Abstract
Nine synthetic amphiphilic phenolic lipids, varied in phenolic moiety (caffeoyl/dimethylcaffeoyl) and fatty acid chain lengths (8–18) were characterized by differential scanning calorimetry (DSC), temperature-ramp Fourier transform infra-red spectroscopy (FT-IR) and atomic force microscopy (AFM). FT-IR and DSC results revealed that the physical state and lateral packing of synthetic molecules were largely governed by fatty acyls. The critical micelle concentrations (CMC) of synthetic lipids was in the range of 0.1 mM to 2.5 mM, affording generation of stable oil-in-water emulsions; as evidenced by the creaming index (<5%) of emulsions stabilized by compounds C12‒C16, and C12a‒C16a after 7 days’ storage. AFM analysis revealed that compound C14 formed stable double-layers films of 5.2 nm and 6.7 nm. Application studies showed that formulations stabilized by synthesized compounds containing 30% fish oil had superior physical and oxidative stability compared to formulations containing commercial emulsifiers or their mixtures with phenolic acids. Moreover, the synthetic compounds were non-toxic against in vitro transformed keratinocytes from histologically normal skin and Caco-2 cell lines. This study demonstrates the relevance of using a natural hydroxycarboxylic acid as a flexible linker between natural antioxidants, glycerol and fatty acids to generate multifunctional amphiphiles with potential applications in food, pharmaceutical and cosmetic industry.
Similar content being viewed by others
Introduction
Fats and oils, carbohydrates, carboxylic acids and phenolic acids have been demonstrated to be valuable raw materials for the development of functional materials for various applications1,2,3,4,5,6,7,8,9. However, the aforementioned raw materials are either hydrophilic (e.g. carboxylic acids, carbohydrates and phenolic acids) or hydrophobic (fats, oils and fatty acids) which limit their application in foods, drugs and cosmetics, where amphiphilic molecules are needed1,2,3,4,5,6,7,8,9.
In a previous work, the rational design of a 2-step synthetic approach permitted the assembling of natural building blocks into a single amphiphilic agent to yield antioxidant emulsifiers (Fig. 1)10. Malic acid was used as flexible linker between natural phenolic acids and monoacylglycerols. The resulting compounds differed from any phenolipids previously reported due to the presence of a hydroxycarboxylic acid moiety, which potentially enable interactions with macromolecules11, and thereby further stabilize droplets of lipophilic ingredients enclosed in the hydrophobic core. Therefore, the compounds are expected to be used as high capacity delivery cargos (e.g. ~70% fish oil) for lipophilic ingredients due to their multifunctional properties (surface activity and antioxidant property), and ability to interact with macromolecules. In addition, due to structural similarities to other compounds in the literature1,2,3,4,5,6,7,8,9, the synthesized amphiphiles could potentially find applications not only in food but also in cosmetics, or as nanocarriers. For instance, longer alkyl chain derivatives of this series of compounds could be applied in cosmetic formulations as protective skin barriers5,6.
Thus, the aim of this work was to further characterize malic acid esters of monoglycerides through multiple physicochemical techniques including Differential Scanning Calorimetry (DSC), temperature-ramp Fourier Transform Infrared spectroscopy (FTIR), and Atomic Force Microscopy (AFM) and confirm their great potential for industry applications. Cytotoxicity assays of the synthesized compounds on a normal cell line established from the skin of a 62-years old Caucasian man were also performed. Moreover, the functionality of the compounds was investigated by measuring the ability of the amphiphilic lipids to stabilize 30% oil-in-water emulsions. Furthermore, the antioxidant potency of the amphiphilic compounds in emulsions was determined using thiobarbituric acid reactive substances (TBARS).
Results and Discussion
Synthesis and characterization
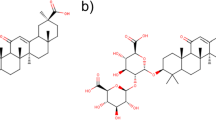
Six compounds (C8, C10, C12, C14, C16 and C18) based on 3,4-dimethoxy-cinnamic acid and three compounds (C12a, C14a and C16a) based on caffeic acid (Fig. 1) were synthesized and fully characterized. Synthesis was carried out following a procedure previously described in the literature10. Briefly, phenolic acids were respectively made react with malic acid to form a phenoleoyl-malic acid anhydride, which was then coupled to monoglycerides by a ring opening mechanism4. The structures of all synthesized compounds were accurately identified by use of MS and NMR (1H, 13C), and further characterized by use of DSC, FT-IR and atomic force microscopy (AFM). In addition, the ability of the compounds to function as emulsifiers was tested by measuring creaming index. The ability of the compounds to inhibit lipid oxidation in emulsions was further determined. Moreover, the cytotoxicity of the compounds was evaluated using in vitro transformed keratinocytes from histologically normal skin (HaCAT) and human colorectal adenocarcinoma (Caco-2).
Thermal analysis by use of Differential Scanning Calorimetry
The amphiphilic lipids were characterized by means of DSC to determine their melting transition temperatures. This is relevant for applications as emulsifiers12, skin lipids5,6, or nanocarriers9. No clear melting transition temperatures were observed for compounds C10‒C16 when temperature scanning from was done −60 °C to 60 °C. On the other hand, a melting point of 38 °C was observed for compound C18 (Figure S1). DSC results suggest that multi-acylation with bulky groups (in the form of phenolic and malic acids) of the glycerol backbone of monoglycerides frustrates the molecular packing of the resulting compounds leading to disorganized structures6. However, elongating the hydrocarbon chain to 18 carbons sufficiently increased the van der Waals interaction of the hydrocarbon chain to overcome the internal repulsions of the ester and carboxylic functional groups, and hence promoted a better organization of the molecules5,6 consequently leading to a melting point above the room temperature (See Table 1 for a summary of the physicochemical properties of the synthesized compounds).
Determination of lateral organization of alkyl chains of amphiphiles
Even though DSC provides valuable information about the thermal transitions of compounds, little is gained about their molecular organizations9. FT-IR is a powerful tool to acquire detailed information about the conformations and molecular orientations of alky side chains of lipids13. The aim of the FT-IR study was to establish further structure-activity relationships for the amphiphilic lipids as this knowledge is relevant for applications of the compounds as emulsifiers9, skin lipids5,6 or as agents for drug delivery9. The CH2 bending bands (∼720‒725 cm−1) and CH2 symmetric stretching bands (∼2920 cm−1 and 2850 cm−1) were of particular interest as these regions provide information regarding lipid chain organization. The CH2 rocking region (720–740 cm−1) of synthesized compounds C8, C10, C12, C14, C16, C12a, C14a and C16a at 28 °C displayed a single peak indicating that these compounds present hexagonal packing (Table 1 summarizes the packing modes of the various amphiphilic lipids), which suggest less organized lipid structures. However, compound C18 showed a doublet at 720 and 740 cm−1, which indicates orthorhombic packing, a more organized conformation of the molecules(Figure S2 shows the rocking region of C10 and C18 as an example). The orthorhombic packing behavior displayed by compound C18 suggests that long hydrocarbon chains (>16) are required to generate enough van der Waals interactions to compensate for the intermolecular repulsions from the ester and carboxylic functional groups in the molecules. This finding corroborates previous reports from other authors5,14,15, and shows that the lengths of hydrocarbon chains affect molecular organization of amphiphilic lipids. In addition, compound C18 displayed peaks at lower frequencies in the CH2 symmetric and asymmetric stretching regions (2848 cm−1 and 2916 cm−1, respectively) compared to compounds C10 to C16 (2852-2854 cm−1 and 2921–2923 cm−1, respectively) suggesting a more organized lipid chain conformation. Frequency shifts in symmetric and asymmetric stretching of hydrocarbon chains can also be used to predict conformational changes under thermal stress. High frequencies indicate disordered chain packing modes while low frequencies indicate that the hydrocarbon chains are in the well-ordered all-trans conformation16. Hence, the FT-IR results showed that the alkyl chains of C18 were in the all-trans conformation at 28 °C. This highly organized conformation is observed up to 46 °C degrees where the lipids chains become disordered (Fig. 2). In other words, compound C18 is able to maintain its molecular organization until 46 °C where disorderliness sets in. This result implies longer derivatives (>C22:0) of malic acid esters of monoglycerides can potentially be excellent skin barrier material and can therefore find value in cosmetic formulations, where tight molecular organizations are essential5,6,17.
On the contrary, the rest of the compounds (C8, C10, C12, C14, C16, C12a, C14a and C16a) displayed disorganized lipids chains at room temperature with peaks above 2852 cm−1 and 2921 cm−1 in the FTIR spectra. The latter is in agreement with DSC analysis where only C18 displayed a melting point above 25 °C and the rest of the compounds being liquid at this temperature. The similarities in terms of the molecular organization and physical state of compounds C8‒C16 to other amphiphilic compounds6,12 reported suggest that they could find application as emulsifiers in foods12 and cosmetics6. Previous studies showed that amphiphilic lipids with short alkyl side chains are more digestible in the gastrointestinal tract compared to amphiphiles with longer alkanyl side chains18. Therefore, C8‒C16 derivatives could favor more rapid digestion by gastrointestinal tract (GIT) lipases and could be applied for the encapsulation and hence rapid release of omega-3 oils in the GIT.
Surface activity of amphiphiles
The application of a surfactant depends on its surface active properties. One of the analytical methods to determine the surface activity of emulsifiers is by measuring emulsion stability19 (creaming index, CI) as function of time of storage. In this work, the CI of oil-in-water emulsions were determined as a function of time and correlated to CMC (Figure S3 in supporting information) of the amphiphilic lipids. CI was generally low during early days of storage (day 0 to day 3), increased gradually to a maximum and then remained stable (Fig. 3). Emulsions stabilized by compounds C12, C12a, C14, C14a, C16 and C16a had comparable CI as commercial DATEM, and were thermodynamically stable throughout the period of storage (0% CI). However, creaming was highest in emulsions stabilized by compound C18 (37.2%) followed by compound C8 (15.1%) and then compound C10 (10.35%) after 7 days of storage, suggesting that there was no correlation between the length of the hydrocarbon chain and the CI of the different phenolic emulsifiers.
It is interesting to note that certain ranges of CMC values favored emulsion stability. Compounds C12, C12a, C14, C14a, C16 and C16a, which formed the most stable emulsions, had CMC values in the range of 0.1 mM to 2.5 mM. As depicted in Fig. S3, the change of in CMC is a function of carbon chain length of fatty acyl moiety. CMC was found to decrease with increasing length of the hydrocarbon chain of amphiphilic lipids. This suggests that a decrease in hydrophobicity of amphiphilic lipids with the same head group is accompanied by an increase in CMC, a trend similar to results from other works20,21. However, structural features such as size, conformation, and configuration of surfactants also play important roles in determining CMC of surfactants22.
In the broader interpretation of these results, it could be explained that compound C18 being more hydrophobic had a very low CMC (0.01 mM) and consequently, a poor surface activity as evidenced by CI measurements. On the contrary, C8 and C10 were very hydrophilic and presented high CMC values (10 mM and 5 mM, respectively). Hence, C8 and C10 will preferentially reside in the aqueous phase of emulsions and that explains the high CI in emulsions stabilized by C8 and C10. In summary, the CI and CMC data suggest that the compounds could be categorized into three main groups namely, the highly hydrophilic group (C8 and C10) with high CMC and good surface activity, the surface active group (C12‒C16) with intermediate CMC, and the highly hydrophobic group (C18) with very low CMC and very poor surface activity.
Determination of antioxidant properties of amphiphiles
Compounds C12a‒C16a being caffeic acid derivatives are expected to be potent antioxidants and could be used as encapsulating agents for oils or drugs sensitive to oxidation. Therefore, an investigation of the ability of the synthesized compounds to inhibit lipid peroxidation in fish oil enriched emulsions was carried out for the most surface active compounds (C12a, C14a and C16a). In addition, an emulsion stabilized with commercial DATEM was used as a control while another emulsion stabilized with commercial DATEM but with added caffeic acid was used for comparison.
As displayed on Fig. 4, all the tested compounds inhibited lipid oxidation in emulsions. For instance, compared with a commercial DATEM with no antioxidant activity, there was only 19.26 ± 0.05% oxidation in emulsions prepared with C14a after 7 days of storage. However, oxidations were significantly (p < 0.05) higher in emulsions prepared with compounds C12a and C16a compared to emulsions stabilized with C14a. In emulsions, transition metals and other agents which promote lipid oxidation, are present in the aqueous phase, and come into close contact with lipids on the surface of oil droplets22. Thus, compound C14a, being an excellent surface active agent as demonstrated by the creaming stability, is expected to be highly concentrated at the oil-water interface and offer a better protection against lipid oxidation.
The degree of oxidation observed in emulsions stabilized by the new compounds are significantly (p < 0.05) lower than the 20.8 ± 2.7% reported for 20% omega-3 oil-in-water emulsions stabilized by dodecenyl succinic anhydride modified alginate2. Furthermore, lipid oxidation was high in emulsions prepared with mixtures of commercial DATEM and caffeic acid compared to emulsions prepared with the phenolic emulsifiers C12a, C14a and C16a. This could be due to a higher concentration of the hydrophilic caffeic acid in the aqueous phase and consequently, a lower concentration at the oil-water interface of the emulsions stabilized with commercial DATEM. Moreover, a high-density of phenolic acids at oil-water interface sets a more effective barrier to prevent penetration of preformed free radicals into the core of encapsulated oil.
In other words, this result provides evidence that this group of synthetic molecules elaborately integrates multi-function into a single molecule by bringing otherwise water-soluble phenolic acids into the interface where lipid oxidation occurs. This agrees with a previous report22 that shows significantly higher lipid peroxides in emulsions stabilized by Tween 20 with added erythorbic acid than in emulsions stabilized with the surface active compound erythorbyl laurate. To the best of our knowledge, the amphiphilic molecules here presented, are the first series of molecules which are sufficiently rich in hydrophilic, hydrophobic and antioxidant moieties that can find value in a wide range of applications.
Atomic Force Microscopy of monolayers
The results from CI determinations and antioxidant activity assay showed that C14 was the optimal molecule for delivery of high loads of omega-3 oils. C14 was therefore chosen for further studies using AFM (Fig. 5 and Figure S4). The Langmuir isotherm of compound C14 showed that monolayer collapse occurred at surface pressures of 39 mN/m. Therefore, a pressure in the liquid condensed phase (~22 mN/m) was selected to transfer monolayers of C14 onto a solid hydrophilic mica substrate for AFM studies. AFM images showed that compound C14 was prone to form stable double-layer films (Film thickness = 5.2 nm and 6.7 nm). Nevertheless, monolayers of 2.3 nm of thickness were also observed. The molecular length of compound C14 from the methyl group of the fatty acid chain to the hydroxyl moiety at the para-position of caffeic acid is approximately 3.4 nm, when the fatty acid chain is fully extended. Therefore, it can be inferred that at P = 22 mN/m mixtures of monolayers and double-layer are present. Since the film thickness found was 2.3 nm but the theoretical length of a molecule of C14 is 3.4 nm, it can be concluded that the molecules are tilted relative to the mica substrate13,23 The significance of the ability of C14 molecules to form monolayers means the hydrophobic core could be loaded with lipophilic ingredients such as omega-3 oils making C14 an excellent delivery vehicle for bioactive compounds9. In addition, a film thickness of 6.7 nm, which is about two molecular lengths of compound C14, indicates the presence of double layer9. This result demonstrated that compound C14 has the potential to form liposome structure useful for encapsulation of hydrophilic compounds of medicinal interest.
Fast Fourier transform filtering (FFTT) was applied to the AFM images of C14 to evaluate possible patterns in periodicity and directions of molecular alignments6. However, contrary to results observed for glycerol monobehenate6 and lignoceric acid24, FFTT result (Fig. 5b) revealed no periodicity patterns for sample C14 which demonstrates that the observed stripes in Fig. 5d for AFM images of compound C14 are a result of the imaging process rather than a property of the system.
Toxicity evaluation of amphiphilic lipids
Since consumers prefer ingredients from natural sources, efforts are continually being made to replace synthetic ingredients with their natural counterparts25. Thus, to evaluate the hypothesis that joining compounds from natural source will lead to non-toxic emulsifiers, cytotoxicity assays of the synthesized compounds using in vitro HaCat and Caco-2 cell lines were performed. The results show that cell proliferation was 100% with non-noticeable cell death when cultured in increasing concentrations of solutions of the new amphiphiles (from 0μM to 50μM) (Fig. 6A,B). These results support the view that assembling natural molecules into a single multifunctional compound may lead to non-toxic agents.
Conclusions
A new series of phenolic-containing amphiphilic compounds were synthesized from renewable raw materials. The compounds were physicochemically characterized by use of DSC, Temp-ramp FTIR and AFM. FT-IR studies revealed that the compounds adopt a hexagonal packing with the exception of compound C18 which exists in the tight orthorhombic packing mode. The orthorhombic packing mode of C18 suggests that it could be applied as encapsulation matrix25 for oxidation sensitive drugs and that longer derivatives of malic acid esters of monoglycerides can find value as skin protective barrier material for cosmetic formulations similar to other long chain amphiphiles reported5,6. Therefore synthesis of ultra-long chain derivatives of these compounds could provide novel functional skin lipids.
In addition, the surface-active properties of the compounds were confirmed by their ability to form stable fish oil-in-water emulsions as measured by creaming index. Compounds C12‒C16 & C12a‒C16a had excellent surface activity with very stable emulsions (CI < 5%) even after 30 days of storage. Moreover, compounds C12a, C14a and C16a significantly (p < 0.05) inhibited lipid oxidation in emulsions compared to mixtures of commercial DATEM and caffeic acid because of their high surface activity, and preferential location of the phenolic moiety at the oil-water interface where lipid oxidation occurs. However, lipid oxidations were significantly higher (p < 0.05) in emulsions prepared with compounds C12a (25.72 ± 0.03% and C16a (39.04 ± 0.02%) compared to emulsions stabilized by compound C14a (19.26 ± 0.05%) suggesting that C14a was highly concentrated at the interface of the oil-in-water emulsions. Compared to other antioxidant emulsifiers reported, compounds C12a, C14a and C16a are comparable or even superior at protecting omega-3 oils against oxidation2. Therefore, compounds C12a‒C16a represent new materials for protecting oxidative sensitive fish oils against chemical deterioration.
Atomic force microscopy of Langmuir-Blodgett films of the optimal surface active and antioxidant compound (C14) on mica substrate revealed the formation of stable double-layer films (Film thicknesses of 5.2 nm and 6.7 nm), and monolayer films of 2.3 nm thickness. It can be inferred that the ability of these compounds to form stable monolayer films in addition to their superior surface activity and antioxidant properties mean that they can be applied for the delivery of lipophilic ingredients with high load capacity. This is an improvement compared to conventional emulsifiers or stabilizers for delivery of bioactives2,22,27. Moreover, since the compound is capable of forming stable double layers of 5.2 nm and 6.7 nm, it can potentially form liposome structures and find application in the encapsulation of hydrophilic compounds prompt to oxidation.
Furthermore, cytotoxicity assays using HaCAT showed that the new multifunctional amphiphiles are non-toxic and could be used as ingredients for food, cosmetics or in pharmaceutical formulations. This is the first study to use natural a hydroxycarboxylic acid as a flexible linker between natural antioxidants, glycerol and fatty acids to generate a new series of multifunctional amphiphiles with potential applications in food, drug and cosmetic applications.
Materials and Methods
Glycerol, caffeic acid, 3,4-dimethoxycinnamic acid, 2,2-diphenyl-1 picrylhydrazyl (DPPH), tricholoroacetic acid (TCA), thiobarbituric acid (TBA), sodium salicylate >99.5% (C7H5NaO3), pyrene, H2O2, pyridine (anhydrous, >99%) and all other chemicals were purchased from Sigma-Aldrich (St. Louise, USA). 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid (C11-BODIPY581/591)) was obtained from Molecular probes (Leiden, The Netherlands), and Diacetyltartaric acid esters of mono- and di-glycerides (DATEM) was obtained as a gift from Dupont Nutrition and Health (Brabrand, Denmark). All water used was de-ionized water obtained from a Milli-Q (Millipore, MA) system. Structural elucidation of target compounds was done using a Bruker Maxis Impact electrospray ionization quadrupole time-of-flight mass spectrometer (ESI-QTOF-MS).1H and 13C NMR spectroscopy were carried out on a Bruker Avance III spectrometer at 400 MHz. FT-IR Spectra were recorded using an ATR-FTIR (PIKE, Madison, WI; Bruker, Ettlingen, Germany). TLC was used to monitor the progress of the reaction using a solvent mixture of diethylether:petroleum ether:acetic acid (85:15:1 v/v/v).
General procedures for synthesis of amphiphilic lipids
Synthesis of monoglycerides
Monoglycerides of various fatty acid chain lengths (8, 10, 12, 14, 16 and 18) were synthesized using Novozyme 435 (Candida antarctica lipase B) in t-BuOH. A typical reaction consisted of 2 mmol of fatty acid, 24 mmol of glycerol, Novozym 435 (8% of total weight of equivalent amount of fatty acid) and 200 mg molecular sieves (3 Å, activated by heating up to 180 °C for 8 h) in 5 mL t-BuOH, a reaction time of 4 h and magnetic stirring at 360 rpm. The reactions mixtures were incubated in 50 ml glass jacketed reactors at a temperature of 40 °C for 30 min before the addition of the lipase.
After the reaction, the mixture was extracted with 50 mL DCM, washed three times with saturated Na2CO3 solution to remove the unreacted fatty acid, and then three times with a saturated NaCl solution. After removal of the trace amounts of water with anhydrous sodium sulfate, the organic phase was separated and the solvent was removed by rotatory evaporation under vacuum.
Synthesis of phenolic acids-hydroxycarboxylic acid adducts (compounds 1a & 1b)
Approximately 2 g of 3,4-dimethoxycinnamic acid or caffeic acid was placed in a 250 ml two-necked round bottom flask fitted with a condenser and the flask placed in an oil-bath at a constant temperature of 90 °C with mechanical agitation at 360 rpm. Thionyl chloride was gradually added from a dropping funnel through the open end of the flask over a period of 30 min and the reaction maintained for 2 h at 90 °C and the excess thionyl chloride distilled off. After cooling to room temperature malic acid (mole ratio of malic acid: phenolic chloride, 1:2) was added. The mixture was warmed to 70 °C for 4 h with stirring at 360 rpm. After cooling to room temperature, the resulting product thoroughly washed with diethylether to remove excess phenolic acid and dried under nitrogen to obtain a white powder (Compound 1a: 3,4-dimethoxy-cinnamoyl malic anhydride and Compound 1b: 3,4-dihydroxy-cinnamoyl malic anhydride). Though this procedure employs less environmentally friendly reagents and solvents, we are currently developing a greener alternative reaction employing biocatalysts.
Compound 1a. White powder. HRMS: calculated for C15H16O8Na [M + Na] + :347.0737; found: 347.0745.
Compound 1b. White powder. HRMS: calculated for C13H12O8Na [M + Na] + : 319.0424; found: 319.0487.
Synthesis and structural identification of phenolic emulsifiers
To a solution of the respective monoacylglycerol (1.78 g) in 5 ml anhydrous DMF, compound 1a or compound 1b (0.5 g) was added. The reaction mixture was cooled to 0 °C and 0.002 moles of dry pyridine was added. Stirring was continued at 360 rpm under nitrogen for 30 min, and then at room temperature for 16 h. After completion of the reaction, 2 N HCl was added at 0 °C with vigorous stirring and the mixture extracted with ethyl acetate. The organic phase was washed three times with brine, dried over anhydrous sodium sulfate, filtered and vacuum dried to yield the desired product. A total of 9 compounds were synthesized by this procedure. Six compounds (C8, C10, C12, C14, C16 and C18) based on 3,4-dimethoxy-cinnamic acid and three compounds (C12a, C14a and C16a) based on caffeic acid (Fig. 1). Detailed spectroscopy data can be found elsewhere10.
Physico-chemical characterization of synthesized compounds
Differential scanning calorimetry measurement
The thermal properties of the compounds were analyzed using Differential Scanning Calorimetry (DSC) instrument on Pyris 6 system (Perkin-Elmer Cetus, Norwalk, USA). Compounds were dried under low pressure over night, and then encapsulated in aluminum pans. The measurement was under an atmosphere of nitrogen with flow of 20 mL/min. The heating and cooling profile was: 1) initial temperature 0 °C; 2) ramp 10 °C /min to 60 °C; 3) isothermal for 5 min; 4) ramp 10 °C /min to −60 °C; 5) isothermal for 5 min; and 6) ramp 10 °C /min to 60 °C. The DSC scans were evaluated by using MicroCal Origin 9.0 software.
Temp-Ramp- Fourier transform infrared spectroscopy measurement
Fourier transform infrared spectroscopy (FT-IR) was applied to determine the molecular organization of the amphiphilic lipids. Spectra were recorded using an ATR-FTIR (PIKE, Madison, WI; Bruker, Ettlingen, Germany). The synthesized compounds were dried under low pressure over night, and then pressed onto a ZnSe ATR crystal mounted in a trough plate. The ATR crystal was coupled with an Auto Pro Temperature Controller (Pike Technologies, Madison, WI) for gradual heating of the crystal from 30 °C to 60 °C. Spectra were collected with a spectral resolution of 4 cm−1 with 8 scans over the range of 3500–650 cm−1. The FTIR spectra were analyzed by using MicroCal Origin 9.0 software.
Determination of critical micelle concentration of emulsifiers
Critical Micelle Concentration (CMC) values of the synthetic compounds were determined by pyrene fluorescence method28 using a Varian Cary Eclipse Fluorescence spectrometer (Agilent Technology, California, USA). The respective solutions were prepared using water previously saturated with pyrene (1 µM final concentration) in different concentrations (0.000001, 0.00001, 0.0001, 0.001, 0.01, 0.1, 1, 2.5, 5, 10, 15, 20 mM). Emission spectra of pyrene were obtained by exciting the samples at 334 nm. The fluorescence intensity ratio of I1/I3 (I1 = 373 nm, I3 = 383 nm) was plotted against sample solution concentration. The concentration at which the first break occurs was referred as the CMC value of the compound in water. Measurements were determined in triplicate.
Atomic Force Microscopy
Langmuir-Blodgett studies was carried out for compound C14, being the ultimate compound with both surface active and antioxidant properties, according to the method of Correa et al.29 with slight modications. Experiments were performed in aqueous solutions at neutral pH. Briefly, 20 µl of 2 mg/mL solution of C14 in chloroform:methanol (9:1, v/v) was applied to the aqueous phase and allowed to evaporate for a period of 20 min. Thereafter, the barriers were compressed at a constant rate of ~9 Å/ (chain min) until film collapsed. After stable conditions were attained, deposition of the corresponding film was carried out onto a hydrophilic mica support at a pressure just before film collapsed. The deposited monolayers on mica were left to air dry overnight. AFM images were acquired at ambient conditions by air tapping mode using a silicon tip on a micro cantilever (Olympus Inc., Japan) with a spring constant of 26 N/m and resonant frequency of 300 kHz. All measurements were performed in the center of the sample. Analyses were done in duplicates.
Formulation of oil-in-water delivery emulsions
The synthetic compounds were evaluated as emulsifiers for fish oil-in-water emulsions and compared to commercial DATEM. Emulsions consisting of 30.0% fish oil were prepared using 1.5 mL (0.1 M) of emulsifier solutions in phosphate buffer (pH 7.0). Coarse emulsions were first created by homogenization of lipid and aqueous phases (PRO250, PRO Scientific, Oxford, USA) at high speed for two minutes at room temperature. Further reduction particle size was achieved by use of a probe sonicator (Branson sonifier 250, Branson ultrasonics, Danbury, US) for a period of 3 min.
Creaming stability of emulsions
To examine their creaming stability, duplicate emulsions were transferred to clear, screw-capped 15 mL vials (diameter 1.8 cm) and stored at 5 °C. By aid of daily digital photographs, the phase separation in each emulsion was monitored over 7 days. The extent of creaming was characterized by the creaming index (CI) according to Equation (2), where HA is the height of the lower, aqueous phase, and HE is the total height of emulsion30).
Determination of lipid oxidation in emulsions
The ability of the new emulsifiers to inhibit lipid oxidation in emulsions was evaluated using Thiobarbituric acid-reactive species (TBARS) assay. TBARS were used to measure the formation of malondialdehyde, a major product of lipid oxidation31, in emulsions after one week of storage. A solution of TCA-TBA-HCl was prepared by mixing 15 g of TCA, 375 mg TBA, 1.76 ml 12 N HCl, and 82.9 ml water. Two millimeters of this solution was mixed with 20 µl of the emulsion sample previously in 1 ml of distilled water. Thereafter, the mixture was heated at 100 °C for 15 min, cooled to room temperature within 10 min under a running tap water, and centrifuged at 2000×g for 15 min22. TBA formed colored complexes with the secondary oxidation products, which were detected in a UV-visible spectrophotometer (Cary 50Bio, Varian, Australia) at 532 nm. The relative degree of oxidation in each emulsion was calculated with respect to the degree of oxidation in emulsions with commercial DATEM, according to Equation (3), where AS is the absorbance of samples with added stabilizer, and A0 is the absorbance of samples with no added emulsifier. Measurements were taken on duplicate emulsion samples.
Statistical analysis
Data processing was performed in Microsoft Excel 2010. All measurements were conducted in duplicates/triplicates, and the results are reported as means ± standard deviations. One-way analysis of variance (one-way ANOVA) was performed using Microsoft Excel Analysis Toolpak (2010) to identify significant differences between groups (p < 0.05).
Toxicity evaluation of amphiphilic lipids
Cell Culture
An aneuploid immortal keratinocyte cell line from adult human skin, HaCat was cultured in RPMI (Sigma, Madrid, Spain) medium supplemented with 10% heat-inactivated FBS and a human colorectal adenocarcinoma cell line, Caco-2 in minimum essential medium Eagle supplemented with 15% fetal bovine serum, 25 mM HEPES. Both media were added with 1% antibiotic/antimycotic solution (100 units mL−1 of penicillin, 100 μg mL−1 of streptomycin and 0.25 μg mL−1 of amphotericin B) (all from Sigma). Cells were kept at 37 °C in a humidified atmosphere with 5% CO2 and harvested by trypsinization (0.25% (w/v) trypsin-EDTA4Na) twice a week.26
Sulfo-rhodamine B assay
The effect of compounds on the growth of the human keratinocyte cell line (HaCat) and Caco-2 cells was assessed according to the procedure adopted by the US National Cancer Institute in the “In vitro Anticancer Drug Discovery Screen” that uses the protein-binding dye SRB to assess cell growth. HaCat and Caco-2 cells were first grown into 96-well plates at a cellular density of 1.5 × 105 cell/mL in RPMI (Sigma, St. Louis, MO) medium and allowed to grow for 24 h. Thereafter, cells were incubated with a four-serial concentration of the compounds (6.3, 12.5, 25, 50 µM), for 48 h, with a maximal solvent concentration (ethanol) of 0.05%. TCA (50% solution) was then added and after 1 h at 4 °C, the plates were washed four times with deionized water and allowed to dry overnight. The wells were then stained with 0.4% solution of SRB for 30 min in the dark. The excess of staining solution was washed out with 1% acetic acid and the bound stain was solubilized with tris-buffer (10 mM, pH 8.0) and the absorbance measured at 492 nm in a microplate reader (Powerwave XS, Bio-Tek Instruments Inc.). Cytotoxicity was determined as percent survival, calculated by the number of treated (T) over the control (C) cells × 100% (% T/C).
References
Balakrishna, M. et al. Synthesis and in vitro antioxidant and antimicrobial studies of novel structured phosphatidylcholines with phenolic acids. Food Chem. 221, 664–672 (2017).
Falkeborg, M. & Guo, Z. Dodecenyl Succinylated Alginate (DSA) as a novel dual-function emulsifier for improved fish oil-in-water emulsions. Food Hydrocoll. 46, 10–18 (2015).
Centini, M. et al. New multifunctional surfactants from natural phenolic acids. J. Agric. Food Chem. 60, 74–80 (2012).
Nandi, S. et al. Novel class of organo- (-hydro-) gelators based on ascorbic acid. Org. Lett. 13(8), 1980–1983 (2011).
Pérez, B. et al. Synthesis and characterization of O-acylated-ω-hydroxy fatty acids as skin-protecting barrier lipids. J. Colloid Interface Sci. 490, 137–146 (2017).
Pérez, B. et al. Ultralong fatty acyl derivatives as occlusive structure lipids for cosmetic applications: synthesis and characterization. ACS Sustainable Chem. Eng. 4, 7137–7146 (2016).
Bhadani, A., Kataria, H. & Singh, S. Synthesis, characterization and comparative evaluation of phenoxy ring containing long chain gemini imidazolium and pyridinium amphiphiles. J. Colloid Interface Sci. 361, 33–41 (2011).
Chen, W., Fan, X., Huang, Y., Liu, Y. & Sun, L. Synthesis and characterization of a pentaerythritol-based amphiphiles star block copolymer and its application in controlled drug release. React. Funct. Polym. 69, 97–104 (2009).
Wei, W. et al. Synthetic ultra-long chain fatty acyl based amphiphilic lipids as a dual function excipient for the production of surfactant-free solid lipid nanoparticles (SL-SLNs): a physico-chemical Study. Green Chem. 18, 3962 (2016).
Anankanbil, S., Pérez, B., Yang, J., Banerjee, C. & Guo, Z. A novel array of interface-confined molecules: assembling natural segments for delivery of multi-functionalities, J. Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2017.08.052. (2017).
Köhler, P. Study of the effect of DATEM. 3: Synthesis and characterization of DATEM components. LWT-Food Sci. Technol. 34, 359–366 (2001).
Köhler, P. & Grosch, W. Study of the effect of DATEM. 1: Influence of fatty acid chain length on rheology and baking. J. Agric. Food Chem. 47, 1863–1869 (1999).
Oncins, G., Torrent-Burgués, J. & Sanz, F. Nanomechanical properties of arachidic acid langmuir-blodgett films. J. Phys. Chem. 112, 1967–1974 (2008).
Kanicky, J. R. & Shah, D. O. Effect of degree, type, and position of unsaturation on the p K a of long-chain fatty acids. J. Colloid Interface Sci. 256(1), 201–207 (2002).
Gibbs, A. G. Lipid melting and cuticular permeability: new insights into an old problem. J. Insect. Physiol. 48(4), 391–400 (2002).
Moore, D. J. & Rerek, M. E. Insights into the molecular organization of lipids in the skin barrier from infrared spectroscopy studies of stratum corneum lipid models. Acta Derm. Venereol. 208, 16–22 (2000).
Bouwstra, J. A. & Gooris, G. S. The Lipid organization in human stratum corneum and model systems. Open Dermatol. J. 4, 10–13 (2010).
Wei, W. et al. Single component solid lipid nanocarriers prepared with ultra-long chain amphiphilic lipids. J. Colloid Interface Sci. 505, 392–401 (2017).
McClements, J. D. & Gumus, E. C. Natural emulsifiers — Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 234, 3–26 (2016).
Kalyanasundraram, K. & Thomas, J. K. Solvent-dependent fluorescence of pyrene-3-carboxaldehyde and its applications in the estimation of polarity at micelle-water interfaces. J. Phys. Chem. 81(23), 2176–2180, https://doi.org/10.1021/j100538a008 (1977).
Smułek, W., Zdarta, A., Milewska, M. & Kaczorek, E. Alkyl polyglucosides as cell surface modification factors: influence of the alkyl chain length. Toxicol. Environ. Chem. 98(1), 13–25 (2016).
Park, K. M. et al. Erythorbyl laurate as a potential food additive with multi-functionalities: interfacial characteristics and antioxidant activity. Food Chem. 215, 101–107 (2017).
Sparr, E., Eriksson, L., Bouswstra, J. A. & Ekelund, K. AFM study of lipid monolayers: III. phase behavior of ceremides, cholesterol and fatty acids. Langmuir. 17, 164–172 (2001).
Ekelund, K., Sparr, E., Engblom, J., Wennerström, H. & Engström, S. An AFM Study of Lipid Monolayers. 1. Pressure-Induced Phase Behavior of Single and Mixed Fatty Acids. Langmuir 15, 6946–6949, https://doi.org/10.1021/la990092+ (1999).
Martínez-Tomé, M. et al. Antioxidant properties of mediterranean spices compared with common food additives. Food Prot. 64(9), 1412–1419 (2001).
Oxley, J. D. Spray cooling and spray chilling for food ingredient and nutraceutical encapsulation. In: Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Ed. (Garti, N. & McClements, D.J.). Woodhead publishing limited, Cambridge, UK. pp 73‒102 (2012).
Yang, J. S., Jiang, B., He, W. & Xia, Y. M. Hydrophobically modified alginate for emulsion of oil in water. Carbohydr Polym. 87(2), 1503–1506 (2012).
Ananthapadmanabhan, K. P., Goddard, E. D., Turro, N. J. & Kuo, P. L. Fluorescence probes for critical micelle concentration. Langmuir. 1, 352–355 (1985).
Mack Correa, M. C. et al. Molecular interactions of plant oil components with stratum corneum lipids correlate with clinical measures of skin barrier function. Exp Dermatol. 23, 39–44 (2014).
McClements, D. J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 47(7), 611–649 (2007).
Cai, L., Cao, A., Aisikaer, G. & Ying, T. Influence of kernel roasting on bioactive components and oxidative stability of pine nut oil. Eur. J Lipid Sci. Technol. 115(5), 556–563 (2013).
Acknowledgements
Technical support from the Interdisciplinary NanoScience Center (iNANO) and the Department of Engineering, Aarhus University is fully acknowledged. Peter R. Ogilby of the Department of Chemistry, Aarhus University is acknowledged for allowing access to equipment in his laboratory. The Danish council for independent research (DFF–4184-00123) is further acknowledged for financial support. B.P. also thanks the Danish Council for Independent Research for her postdoctoral grant 5054-00062B.
Author information
Authors and Affiliations
Contributions
Z.G. (Guo). perceived the project concept, designed molecules of interest and reviewed the manuscript. S.A. and B.P. established the synthesis pathway and S.A. synthesized the compounds. K.M.W. performed Langmuir isotherm and sample deposition. Z.G. (Gao). performed AFM imaging. I.F. and N.M. conducted cell toxicity study of synthetic compounds. S.A. and B.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anankanbil, S., Pérez, B., Fernandes, I. et al. A new group of synthetic phenolic-containing amphiphilic molecules for multipurpose applications: Physico-chemical characterization and cell-toxicity study. Sci Rep 8, 832 (2018). https://doi.org/10.1038/s41598-018-19336-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19336-8
- Springer Nature Limited