotic and stygobiotic species in different ways. The high discharge that occurred in 2005 increased the dominance (and hence decreased diversity and evenness) of non-stygobiotic species (but not of the stygobiotic ones). The earthquake that occurred in 2009 hit this already perturbed community, by inducing a significant decrease in dominance, and a significant increase in diversity and evenness of non-stygobites, which led this group of species to return to the 1997 values. Therefore, the effect of the earthquake on non-stygobiotic species was to re-equilibrate a situation altered by the high discharge in 2005. This pattern can be explained by the colonisation dynamics of these species within the TS system. Non-stygobiotic species occurring in benthic habitats of the TS system (which is an aquitard, with the carbonate bedrock with several fractures and strong upwelling in the sediment matrix overlying the bedrock30) may reach the spring both via surface-water dispersal from downstream and via the aquifer when drifting from the surface recharge area52,53. Therefore spring colonisation by non-stygobites is not strictly dependent on groundwater dynamics52 but is primarily regulated by the sediment texture of the spring-bed characteristics49. Thus, the earthquake might have induced variations in diversity, evenness and dominance of non-stygobites by re-shaping the sediment texture of TS through the effect of an increased discharge, more than by a direct effect of the aquifer dynamics. However, re-arrangements of sediment texture could have been equally produced by the high discharge that occurred in 2005. Thus, the non-stygobiotic species were affected by both the 2005 anomalous discharge and the mainshock-induced high discharge. By contrast, stygobiotic species, being only affected by changes in the karst groundwater flow that is focused to spring outlets, were much more sensitive to the effects of the earthquake than to the anomalous 2005 discharge. In fact, the 2005 anomalous discharge did not change diversity, dominance and evenness of stygobites, probably because it was within the range of the hydrological changes to which these groundwater-dweller species are used to. Post-seismic values in diversity and evenness of stygobites were higher not only in comparison with the values recorded in 2005 (and characterised by a high dominance effect), but also in comparison with 1997 values. Differently from non-stygobiotic species, stygobites reflected more directly the ecological processes that occurred into the aquifer feeding the spring system49,52,53. The 2009 mainshock markedly changed the Gran Sasso groundwater flow34,54,55 as well as water isotope and chemical composition37, and these changes were mirrored by a variation in the stygobiotic assemblage composition30,32 that was observed also in this study. In fact, the increase in stygobiotic diversity recorded in 2012 occurred as a result of a reduction in the abundance of most species30 and was associated with an increase in niche overlap due to the redistribution of animals caused by the earthquake-triggered discharge32.
This scenario is paralleled by variations in species abundance distribution patterns. For the whole community, the lognormal series was identified as the best fit model in the 1997 community, whereas the 2005 community followed the geometric series, and the 2012 community was equally best fitted by both models. The geometric model predicts very uneven abundances, broken stick predicts very even abundances, while lognormal is intermediate and assumes a small number of very rare species56. Low productivity systems were claimed to have uneven species abundance distributions and be well fitted by a geometric series, while high productivity systems are well fitted by lognormal curves and exhibit the highest evenness57. Species abundance distributions was observed to change along a successional gradient in deciduous forest plots in Illinois, USA, with more lognormal, more even communities occurring late in succession just as for productivity, whereas early stages of succession (e.g. following the felling of trees for timber) followed a geometric series58. Since these pioneer observations, lognormal shapes have mainly been associated with “fully censused” communities59 regulated by a large number of biotic and abiotic factors that together produce a lognormal abundance distribution according to the central limit theorem of statistics40,60,61,62.
In the copepods of TS, the 2005 above-average discharge conditions induced a shift in the species abundance distribution from relatively even abundances (expressed by the lognormal model) to uneven abundances (expressed by the geometric series). The effect of the earthquake was to level the strongest differences in species abundances and hence to re-establish a species abundance distribution similar to that recorded in 1997, especially to the detriment of stygobiotic species. In other words, the earthquake decimated the whole community, but the impact was particularly severe on the species-abundance distribution of stygobites, hence allowing an increase in the diversity and evenness of the non-stygobiotic species, thus re-establishing a more balanced species abundance distribution in the whole community. Non-stygobiotic species showed similar patterns of abundance distribution in all three years, whereas stygobiotic species showed a significant increase in the slope of the geometric series between 1997 and 2005 and then a reduction after the earthquake. Thus, changes in the species abundance distribution were mostly driven by the stygobiotic component.
Stygobites and non-stygobites showed important differences also for changes in beta-diversity patterns between years. For the whole community and for the non-stygobiotic species, beta-diversity patterns remained similar between the two pre-seismic years, but changed between pre-seismic and post-seismic years. Thus, non-stygobites were not affected by the higher discharge in 2005, but the earthquake disrupted the previous patterns by increasing beta-diversity. In contrast, for the stygobiotic species, beta-diversity pattern of 2005 was different from those of 1997 and 2012, whereas 2012 beta-diversity pattern was similar to that of 1997. Thus, the effect of the earthquake for the stygobites was that of reconstructing a beta-diversity pattern similar to that observed in 1997. It is counter-intuitive that the earthquake determined an increase in diversity and evenness. However, this unexpected “positive” effect can be explained in consideration of the type of changes in the community structure determined by the seismic event. The earthquake changed species composition (three species disappeared)30 and lowered species abundances that were increased by the 2005 high discharge; this induced a strong population decline of the species that dominated copepod communities, thus allowing a more equitable species-abundance distribution, and hence higher diversity and evenness.
Evidence that natural disasters may severely threaten biodiversity typically refer to population decline due to destruction of resources63,64 whereas effects on communities may be much more complex and, in certain ecosystems, periodic disaster events may be necessary for maintaining or introducing variability in community structure65,66,67.
For example, communities of small mammals inhabiting areas hit by earthquakes, fires, clearcutting, and floods have low relative abundances and high species diversity68,69,70,71. Also, it has been observed that a reduction in food availability immediately after the disturbance might be a reason for low relative abundances69,72. In the case of spring copepods, it is difficult to invoke a reduction in food availability as a main reason for population decline, because particulate organic matter (POM), which constitutes a consistent food supply for copepods along with bacteria73, increased with the earthquake30. Rather, decline in species abundances after the earthquake was mainly due to a strong increase in hydraulic conductivity and to the consequent aquifer dewatering, which massively flushed out individuals of fracture-dwelling species30,32. Moreover, one of the disruptive consequences of the earthquake was that of redistributing the pre-seismic stygobiotic species and causing new co-occurrence patterns and interspecific interactions32. Although the post-seismic community showed structural parameters similar to those of the 1997, species abundances at the level of individual spring of the TS system and their distribution across springs were altered by the earthquake more than by the increased discharge in 2005.
The community dynamics in disturbed areas is highly dependent on the re-colonisation processes from source populations25,74,75. However, copepod re-colonisation did not occur at the TS system for the duration of our study. Changes in the aquifer structure due to the earthquake led to a change in the pre-seismic species patterns. These patterns could not be re-established by colonisation from source pools for two reasons. The first reason is that most stygobites present in the storage subsystems of the aquifer were flushed out during the mainshock; this led to a strong reduction of the populations living in the primary habitat, thus preventing subsequent recolonisation of the springs32. The second reason is related to the fact that discharge remained above average throughout 2012. During a high discharge event, a karst aquifer feeding a spring works like a hydraulic system under pressure76. The pressure in the main drain pushes the groundwater into the less permeable parts of the aquifer, thus producing what is called a “piston effect”, which consists in the propagation of the hydraulic pressure at large distances76. In this aquifer type, which includes large conductive systems with high flow rate and current velocity, most copepods live in the small fractures of annex capacitive subsystems, and, even if good swimmers, they remain somewhat confined to this habitat30,32.
Because of this piston effect, stygobiotic copepods that survived fracture cleaning during the dewatering phase were transported to, and remained trapped in, the less permeable part of the saturated zone30,77,78. Thus, the 2012 post-seismic community was only marginally influenced (if any) by recolonisation processes, and changes in its structure can be substantially attributed only to the effects of the earthquake.
Conclusions
Contrary to expectations, the mainshock of L’Aquila earthquake on 6 April 2009 did not impact negatively on structural parameters of the copepod community, but re-established a more balanced species abundance distribution after the changes induced by the anomalous discharge occurred in 2005. This apparently paradoxical situation is a consequence of the different processes that characterised the stygobiotic and non-stygobiotic species, and highlights the importance of considering species ecology to understand the effects of a catastrophic event, especially when it hits a community comprised of species that differ markedly in their response to environmental changes. However, sorting groundwater taxa into ecological categories is not an easy task and involves a remarkable taxonomic effort, which complicates the study of groundwater ecosystems, when compared with surface-water ecosystems. For example, although identification to species may be important to study the response of Ephemeroptera-Plecoptera-Tricoptera (EPT) to floods79, it seems that, in general, identification to the genus level may be sufficient for defining ecological categories in most surface-water invertebrates80. By contrast, in the groundwater fauna, the same genus may include both stygobiotic and non-stygobiotic species, which have completely different adaptations, trophic roles and colonisation dynamics81. We are aware that ecological studies requiring taxonomic identifications to the species level are onerous, time consuming and can be performed only by trained people; yet our study demonstrates that it is necessary not to jump to misleading conclusions.
Groundwater communities are well known for their low resilience82 and disturbance events that negatively affect their populations may easily lead to local extinction30,82. Most groundwater species are phylogenetic and/or distributional relicts, thus they are species of high conservation concern83. Groundwater habitats are generally considered stable environments, but our study demonstrates that, in fact, they can be suddenly modified by natural changes and that copepod communities can be subject to profound alterations due to occasional, but strong disturbance events represented by earthquakes. Groundwater environments are under a variety of severe anthropogenic pressures, such as pollution and water extraction43. Thus, anthropogenic disturbances occurring in a community already stressed by an earthquake might have extremely negative consequences. Of course, we cannot avoid earthquakes, but we should address any effort to avoid, or at least to reduce, the impact of anthropogenic stressors.
Materials and Methods
Study area and sampling procedures
The TS area is a spring complex at the boundary of the Gran Sasso Aquifer (GSA) located in the Gran Sasso Massif in central Italy (Apennines mountain range), featuring the highest peak south of the Alps (Corno Grande, 2922 m a.s.l.) and characterised by a high- to moderate-altitude montane landscape with low human impact. The GSA is a karstic aquifer with fast-flowing sections (karstic conduits) and interconnected low-flowing water small chambers30. The TS is the largest GSA-fed spring system, receiving ~65% of the GSA discharge35. The short (~15 km) Tirino River originating from TS joins the Aterno-Pescara River before eventually emptying into the Adriatic Sea.
Mean annual discharge at TS was relatively low in the first sampling year (1997: mean ± SD: 5.68 ± 0.21 m3 s−1); it was above-average in the second sampling year (2005: 6.02 ± 0.26 m3 s−1) and was well above average in the third sampling year (2012: 7.14 ± 0.26 m3 s−1) due to a 3-yr rising in discharge caused by the 6.3-Mw 2009 earthquake, before slowly returning to pre-seismic discharge values in summer 201330.
Copepods were collected at eight sampling sites at the TS system adopting a random sampling method with four temporal replicates of three samples of 20 L in each of the eight sampled sites, for a total of 96 samples (and hence 1920 L) in each year. Subsurface samples of water were collected from the springbed (sediment patches and karstic fractures) with a hand-made Bou-Rouch pump32 and mobile pipes hammered at each sampling site. For each replicate, a standardised sample size of 20 L was withdrawn, a volume of water/sediments that is sufficient to obtain reliable estimates of abundance of rare species84. The meiofauna was extracted by filtering the 20-L samples through a hand net (mesh size = 60 µm). Samples were preserved in 80% ethyl alcohol. Individuals were later counted and identified to species level. Species were assigned to two ecological categories: stygobites and non-stygobites. Stygobites (obligate groundwater species) are strictly dependent on groundwater to complete their life cycles, and are drifted or washed periodically to the aquifer outlets following the groundwater flow. Non-stygobites found in the springs are freshwater species that live on the springbed surface, or in sediment interstices (e.g., to avoid predation) or are habitat generalists. Some of them are drifted from surface waters of the recharge area of the aquifer, others can be defined as crenobionts, i.e. species that complete their life cycle in the stable and relatively cold thermal regime of surface spring waters; other are generalist species that can colonise the spring sediments from downstream via the surface hydrological continuum.
Further details about the study site, discharge patterns and sampling procedures are given elsewhere30,36,37. Primary data used in this study have been published in previous papers30,32.
Statistical analysis
Because no single diversity index encompasses all the characteristics of an ideal index85, a combination of them that reflects richness, dominance, evenness, and relative abundance was used. Thus, the following community parameters were calculated to compare pre- and post-seismic communities:
where n i is the abundance of species i and n is the overall abundance (total number of individuals); H′ ranges from 0 (one species dominates the community completely) to high values for communities with many species, each with few individuals.
D varies from 0 (all species are equally present) to 1 (one species dominates the community completely).
where H′ is the Shannon index and S is the total number of species. This index varies from 0 (highest dominance by a single species) to 1 (all species have the same abundance).
i.e. the number of individuals in the dominant species (n max ) divided by n.
Properties of these indices are discussed elsewhere40,86,87,88.
Ninety-five percent confidence intervals for all these indices were computed with a bootstrap procedure with 9999 randomizations. To compare diversity indices of pre- and post-seismic communities, we generated 9999 random matrices with two columns (samples), each with the same row and column totals as in the original data matrix. The probability of obtaining the observed difference by random sampling from a unique parental population was calculated as the number of times that the absolute difference of the indices of a replicate pair exceeded or equalled that of the original samples. Calculations were done using PAST v. 3.089.
We also investigated if the earthquake modified the species abundance distributions (SADs) because the study of SADs allows inferences about patterns in the commonness and rarity of species in a community beyond those that flow from many simple diversity indices and can therefore provide insights into the effects of disturbance on ecological communities90. We modelled SADs using rank-abundance curves40,85. In the abundance-rank representations, all the species in a community are ranked from the most to the least abundant. Each species has a rank, which is plotted on the horizontal axis, while its abundance is plotted on the vertical axis: the abundance for the most abundant species is plotted first, then the next most common and so on until the array is completed by the rarest species.
Several a priori established distributions can be used to model empirical rank-abundance curves59. We compared pre- and post-seismic SADs using a selection of widely applied SAD models that are appropriate for discrete distributions56,91: the geometric series, the broken stick model, the lognormal series, and the Zipf model.
In the geometric series, also known as the niche preemption model, each species takes a constant fraction (α) of the remaining resources and the expected abundance of a species at rank r is:
The only estimated parameter is the preemption coefficient α, which gives the decay rate of abundance per rank, whereas J is the total number of individuals.
The geometric series is the mathematical model used to express the niche preemption hypothesis, in which the sizes of the niche hypervolumes (measured by species relative abundances) are sequentially preempted by the most abundant to the least abundant species. If in the rank-abundance plot a log scale is used for abundance, the species exactly fall along a straight line, according to the equation:
where a is the species abundance, r is the respective rank, and b0 (the intercept) and b1 (the slope) are optimised fitting parameters39. With this approach, it is possible to use the regression slope to compare different species assemblages that follow the same rank-abundance distribution39. Among all proposed SAD models, the geometric series represents the least equitable distribution and it is known to provide a good fit to simple communities characterised by the high dominance of a few species40,85,92. On the opposite, most equitable empirical distributions should be modelled by the broken stick (BS) model93. The BS model is theoretically questionable and communities rarely are correctly characterised by such model88,94. Yet, the BS model is useful in comparative analyses because it represents a simple benchmark in opposition to the geometric series.
In the broken stick model, the expected abundance of species at rank r is:
where J is the total number of individuals and S is the total number of species in the community95. In the BS there are no fitted parameters.
Note that another species abundance distribution model widely used in community ecology for communities dominated by few species is the log-series96,97,98. However, the geometric series and the log-series abundance distributions are interrelated and are two representations of, essentially, the same underlying abundance distribution97,99.
The lognormal is one of the most commonly used models for describing SADs100. It has been derived as a null form of the distribution resulting from the central limit theorem97, and it is classified among the purely statistical models56, but can be the limit of population dynamics101, or niche partitioning102,103.
The lognormal model assumes that the logarithmic abundances are distributed normally:
where µ and σ are, respectively, the mean and standard deviation of the variable’s natural logarithm, and Φ is a normal deviate.
The Zipf distribution (which is a type of power law probability distribution based on branching processes56) is:
where p1 is the fitted proportion of the most abundant species, and γ is a decay coefficient.
Following current best practices in the study of species abundance distributions98,104, we used maximum likelihood estimation to fit models105,106,107 and likelihood-based model selection to compare models108. The lognormal and Zipf models were fitted using generalized linear models with logarithmic link function. The preemption model was fitted as a non-linear (quasi Newton) algorithm. Since species abundances were expressed as count data, we used the Poisson error. We used the Akaike Information Criterion (AIC) to compare the fits of the different models98,104,108. All models were fitted and compared using the R package ‘vegan’ version 2.4-3109.
For communities that followed the geometric series, we also used the regression approach described above and tested the equality of slope with an ANCOVA approach using R110. We conducted all the analyses for all species and for stygobites and non-stygobites separately for the whole TS system.
Finally, we investigated how beta-diversity varied between years. To express between-spring beta-diversity we used the Morisita index, which is suggested as the most appropriate for quantitative data111. We tested for differences in average beta-diversity values between years using paired t-tests. Then, we correlated matrices of between-spring beta-diversity values using Mantel tests (Pearson correlation coefficient, 10000 permutations) to assess if patterns were similar between years. Calculations were done with the R package ‘vegan’ version 2.4-3109.
References
Pickett, S. T. A. & White, P. S. The Ecology of Natural Disturbance And Patch Dynamics (Academic Press, 1985).
Hobbs, R. J. & Huenneke, L. F. Disturbance, diversity and invasions: implications for conservations. Cons. Biol. 6, 324–337 (1992).
Sousa, P. W. The role of disturbance in natural communities. Annu. Rev. Ecol. Evol. S. 15, 353–391 (1984).
Mena, J. L. & Medellin, R. A. Small mammal assemblages in a disturbed tropical landscape at Pozuzo, Peru. Mammal. Biol. 75, 83–91 (2010).
Byrnes, J. E. et al. Climate-driven increases in storm frequency simplify kelp forest food webs. Glob. Change. Biol. 17, 2513–2524 (2011).
Emanuel, K. A. Increasing destructiveness of tropical cyclones over the past 30 years. Nature 436, 686–688 (2005).
Westerling, A. L., Hidalgo, H. G., Cayan, D. R. & Swetnam, T. W. Warming and earlier spring increase western US forest wildfire activity. Science 313, 940–943 (2006).
International Federation of Red Cross and Red Crescent Societies. World Disasters report 2009: focus on early warning and early action, http://www.ifrc.org/Global/WDR2009-full.pdf (2009).
Avouac, J.-P. Earthquakes: Human-induced shaking. Nat. Geosci. 5, 763–764 (2012).
Amos, C. B. et al. Uplift and seismicity driven by groundwater depletion in central California. Nature 509, 483–486 (2014).
Clarke, H., Eisner, L., Styles, P. & Turner, P. Felt seismicity associated with shale gas hydraulic fracturing: the first documented example in Europe. Geophys. Res. Lett. 41, 8308–8314 (2014).
Zhang, J. et al. Impact of the 2008 Wenchuan earthquake on biodiversity and giant panda habitat in Wolong Nature Reserve, China. Ecol. Res. 26, 523–531 (2011).
Lindenmayer, D. B., Likens, G. E. & Franklin, J. F. Rapid responses to facilitate ecological discoveries from major disturbances. Front. Ecol. Environ. 8, 527–532 (2010).
Fattorini, S. Effects of fire on tenebrionid communities of a Pinus pinea plantation: a case study in a Mediterranean site. Biodivers. Conserv. 9, 1237–1250 (2010).
Bixby, R. J. et al. Fire effects on aquatic ecosystems: an assessment of the current state of the science. Fresh. Sci. 34(4), 1340–1350 (2015).
Valle Ferreira, L. Effects of flooding duration on species richness, floristic composition and forest structure in river margin habitat in Amazonian blackwater floodplain forests: implications for future design of protected areas. Biodivers. Conserv. 9(1), 1–14 (2000).
Milner, A. M., Robertson, A. L., McDermott, M. J., Klaar, M. J. & Brown, L. E. Major flood disturbance alters river ecosystem evolution. Nat. Clim. Change 3, 137–141 (2013).
Kwit, C., Platt, W. J. & Slater, H. H. Post-hurricane regeneration of pioneer plant species in South Florida subtropical hardwood hammocks. Biotropica 32(2), 244–251 (2000).
Hou, A. et al. Pathogen indicator microbes and heavy metals in Lake Pontchartrain following Hurricane Katrina. Environ. Sci. Technol. 40(19), 5904–5910 (2006).
McKee, K. & Cherry, J. Hurricane Katrina sediment slowed elevation loss in subsiding brackish marshes of the Mississippi River delta. Wetlands 29(1), 2–15 (2009).
Myster, R. W. & Malahy, M. P. Tornado effects on damage, resprouting and spatial heterogeneity in the Cross Timbers ecotone of Oklahoma, USA. J. Plant. Ecol. 3(3), 157–163 (2010).
Fraver, S. Forest structure following tornado damage and salvage logging in northern Maine, USA. Can. J. For. Res. 47(4), 560–564 (2017).
Fattorini, S. Biogeographical kinetics on mainland and island volcanoes. J. Biogeogr. 37, 2158–2168 (2010).
Fattorini, S. & Borges, P. V. A. Biogeographical kinetics on an island volcano (Capelinhos, Azores): fast colonization rates and dominance of arthropod exotic species. Insect Conserv. Divers. 5, 358–366 (2012).
Lallementa, M. et al. Rising from the ashes: changes in salmonid fish assemblages after 30 months of the Puyehue–Cordon Caulle volcanic eruption. Sci. Total Environ. 541, 1041–1051 (2015).
Urabe, J. & Nakashizuka, T. Ecological Impacts Of Tsunami On Coastal Ecosystems. Lessons From The Great East Japan Earthquake Ecological Research Monograph (Springer, 2016).
Miura, O. et al. Ecological and genetic impact of the 2011 Tohoku Earthquake Tsunami on intertidal mud snails. Sci. Rep. 7, 44375 (2017).
Brancelj, A. et al. Consecutive earthquakes temporarily restructured the zooplankton community in an Alpine Lake. Int. J. Lim. 48, 113–123 (2012).
Jaramillo, E. et al. Ecological implications of extreme events: footprints of the 2010 earthquake along the Chilean coast. PLoS One 7(5), e35348 (2012).
Galassi, D. M. P. et al. Earthquakes trigger the loss of groundwater biodiversity. Sci. Rep. 4, 6273 (2014).
Sepúlveda, R. D. & Valdivia, N. Localised effects of a mega-disturbance: spatiotemporal responses of intertidal sandy shore communities to the 2010 Chilean earthquake. Plos One 11(7), e0157910 (2016).
Fattorini, S. et al. Earthquake-related changes in species spatial niche overlaps in spring communities. Sci. Rep. 7, 443 (2017).
Jackson, J. & McKenzie, D. Active tectonics of the Alpine–Himalayan Belt between western Turkey and Pakistan. Geophys. J. Int. 77(1), 185–264 (1984).
Adinolfi Falcone, R. et al. Changes on groundwater flow and hydrochemistry of the Gran Sasso carbonate aquifer after 2009 L’Aquila earthquake. Ital. J. Geosci. (Boll. Soc. Geol. It.) 131, 459–474 (2012).
Barbieri, M., Boschetti, T., Petitta, M. & Tallini, M. Stable isotopes (2H, 18O and 87Sr/86Sr) and hydrochemistry monitoring for groundwater hydrodynamics analysis in a karst aquifer (Gran Sasso, central Italy). Appl. Geochem. 20, 2063–2081 (2005).
Fiasca, B. et al. The dark side of springs: what drives small-scale spatial patterns of subsurface meiofaunal assemblages? J. Limnol. 73, 71–80 (2014).
Petitta, M., Caschetto, M., Galassi, D. M. P. & Aravena, R. Dual-flow in karst aquifers toward a steady discharge spring (Presciano, Central Italy): influences on a subsurface groundwater dependent ecosystem and on changes related to post-earthquake hydrodynamics. Environ. Earth. Sci. 73, 2609–2625 (2015).
Tokeshi, M. Species abundance patterns and community structure. Adv. Ecol. Res. 24, 112–186 (1993).
Fattorini, S. A simple method to fit geometric series and broken stick models in community ecology and island biogeography. Acta Oecol. 28, 199–205 (2005).
Magurran, A. E. Measuring Biological Diversity (Blackwell Publishing, 2004).
Galassi, D. M. P., Stoch, F., Fiasca, B., Di Lorenzo, T. & Gattone, E. Groundwater biodiversity patterns in the Lessinian Massif of northern Italy. Freshwater Biol. 54(4), 830–847 (2009).
Di Lorenzo, T. & Galassi, D. M. P. Agricultural impact on Mediterranean alluvial aquifers: Do groundwater communities respond? Fundam. Appl. Limnol. 182(4), 271–282 (2013).
Di Lorenzo, T., Cifoni, M., Lombardo, P., Fiasca, B. & Galassi, D. M. P. Ammonium threshold values for groundwater quality in the EU may not protect groundwater fauna: evidence from an alluvial aquifer in Italy. Hydrobiologia 743(1), 139–150 (2015).
Korbel, K., Chariton, A., Stephenson, S., Greenfield, P. & Hose, G. C. Wells provide a distorted view of life in the aquifer: implications for sampling, monitoring and assessment of groundwater ecosystems. Sci. Rep. 7, 40702 (2017).
Manga, M. Using springs to study groundwater flow and active geologic processes. Annu. Rev. Earth Planet. Sci. 29, 201–228 (2001).
Cantonati, M., Gerecke, R., Jüttner, I. & Cox, E. J. Springs: neglected key habitats for biodiversity conservation. J. Limnol. 70, 1–187 (2011).
Cantonati, M., Füreder, L., Gerecke, R., Jüttner, I. & Cox, E. J. Crenic habitats, hotspots for freshwater biodiversity conservation: toward an understanding of their ecology. Freshwater Sci. 31, 463–480 (2012).
Spitale, D., Leira, M., Angeli, N. & Cantonati, M. Environmental classification of springs of the Italian Alps and its consistency across multiple taxonomic groups. Freshwater Sci. 31, 563–574 (2012).
Stoch, F. et al. Exploring copepod distribution patterns at three nested spatial scales in a spring system: Habitat partitioning and potential for hydrological bioindication. J. Limnol. 75(1), 1–13 (2016).
Bottazzi, E. et al. Spatial and seasonal distribution of invertebrates in Northern Apennine rheocrene springs. J. Limnol. 70, 77–92 (2011).
Hahn, H. J. Studies on classifying of undisturbed springs in southwestern Germany by macrobenthic communities. Limnologica 30, 247–259 (2000).
Mori, N. & Brancelj, A. Differences in aquatic microcrustacean assemblages between temporary and perennial springs of an alpine karstic aquifer. Int. J. Speleol. 42(3), 257–266 (2013).
Mori, N., Kanduč, T., Opalički Slabe, M. & Brancelj, A. Groundwater drift as a tracer for identifying sources of spring discharge. Groundwater 53(1), 123–132 (2015).
Amoruso, A. et al. Impact of the 6 April 2009 L’Aquila earthquake on groundwater flow in the Gran Sasso carbonate aquifer, Central Italy. Hydrol. Process. 25, 1754–1764 (2011).
Amoruso, A., Crescentini, L., Petitta, M. & Tallini, M. Parsimonious recharge/discharge modeling in carbonate fractured aquifers: The groundwater flow in the Gran Sasso aquifer (Central Italy). J. Hydrol. 476, 136–146 (2013).
McGill, B. J. et al. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10, 995–1015 (2007).
Whittaker, R. H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 30, 279–338 (1960).
Bazzaz, F. A. Plant species diversity in old-field successional ecosystems in southern Illinois. Ecology 56, 485–488 (1975).
Ulrich, W., Ollik, M. & Ugland, K. I. A meta-analysis of species-abundance distributions. Oikos 119, 1149–1155 (2010).
Hill, J. K. & Hamer, K. C. Using species abundance models as indicators of habitat disturbance in tropical forests. J. Appl. Ecol. 35, 458–460 (2003).
Connolly, S. R., Hurghes, T. P., Bellwood, D. R. & Karlson, R. H. Community structure of corals and reef fish at multiple scales. Science 309, 1363–1365 (2005).
Šizling, A. L. et al. Species abundance distribution results from a spatial analogy of central limit theorem. PNAS 106, 6691–6695 (2009).
Gould, L., Sussman, R. W. & Sauther, M. L. Natural disasters and primate populations: the effects of a 2-year drought on a naturally occurring population of ring-tailed lemurs (Lemur catta) in southwestern Madagascar. Int. J. Primatol. 20, 69–84 (1999).
Lai, Y.-C., Shieh, B.-S. & Kam, Y.-C. Population patterns of a riparian frog (Rana swinhoana) before and after an earthquake in subtropical Taiwan. Biotropica 39, 731–736 (2007).
Connell, J. H. Diversity in tropical rain forests and coral reefs. Science 199(4335), 1302–1310 (1978).
Tilman, D. The benefits of natural disasters. Science 273(5821), 1518 (1996).
Vittoz, P., Stewart, G. H. & Duncan, R. P. Earthquake impacts in old-growth Nothofagus forests in New Zealand. J. Veg. Sci. 12, 417–426 (2001).
Kirkland, G. L. Jr. Patterns of initial small mammal community change after clearcutting of temperate North American forests. Oikos 59, 313–320 (1990).
Fisher, J. T. & Wilkinson, L. The response of mammals to forest fire and timber harvest in the North American boreal forest. Mammal Rev. 35, 51–81 (2005).
Raybuck, A. L. Short-term response of small mammals following oak regeneration silviculture treatments. Forest Ecol. Manage. 274, 10–16 (2012).
Li, B., Ran, J. H., Yue, B. S., Zhang, M. & Wu, Y. J. Non-volant small mammals in landslides caused by the Wenchuan earthquake in a fragmented forest of Sichuan, China. Pak. J. Zool. 47, 535–544 (2015).
Hopkins, M. E. Mantled howler (Alouatta palliata) arboreal pathway networks: relative impacts of resource availability and forest structure. Int. J. Primatol. 32, 238–258 (2011).
Schmid-Araya, J. S., Schmid, P. E., Tod, S. P. & Esteban, G. F. Trophic positioning of meiofauna revealed by stable isotopes and food web analyses. Ecology 97(11), 3099–3109 (2016).
Ulrich, W. & Ollik, M. Frequent and occasional species and the shape of relative abundance distributions. Div. Distr. 10, 263–269 (2004).
Shenko, A. N., Bien, W. F., Spotila, J. R. & Avery, H. W. Effects of disturbance on small mammal community structure in the New Jersey, Pinelands, USA. Integr. Zool. 7, 16–29 (2012).
Milanović, P. Hydraulic properties of karst groundwater and its impacts on large structures in H2Karst Research in Limestone Hydrogeology (ed. Mudry, J., Zwalhen, F., Bertrand, C. & LaMoreaux, J.W.) 19–47 (Springer, 2014).
Di Lorenzo, T. et al. Dynamics of groundwater copepod assemblages from the Mazzoccolo karstic spring (central Italy). Meiofauna Marina 14, 97–103 (2005).
Fattorini, S. et al. Trapped in the web of water: Groundwater-fed springs are island-like ecosystems for the meiofauna. Ecol. Evol. 6, 8389–8401 (2016).
Monk, W. A. et al. How does macroinvertebrate taxonomic resolution influence ecohydrological relationships in riverine ecosystems. Ecohydrol. 5, 36–45 (2012).
Guerold, F. Influence of taxonomic determination level on several community indices. Water Res. 34, 487–492 (2000).
Galassi, D. M. P. Groundwater copepods: diversity patterns over ecological and evolutionary scales. Hydrobiologia 453(1), 227–253 (2001).
Gibert, J., Danielopol, D. L. & Stanford, J. A. Eds Groundwater Ecology (Academic Press, San Diego, 1994).
Galassi, D. M. P., Dole-Olivier, M.-J. & De Laurentiis, P. Phylogeny and biogeography of the genus Pseudectinosoma, and description of P. janineae sp. n. (Crustacea, Copepoda, Ectinosomatidae). Zool. Scr. 28, 289–303 (1999).
Dole-Olivier, M.-J. et al. Assessing invertebrate assemblages in the subsurface zone of stream sediments (0-15 cm deep) using a hyporheic sampler. Water Resources Res. 50, 453–465 (2014).
Magurran, A. E. Ecological Diversity And Its Measurements (Princeton University, 1988).
Krebs, C. J. Ecological Methodology, Second edition. (A. Wesley Longman, 1999).
Legendre, P. & Legendre, L. Numerical Ecology. Second edition. Developments In Environmental Modelling (Elsevier, 1998).
Hayek, L. C. & Buzas, M. A. Surveying Natural Populations. Quantitative Tools for Assessing Biodiversity (Columbia Univ. Press, 2010).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST—PAlaeontological STatistics, ver. 1.89. Palaeontol. Electron. 4(1), 1–9 (2001).
Matthews, T. J. & Whittaker, R. J. On the species abundance distribution in applied ecology and biodiversity management. J. Appl. Ecol. 52, 443–454 (2015).
Morlon, H. et al. Taking species abundance distributions beyond individuals. Ecol. Lett. 12, 488–501 (2009).
Giller, P. S. Community Structure And The Niche (Chapman and Hall, 1984).
Higgins, C. L. & Strauss, R. E. Modelling stream fish assemblages with niche apportionment models: patterns, processes, and scale dependence. T. Am. Fish. Soc. 137, 696e706 (2008).
Wilson, J. B. Would we recognise a broken-stick community if we found one? Oikos 67, 181–183 (1993).
Pielou, E. Ecological Diversity (Wiley, 1975).
Baldridge, E., Harris, D. J., Xiao, X. & White, E. P. An extensive comparison of species-abundance distribution models. PeerJ 4, e2823 (2016).
May, R. M. Patterns of species abundance and diversity in Ecology And Evolution Of Communities (eds Cody, M. L. & Diamond, J. M.) 81–120 (Harvard University Press, 1975).
Matthews, T. J. & Whittaker, R. J. Fitting and comparing competing models of the species abundance distribution: assessment and prospect. Front. Biogeogr. 6, 67–82 (2014).
Solé, R. V., Alonso, D. & Saldaña, J. Habitat fragmentation and biodiversity collapse under in neutral communities. Ecol. Complex. 1, 65–75 (2004).
McGill, B. J. A test of the unified neutral theory of biodiversity. Nature 422, 881–885 (2003).
Engen, S. & Lande, R. Population dynamic models generating species abundance distributions of the gamma type. J. Theor. Biol. 178, 325–331 (1996).
Bulmer, M. On fitting the Poisson lognormal distribution to species-abundance data. Biometrics 30, 101–110 (1974).
Sugihara, G. Minimal community structure: an explanation of species abundance patterns. Amer. Nat. 116, 770–787 (1980).
Connolly, S. R. et al. Commonness and rarity in the marine biosphere. Proc. Natl. Acad. Sci. USA 111, 8524–8529 (2014).
Clark, R., Cox, S. & Laslett, G. Generalizations of power-law distributions applicable to sampled fault-trace lengths: model choice, parameter estimation and caveats. Geophys. J. Int. 136, 357–372 (1999).
Newman, M. E. Power laws, Pareto distributions and Zipf’s law. Contem. Phys. 46, 323–351 (2005).
White, E. P., Enquist, B. J. & Green, J. L. On estimating the exponent of power-law frequency distributions. Ecology 89, 905–912 (2008).
Burnham, K. P. & Anderson, D. R. Model Selection And Multimodel Inference. A Practical Information-Theoretic Approach, 1–488 (Springer, 2002).
Oksanen Jari, O. et al. Package ‘vegan’. R package version 2.4-3, https://CRAN.R-project.org/package=vegan (2017).
R Core Team. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.Reproject.org/ (2016).
Barwell, L. J., Isaac, N. J. B. & Kunin, W. E. Measuring β-diversity with species abundance data. J. Anim. Ecol. 84(4), 1112–1122 (2015).
Acknowledgements
We are grateful to B. Fiasca and A. Di Cioccio for their help in copepod sampling and identification and to S. West for language suggestions. The project was funded by the European Community (LIFE12 BIO/IT/000231 AQUALIFE).
Author information
Authors and Affiliations
Contributions
S.F. had the idea; T.D.L. checked the ecology of the species; S.F. performed the statistical analyses; D.M.P.G. led the study; all authors discussed the results and contributed to writing the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fattorini, S., Di Lorenzo, T. & Galassi, D.M.P. Earthquake impacts on microcrustacean communities inhabiting groundwater-fed springs alter species-abundance distribution patterns. Sci Rep 8, 1501 (2018). https://doi.org/10.1038/s41598-018-20011-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20011-1
- Springer Nature Limited
This article is cited by
-
Impact of climate change on biodiversity loss: global evidence
Environmental Science and Pollution Research (2022)
-
Climate change, insurance market, renewable energy, and biodiversity: double-materiality concept from BRICS countries
Environmental Science and Pollution Research (2022)
-
The impact of nitrate on the groundwater assemblages of European unconsolidated aquifers is likely less severe than expected
Environmental Science and Pollution Research (2021)
-
Spatial distribution of stygobitic crustacean harpacticoids at the boundaries of groundwater habitat types in Europe
Scientific Reports (2020)