Abstract
The water regime is often the primary force driving the evolution of freshwater lakes, but how soil microbes responded to this process is far from understood. This study sampled wetland soils from a shallow lake that experienced water regime changes, Poyang Lake of China, to explore the features of bacterial and fungal community in response to water level changes. The soil physicochemical properties, T-RFLP based community structures and soil activities (including basal respiration, microbial biomass and enzymes) were all determined. Soil microbial eco-function was captured by testing the carbon metabolism with Biolog-Ecoplate. The results showed remarkable influence of the water level gradients on the soil physicochemical properties, microbial community structures and soil activities. However, the carbon utilization profile exhibited weak connections with the environmental variables and microbial community structures (p > 0.05). The microbial activities were significantly correlated with both bacterial and fungal community structures. Our results also emphasized the ascendant role of the deterministic process in the assemblages of microbial community structures and functions in wetland. In conclusion, this study revealed the discrepancy between community structures and eco-functions in response to water level gradients, and a relatively stable eco-function helped to maintain the ecosystem function of wetland from a long-term perspective.
Similar content being viewed by others
Introduction
Freshwater lakes provide important ecological and economic value, including local climate regulation, a habitat that supports biodiversity, and resources for economic development. Lake wetlands are an important functional habitat that often contain the highest productivity and microbial activity in the lake region. They are also regions susceptible to environmental disturbances, including changes in hydrology, water quality, and vegetation1. However, the important ecological contributions to biogeochemical cycling by lake wetlands have been underestimated in a global view due to their small proportional area2,3,4. Because of these important contributions, it is crucial to understand how environmental disturbances impact wetland function.
In recent years, global climate change and anthropogenic activities, including the construction of hydropower stations and dams, aquaculture, and agricultural reclamation, have profoundly impacted the water regime of the lake ecosystem. Water regime changes may strongly affect the wetland microbial composition as well as functional activity in terms of biogeochemical cycling and energy flux5. Altered hydrological and physicochemical characteristics of the waters and soils have been documented to affect the plant, animal, and microbial communities6,7,8,9. Many studies have described shifts in the soil microbial community composition or community functional activity, but most did not investigate changes in the community composition and function together10,11,12,13; thus, it remains unclear to what extent community structure determines a functional profile in the different environments14,15. Moreover, shifts in the community composition do not always cause changes in the community functional processes16. When facing environmental or anthropogenic disturbances, the microbial community structure and function may respond separately or concurrently17,18. Studies that quantify changes in the community structure and function in response to disturbance have been few up to now, and much work is needed to understand these changes.
In addition to the uncertain link between the microbial community structure and function, there is considerable debate about what drives microbial community assembly. The most frequently proposed mechanisms concerning community assembly and the spatial distribution of soil microbial communities are niche-related (deterministic) and neutral (stochastic) processes19,20,21,22,23. These processes may occur at different scales24, and many surveys showed the combined effects of both theories25. pH26, soil moisture27, phosphate and the C:N ratio28 were all important drivers in the deterministic process, while the dispersal limitation of the stochastic processes were noted in Actinobacteria29, AM fungi21, and aquatic bacterial communities30. While studies have quantified the relative contributions of niche and neutral processes to community assembly, very few have compared these processes in variable habitats, such as lake wetlands that experience periodic fluctuations in hydrology. In the highly complex and dynamic wetland lake ecosystem, there is a lack of understanding regarding the link between community structure and function and the influence of the niche or neutral community assembly processes. Increasing our understanding of these factors may help with wetland management and preservation.
The objective of this study was to investigate how water level gradients (or hydroperiods) affect the wetland microbial community composition and community ecological functions. We also wanted to quantify the relative influence of the deterministic and stochastic processes in structuring the spatial distribution patterns of the wetland microbial communities. We hypothesized that the microbial functions would be more stable than the composition in coping with the water level gradients and that the deterministic process would play a greater role than the stochastic process in structuring the microbial community assembly. We sampled wetland soils with different water level gradients from the lakeside of Poyang Lake, the largest freshwater lake of China31. Soil physicochemical properties, microbe-related soil activities (enzymes, biomass and respiration), bacterial and fungal community composition and Eco-plate carbon utilization profiles were all measured and analysed. The variance partitioning method was also used to determine the impact of the deterministic and stochastic processes in this study.
Results
The impact of water gradients on soil properties and vegetation communities
Water level gradients were associated with differences in several shoreline wetland soil properties, including SM (soil moisture), TOC (total organic carbon) and TP (total phosphorus). The SM decreased from the H1 plot to the H6 plot, along with an increase in the water level gradients. Meanwhile, the soil pH values showed significant differences between the near water plots (H1-H3) and remote water plots (H4-H6) (p < 0.05). However, most soil nutrient-related parameters failed to exhibit a clear, constant gradient, including AFDM, TN and NO3-N (Table 1). The TOC content divided all the tested plots into two groups; H1-H4 had less TOC than the H5-H6 group. The H1 plot persisted in having a very high NH4-N content (23.54 ± 1.00 mg/kg), which was approximately 4–8 times higher than that in the other plots (3.01–6.24 mg/kg).
The flooding hydroperiod differed among the plots, and the lowest H1 plots were submerged all year, whereas the highest H6 plots were only submerged for approximately one month (Table 1). The vegetation distribution was quite distinct. All plots could be divided into three groups based on the species number and abundance, the naked group (H1-H2), sparse group (H3-H4) and dense group (H5-H6). Greater plant growth (H5 and H6) was associated with drier conditions, which were observed in the plots furthest from the lake surface, while the long-term flooding conditions of the H1 and H2 plots repressed plant growth.
Microbial community profiles from T-RFLP
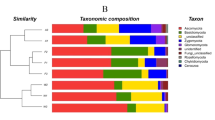
A total of 388 bacterial TRFs and 420 fungal TRFs were detected by T-RFLP, and approximately 80% of the total TRFs remained for the bacterial and fungal community after quality control, including 147 bacterial TRFs and 206 fungal TRFs, respectively. The cluster analysis of the bacterial community revealed three different groups of study plots: the H6 plot was primarily separated from the others, and then, the H1 and H5 plots were also separated from the H2, H3 and H4 plots (Fig. 1). However, the clustering result of the fungal community was inconsistent with that of the bacterial community, and the H5 and H1 plots were distinguished from the H2, H3, H4 and H6 plots. Four of the community diversity indexes were evaluated, but no unified tendency was extracted (Fig. 1 and Table S1).
Microbial-related activities and carbon utilization of soil microbial communities
The soil enzyme activity of BX, BG, NAG and PHOS varied among the plots, and the distribution profiles of each enzyme also differed. Generally, H5 and H6 plots had higher activities than the other plots for all the enzymes except for BG. The activities of BX, NAG and PHOS were highest in the H5 plot (p < 0.05), and their highest values were 40.23 ± 10.53, 54.97 ± 5.71 and 875.78 ± 90.52 μmol h−1 g−1, respectively. The H1, H2 and H5 plots all had higher BG activity than the other plots, including H6 (Table 2). The H5 and H6 plots had significantly higher microbial biomass carbon (MBC) and nitrogen (MBN) than the other plots (p < 0.05). The MBC contents were similar among H1-H4, but the MBN contents exhibited a profile of H1 > H4 > H3 > H2. The comparison of the soil basal respiration (BR) only distinguished the H2 plot with the lowest value. The distribution profile of qCO2 (h−1) was similar to that of MBC; the H5 and H6 plot values were higher than those of the H1-H4 plots (Table 2). The microbial carbon (MBC/TOC) and nitrogen (MBN/TN) were both higher in the H5 and H6 plots, and H1-H4 were similar except for H2, which had the lowest microbial nitrogen content at 0.14 ± 0.09% (Table 2).
Generally, three groups of AWCD levels from the Biolog Ecoplate could be partitioned: H5 with the highest utilization efficiency, H1 with the lowest efficiency, and the others with moderate efficiency (Figure S1). A detailed comparison of the different carbon source utilization profiles separated H5 and H6 from the others, and H4 was also separated from H1-H3 (Fig. 2).
Factors driving the assemblage of the soil microbial community
Soil microbial communities were associated with various environmental factors, including local physicochemical properties and geographical heterogeneity. We tested the correlation between the community profiles and related variables using Pearson correlation, and the significant results are shown in Table 3. Both the soil microbial biomass (MBC/MBN) and microbial carbon/nitrogen were positively correlated with AFDM, TOC, TN and the water level gradients but negatively correlated with SM, TP, pH and hydroperiod. Metabolic profiles showed an inverse relationship compared to the microbial biomass. Most of the enzyme activities were positively correlated with AFDM, TOC, TN, MBC, MBN and the water level gradients but negatively correlated with SM, TP, pH and hydroperiod, except for BG, which seemed to be less affected. Bacterial and fungal TRFs showed a positive correlation with qCO2, pH and TP but were negatively correlated with most soil nutrient parameters. The bacterial Shannon index was positively affected by TP and pH but negatively affected by TOC, while the fungal Shannon index was positively affected by NO3-N but negatively affected by most soil nutrient parameters. The aboveground vegetation also showed comprehensive relationships with many factors, with the positive factors including MBC, MBN, BX, NAG, PHOS, TOC, AFDM and WH and the negative factors including qCO2, SM, pH, NH4-N and hydroperiod (Table 3).
The structure and function of the soil microbial communities differed among plots, and many environmental factors contributed to these differences (Fig. 3). For bacteria, the distance-based similarity matrix of the community composition was significantly affected by most of the environmental factors, except for TN, NH4-N and NO3-N. The water level gradient (WH) was the most significant variable for the H6 plots, while AFDM and TOC were the most significant for the H5 plots and pH and TP for the H2-H4 plots (Fig. 3A). The fungal community patterns were different from the bacterial patterns among the plots, and all the factors except for TN, NH4-N and AFDM were significant (p < 0.05). The H1 and H5 plots were separated from the other plots. NH4-N, SM, and hydroperiod were the most significant contributors to the H1 plot, and AFDM and TOC were the most significant contributors to the H5 plot (Fig. 3B). The carbon utilization profile among the plots was also affected by many environmental factors, but TN, NH4-N, NO3-N and pH all failed to pass the Monte Carlo test with Bonferroni correction. The H5 and H4 plots were separated from the other plots, and the contribution of AFDM and TOC to the H4 plots was shown (Fig. 3C).
The discrepancy between the microbial community structure and functional traits in response to the environmental variables
There were differences between the community composition and the functional traits in response to the environmental variables. Environmental variables significantly correlated with both the bacterial and fungal community composition but were not correlated with carbon utilization variables of the soil microbes (Fig. 4a–d). The carbon utilization patterns were so consistent that neither the bacterial nor fungal community structure could be clearly attributed to it (Fig. 4e,f). Soil enzyme activities were correlated with environmental variables and with both the bacterial and fungal community compositions (Fig. 4c,g,h). All the above results represented a distinct discrepancy between the microbial community composition and the functional traits in response to the environmental variables.
Variance partitioning of the microbial community structures and functional traits
The variance partitioning method was employed in this study to quantify the relative influence of the stochastic and deterministic processes on the soil bacterial and fungal community compositions as well as on their related functional activity. The combined effect of all the significant environmental variables accounted for 22% and 30% of the total variation in the bacterial and fungal community compositions, respectively, which were larger than the effect of spatial distances, for which 5% and 6% contributions were found, respectively (Fig. 5a,b). For both the bacterial and fungal communities, the combined variables explained less than 40% of the total variation, which implied that other unnoticed factors also contributed to community variation. The environmental effect was also demonstrated to be a dominant player in structuring the functional activity profiles of carbon utilization and soil activities, rather than the spatial distances (Fig. 5c,d). For the carbon utilization profile, the combined environmental factors explained 33% of the total variation, which was higher than the 8% from the spatial effect (Fig. 5c). The profile of the soil activities was mostly governed by the environmental variables (33%) and the related interaction with the spatial distances (62%), while the pure effect of spatial distances was not detected. In addition, the 5% residuals meant that the existing parameters were sufficient to explain the variation in the soil activities (Fig. 5d).
The proportion of variance in the community composition (a for bacterial community, b for fungal community) and functional traits (c for carbon utilization profiles, d for soil activities) explained by the environmental components (X1) and spatial distance (X2). Each diagram represents the variation in a given dissimilarity metric partitioned into the relative effects of each component or combination of components. Unexplained variation (residuals) is marked on each plot.
Discussion
Water fluctuation can strongly affect the lake ecosystem, and wetland soil, as well as the soil microorganisms, always bear the brunt of it. Soil water content varies with water level gradients, and most nutrient elements also exchange with water. Ventilation conditions and redox potentials also shift; thus, the structure and function of the soil microbial community alters. The patterns documented in this study indicate a complex relationship between the microbial community composition and function and note that there is still much to understand regarding the complex relationships between the biotic and abiotic factors.
In this study, soil moisture (SM) gradients were inversely correlated with water level gradients (WH), and the pH values exhibited a unimodal pattern, which was high in the H3 plots. In general, the high SM plots had higher pH values. Both the SM and pH were important factors that affected the soil microbial community composition, and the pH values have been shown to serve as indicators that predict the soil bacterial community26,32, while excessive water content in the soil was considered to be a limiting factor for microbial processes33. The high SM and pH values in the near water plots illustrated that higher pH values were associated with lake waters, and nitrogen influx into soils of submerged plots was the potential cause for high pH. This pattern was also noted in previous studies that documented water movements transporting nutrients into the sediment in natural freshwater systems34. The higher accumulation of AFDM and TOC in higher elevation plots was associated with an increase in the microbial biomass. In contrast to submerged soils, the desiccation process and increased vegetation encouraged the accumulation of organic matter and the greater microbial growth35.
All enzymes peaked in the H5 plots of this study, and the carbon utilization activity of H5 also exceeded those of the other plots (Table 2 and Figure S1). This pattern revealed the highest microbial activity in the H5 plots, followed by the H6 plots and H1-H4 plots. This distinctly separated trend was also found in the profiles of microbial community composition and carbon utilization among the H5, H6 and other plots (Fig. 3). Microorganisms can regulate enzyme production according to existing substrates in the environment, and the accumulated plant organic matter satisfied the increased costs of the microbial biomass and enhanced enzyme production in the H5 and H6 plots36. Meanwhile, the strong correlation between the fungal community composition and soil enzyme activity may reveal the equal importance of bacteria and fungi in soil activity.
Microbial biomass C/N (MBC and MBN) and microbial C/N ratios (MBC/TOC and MBN/TN) were both higher in the H5 and H6 plots, and relatively higher values were observed in the H1 plots compared to the other three plots (H2-H4). The continual flooding conditions of the H1-H4 plots suppressed the massive growth of the soil microorganism, and the higher biomass in the H1 plots may be associated with the aquatic bacterial communities37. Despite the differences in the microbial biomass, basal respiration among the plots was consistent, and the water gradients, as well as microbial biomass, did not greatly influence the soil basal respiration. Although previous studies noted the impact of vegetation on soil basal respiration, this impact was not found in this study38. We propose that this stable phase of soil basal respiration was covered up by mixing the susceptible surface layer and stable deep layer of the sampled soil cores, and the cold temperature of the sampling seasons may also contributed to it. Along the water gradients, different succession stages of the microbial communities and vegetation developed, and both were driven by water level fluctuations. Although the microbial carbon metabolic profile seemed less influenced by the environmental gradients, the impact of vegetation was observed in shaping the community structure and function by enhanced nutrient cycling39,40,41. Soil basal respiration, enzyme activities and microbial biomass all showed correlations with the soil properties and microbial community composition, but this finding varied from other reports that documented a slow response of the community composition to environmental changes42. The great variation range of the environmental conditions, as well as the regular water regime, were considered to be a major cause of the active response of soil biotic and abiotic variables to water fluctuation in this study43.
The soil microbial community structure and function showed different response patterns to water gradients. The community structure seemed more sensitive to water gradients, while the carbon metabolism was more stable (Fig. 4). Compared to fungi, there was a stronger correlation between the bacterial community composition and water level. Soil moisture was documented as a major factor influencing the microbial community structure and enzyme activities in this study (Table 3), and a similar conclusion was obtained from the forest soil samples that also revealed a close relationship between the microbial community composition and soil moisture27. Considering the connection between the microbial community composition and microbial activity, the microbial biomass, soil respiration and soil enzymes were all identified as indicators of soil microbial activity41,44. Soil microbial activity was correlated with the microbial community structure and showed an active response to environmental changes45.
However, for carbon metabolism, no obvious connection was found with either environmental characteristic or microbial community structure (Fig. 4e,f). This independent profile may play important roles in the wetland ecosystem, including maintaining a stable and balanced carbon transformation process and providing a resilient mechanism allowing the ecosystem function to be maintained when facing diverse environmental disturbances. There were two possible explanations for this metabolic independence that we considered. Soil nutrient levels are considered important factors that influence the stability of soil biological functions. This is true especially for TOC, as higher TOC is helpful for maintaining the original function of soil microbial communities in response to drying-rewetting pressures46. Another common hypothesis for this independence is functional redundancy. While a small group of functions are regulated by only a small proportion of the microbial community with specialized physiological pathways, the existence of relatively ubiquitous organisms will mask these minor differences in the community structure-function relationships47,48,49.
Simplified variance partition models have revealed the dominance of the deterministic process on the assemblage of the soil microbial community and extensions on soil activity and microbial functions50. This result may emphasize the importance of local scale environmental variables and highlight the high variability and complexity of the soil microbial community in wetlands. Environmental properties create abiotic stress on soil microbes and strengthen the interactions among species, which result in more specialized niches. The significant difference among the heterogeneous environmental gradients filtered the microbial composition and hindered the stochastic processes20,30.
In conclusion, this study sheds light on the role of the soil microbial process within the function of the wetland ecosystem, documents the response of the microbial community composition and functional activity to water level gradients, and reveals the assemblage processes of bacterial and fungal communities. We also speculate that a decline of the lake water level would lead to stimulated microbial growth and activity and would alter the structure and function of soil microbial communities associated with Poyang Lake wetlands. In addition, lake water shrinkage would induce plant succession towards the lake centre and would strengthen organic matter accumulation and mineralization, but the basal respiration and carbon utilization would remain stable. Increased understanding of the impact of environmental conditions on microbial community composition and functional activity will help us to develop better management strategies in the face of a changing climate.
Methods
Study design and sampling procedures
As the biggest freshwater lake of China, Poyang Lake is in the northern part of Jiangxi province, connects to the downstream Yangtze River, and acts as an important reservoir for flood regulation31. Due to the high throughput water conditions of Poyang Lake, the water areas have varied dramatically, with an annual water level fluctuation of over 10 metres. Lake wetlands were recorded as internationally important wetland habitats by the United Nations in 19927. In recent years, the water regime of Poyang Lake has changed, resulting in reduced water levels (hydroperiod) and extended dry seasons. Banghu Lake, a dish-shaped sublake of Poyang Lake, was selected as the field site for this study. Banghu Lake was sensitive to water level changes, and the riparian vegetation varied according to site elevations51 (Fig. 6A).
Study field and sampling sites in Lake Poyang of China. The location of the study field in Lake Poyang is shown in (A) (generated by ArcGIS 10.2, www.esri.com), and three sampling belts with six plots were placed as shown in (B); the vertical distance to the water level is demonstrated in (C).
We selected three transects along a shore-side wetland area. Each transect (500 m apart) had six plots (H1-H6, 10 m*10 m) for sampling (shown in Fig. 6B and C). The H1 plot was the lowest water point that was continually submerged in lake water, and the other plots were distributed along the wetland slope with increased elevation. The H1 and H2 plots were bare without any vegetation, the H3 and H4 plots had sparse, small plants, and the H5 and H6 plots were covered by lush forbs and graminoids.
Surface soil samples (0–15 cm) were collected in March 2013 (this time of year has the lowest yearly water level and the broadest shoreline as well as the ideal water gradients) with an aseptic soil sampler. A total of 10 independent soil cores (2.5 cm in diameter) were randomly collected within each plot and pooled as one sample. Samples collected from the 18 plots were transferred to the laboratory in an ice-cold box. In the lab, each of the soil samples were subdivided into two parts, one part for physicochemical analysis and Biolog Ecoplate assay and the other part for molecular analysis (stored at −80 °C). Vegetation species and abundance were investigated by measuring the number of species (vegetation species) and strains (vegetation abundance) of several 1 m2 areas for each plot.
Physicochemical analysis
We measured the soil moisture (SM), pH, ash free dry mass (AFDM), total organic carbon (TOC), total nitrogen (TN), total phosphate (TP), ammonia nitrogen (NH4-N) and nitrate nitrogen (NO3-N) of all the collected samples (Table 1). The detailed measuring methods for the above parameters were described in a previous study31. NH4-N and NO3-N were extracted from 10 g of wet soil using a 2 M KCl extraction procedure and then measured by a discrete auto analyser (Smartchem 200, Westco, France)10.
DNA extraction and T-RFLP analysis
Total DNA was extracted from 0.5 g soil samples using a PowerSoil DNA isolation kit (MoBio, USA) following the manufacturer’s instructions. T-RFLP analysis was performed after the amplification of the 16 S or 18 S rRNA gene fragments and the enzymatic digestion of the PCR products. The primer pairs of Eub338F/1392 R and ITS1F/ITS4 were used for amplification, and the former primer 338 F and ITS1F were both modified with the 6-FAM label52,53. The resulting enzymatic fragments were purified and sent to Sangon Biotech Co. Ltd. (Shanghai) for testing. The resulting T-RFLP profiles were normalized by peak height, and only the fragments with a relative abundance over 0.5% were used for community analysis54.
Soil microbial activity and functional traits
Soil enzyme analysis
A microplate fluorometric assay method was followed to determine the soil enzyme activity of β-glucosidase (BG), β-D-xylosidase (BX), acid phosphatase (AP) and N-acetyl-β-D-glucosaminidase (NAG)55. Generally, 1 g of soil was added to 125 ml of 50 mM acetate buffer (pH 5.0) and homogenized; then, the sample suspensions were mixed with fluorogenic substrates in 96-well microplates and incubated in the dark at 25 °C for 0.5–2 h. Ten microlitres of 0.5 M NaOH was added to each well to stop the reaction, and the microplates were examined in a Varioskan Flash (Thermo Scientific, USA) with 365 nm excitation and 450 nm emission filters. After correcting for the negative controls and quenching, all the tested enzyme activities were expressed in units of μmol h−1 g−1 dry soil56.
Soil microbial biomass and basal respiration (BR) analysis
All soil samples were stored at 4 °C and measured within three days57. Soil samples were sufficiently homogenized after removal of any visible plant roots or stones, and the microbial biomasses of the carbon and nitrogen (MBC/MBN) were determined following the standard chloroform-fumigation approach58.
Soil basal respiration (BR) was determined by quantifying the carbon dioxide (CO2) released from microbial respiration during 10 days of incubation. Generally, 25 g soil samples were sealed in a confined space with NaOH (0.5 M) to capture the released CO2, and the amount of CO2 was determined by titration with 0.5 M HCl, with barium chloride (0.25 M BaCl2) and phenolphthalein added before titration. The respiration rate was calculated as the average CO2 content respired hourly per kilogram soil59.
Carbon utilization analysis
Heterotrophic microbial activity from the wetland soils was measured as the carbon utilization patterns by Biolog Ecoplate (Hayward, USA)60. Approximately 10 g of soil samples (dry weight equivalent) as dispersed in 90 ml of aseptic normal saline and then diluted 100 times, and 150 μl of diluent was added into each well of the plate. The original optical density (OD) was measured immediately with an automated plate reader (BIOLOG Inc., USA) at 590 nm. After a 25 °C incubation every 12 hours in a constant temperature humidity chamber, the OD was documented, and the procedure lasted up to 10 days to ensure the carbon utilization saturation phase was reached in all the samples61.
Statistical analyses
Statistical analysis of the environmental variables and soil activity parameters among the sampling plots was determined using one-way ANOVA by the SPSS 21.0 software package. The soil microbial community dataset, which was characterized by T-RFLP profiles, was analysed using PAleontological Statistics (PAST) Version 3.11 for community diversity17. Hierarchical clustering analysis was used for the soil microbial community, and the Bray-Curtis dissimilarity matrix of abundance based on RFs was calculated in the R project 3.3.1 (“ape” and “vegan”) with the single method14. All the ordination analysis was done with Canoco 5.0, and the models of PCA and db-RDA were used in this study43. Pearson correlation analysis between the biological observations and environmental variables was assessed in SPSS 21.0. Generalized Linear Models (GLM) and Mantel tests (Pearson’s correlation) in the R project were used for the relationship analysis among the microbial community (bacteria and fungi), environmental variables, geographic distance and vegetation14. To further estimate the relative importance of the deterministic and stochastic processes on the community assemblage of the microbial structure and function, variation partitioning was performed in R with a distance-based approach as previously described62.
References
Keddy, P. A. Wetland ecology: principles and conservation (Cambridge University Press, 2000).
Zedler, J. B. & Kercher, S. Wetland resources: status, trends, ecosystem services, and restorability. Annu. Rev. Env. Resour. 15, 39–74 (2005).
Mitra, S., Wassmann, R. & Vlek, P. L. G. An Appraisal of global wetland area and its organic carbon stock. Currentence 88, 25–35 (2005).
Conrad, R. The global methane cycle: recent advances in understanding the microbial processes involved. Env. Microbiol. Rep. 1, 285–292 (2009).
Weise, L. et al. Water level changes affect carbon turnover and microbial community composition in lake sediments. FEMS microbiol. Ecol. 92, fiw035, https://doi.org/10.1093/femsec/fiw035 (2016).
Tan, Z. & Jiang, J. Spatial-temporal dynamics of wetland vegetation related to water level fluctuations in Poyang Lake, China. Water 8, 397, https://doi.org/10.3390/w8090397 (2016).
Wang, X., Xu, L., Wan, R. & Chen, Y. Seasonal variations of soil microbial biomass within two typical wetland areas along the vegetation gradient of Poyang Lake, China. Catena 137, 483–493, https://doi.org/10.1016/j.catena.2015.10.020 (2016).
Dolinar, N., Šraj, N. & Gaberščik, A. [Water regime changes and the function of an intermittent wetland]. Water and nutrient management in natural and constructed Wetlands (Springer Netherlands, 2010).
Paillisson, J. M., Reeber, S. & Marion, L. Bird assemblages as bio-indicators of water regime management and hunting disturbance in natural wet grasslands. Biol. Conserv. 106, 115–127 (2002).
Ding, X. et al. Spatial distribution of bacterial communities driven by multiple environmental factors in a beach wetland of the largest freshwater lake in China. Front. Microbiol. 6, 129, https://doi.org/10.3389/fmicb.2015.00129 (2015).
Tian, J. et al. Response of archaeal communities to water regimes under simulated warming and drought conditions in Tibetan Plateau wetlands. J. Soil. Sediment. 15, 179–188, https://doi.org/10.1007/s11368-014-0978-1 (2014).
Barnard, R. L., Osborne, C. A. & Firestone, M. K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. The ISME J. 7, 2229–2241, https://doi.org/10.1038/ismej.2013.104 (2013).
Brinson, M. M., Bedford, B. E., Middleton, B., Verhoeven, J. A. & Polunin, N. V. C. [Temperate freshwater wetlands: response to gradients in moisture regime, human alterations and economic status] Aquatic Ecosystems–Trends & Global Prospects (CAB Direct, 2008).
Perez-Valera, E., Goberna, M. & Verdu, M. Phylogenetic structure of soil bacterial communities predicts ecosystem functioning. FEMS microbiol. Ecol. 91, https://doi.org/10.1093/femsec/fiv031 (2015).
Severin, I., Lindstrom, E. S. & Ostman, O. Relationships between bacterial community composition, functional trait composition and functioning are context dependent-but what is the context? PLOS ONE 9, e112409, https://doi.org/10.1371/journal.pone.0112409 (2014).
Isobe, K. & Ohte, N. Ecological perspectives on microbes involved in N-cycling. Microbes. Environ. 29, 4–16, https://doi.org/10.1264/jsme2.ME13159 (2014).
Lima, M. S. et al. Contrasting factors drive within-lake bacterial community composition and functional traits in a large shallow subtropical lake. Hydrobiologia 778, 105–120, https://doi.org/10.1007/s10750-015-2610-9 (2016).
Peralta, A. L., Ludmer, S. & Kent, A. D. Hydrologic history influences microbial community composition andnitrogen cycling under experimental drying/wetting treatments. Soil. Biol. Biochem. 66, 29–37 (2013).
Wiens, J. J. et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324, https://doi.org/10.1111/j.1461-0248.2010.01515.x (2010).
Wang, J. et al. Phylogenetic beta diversity in bacterial assemblages across ecosystems: deterministic versus stochastic processes. ISME J. 7, 1310–1321, https://doi.org/10.1038/ismej.2013.30 (2013).
Dumbrell, A. J., Nelson, M., Helgason, T., Dytham, C. & Fitter, A. H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 4, 337–345 (2010).
Martiny, J. B. et al. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112, https://doi.org/10.1038/nrmicro1341 (2006).
Green, J. & Bohannan, B. J. Spatial scaling of microbial biodiversity. Trends Ecol. Evol. 21, 501–507, https://doi.org/10.1016/j.tree.2006.06.012 (2006).
Feinstein, L. M. & Blackwood, C. B. Taxa–area relationship and neutral dynamics influence the diversity of fungal communities on senesced tree leaves. Environ. Microbiol. 14, 1488–1499, https://doi.org/10.1111/j.1462-2920.2012.02737.x (2012).
Bissett, A., Richardson, A. E., Baker, G., Wakelin, S. & Thrall, P. H. Life history determines biogeographical patterns of soil bacterial communities over multiple spatial scales. Mol. Ecol. 19, 4315–4327, https://doi.org/10.1111/j.1365-294X.2010.04804.x (2010).
Jeanbille, M. et al. Soil parameters drive the structure, diversity and metabolic potentials of the bacterial communities across temperate beech forest soil sequences. Microb Ecol 71, 482–493, https://doi.org/10.1007/s00248-015-0669-5 (2016).
Brockett, B. F. T., Prescott, C. E. & Grayston, S. J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil. Biol. Biochem. 44, 9–20, https://doi.org/10.1016/j.soilbio.2011.09.003 (2012).
Kuramae, E. E. et al. Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS microbiol. Ecol. 79, 12 (2012).
Eisenlord, S. D., Zak, D. R. & Upchurch, R. A. Dispersal limitation and the assembly of soil Actinobacteria communities in a long-term chronosequence. Ecol. Evol. 2, 538–549 (2012).
Van, dG. K. et al. The power of species sorting: local factors drive bacterial community composition over a wide range of spatial scales. P. Natl. Acad. Sci. USA 104, 20404 (2007).
Ma, Y. et al. The distribution pattern of sediment archaea community of the Poyang Lake, the largest freshwater lake in China. Archaea 4, 1–12 (2016).
Lauber, C. L., Strickland, M. S., Bradford, M. A. & Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil. Biol. Biochem. 40, 2407–2415, https://doi.org/10.1016/j.soilbio.2008.05.021 (2008).
Mentzer, J. L., Goodman, R. M. & Balser, T. C. Microbial response over time to hydrologic and fertilization treatments in a simulated wet prairie. Plant Soil 284, 85–100, https://doi.org/10.1007/s11104-006-0032-1 (2006).
Huettel, M., Ziebis, W. & Forster, S. Flow-induced uptake of particulate matter in permeable sediments. Limnol. Oceanogr. 41, 309–322 (1996).
Zoppini, A., Amalfitano, S., Fazi, S. & Puddu, A. Dynamics of a benthic microbial community in a riverine environment subject to hydrological fluctuations (Mulargia River, Italy). Hydrobiologia 657, 37–51 (2010).
Allison, S. D., Weintraub, M. N., Gartner, T. B. & Waldrop, M. P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function (Springer Berlin Heidelberg, 2010).
Unger, I. M., Kennedy, A. C. & Muzika, R.-M. Flooding effects on soil microbial communities. Appl. Soil Ecol. 42, 1–8, https://doi.org/10.1016/j.apsoil.2009.01.007 (2009).
Tang, Y. S. et al. Response of soil microbial community in Jiuduansha wetland to different successional stages and its implications for soil microbial respiration and carbon turnover. Soil. Biol. Biochem. 43, 638–646, https://doi.org/10.1016/j.soilbio.2010.11.035 (2011).
Hortal, S. et al. Soil microbial community under a nurse-plant species changes in composition, biomass and activity as the nurse grows. Soil. Biol. Biochem. 64, 139–146, https://doi.org/10.1016/j.soilbio.2013.04.018 (2013).
Dornbush, M. E. G. Litter, and their interaction affect microbial biomass and soil enzyme activity. Soil. Biol. Biochem. 39, 2241–2249, https://doi.org/10.1016/j.soilbio.2007.03.018 (2007).
Morrissey, E. M., Berrier, D. J., Neubauer, S. C. & Franklin, R. B. Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland. Biogeochemistry 117, 473–490, https://doi.org/10.1007/s10533-013-9894-5 (2013).
Balser, T. C. & Firestone, M. K. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73, 395–415, https://doi.org/10.1007/s10533-004-0372-y (2005).
Andersson, M. G., Berga, M., Lindström, E. S. & Langenheder, S. The spatial structure of bacterial communities is influenced by historical environmental conditions. Ecology 95, 1134–1140 (2014).
Jian, S. et al. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil. Biol. Biochem. 101, 32–43, https://doi.org/10.1016/j.soilbio.2016.07.003 (2016).
Whitaker, J. et al. Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. J. Ecol. 102, 1058–1071 (2014).
Zhao, B., Chen, J., Zhang, J. & Qin, S. Soil microbial biomass and activity response to repeated drying-rewetting cycles along a soil fertility gradient modified by long-term fertilization management practices. Geoderma 160, 218–224, https://doi.org/10.1016/j.geoderma.2010.09.024 (2010).
Griffiths, B. S. et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: an examination of the biodiversity-ecosystem function relationship. Oikos 90, 279–294 (2000).
de Scally, S. Z., Makhalanyane, T. P., Frossard, A., Hogg, I. D. & Cowan, D. A. Antarctic microbial communities are functionally redundant, adapted and resistant to short term temperature perturbations. Soil. Biol. Biochem. 103, 160–170, https://doi.org/10.1016/j.soilbio.2016.08.013 (2016).
Torsvik, V. & Øvreås, L. Microbial diversity and function in soil: from genes to ecosystems. Curr. Opin. Microbiol. 5, 240–245 (2002).
Petchey, O. L. & Gaston, K. J. Functional diversity: back to basics and looking forward. Ecol. Lett. 9, 741–758, https://doi.org/10.1111/j.1461-0248.2006.00924.x (2006).
Zhang, L., Yin, J., Jiang, Y. & Wang, H. Relationship between the hydrological conditions and the distribution of vegetation communities within the Poyang Lake National Nature Reserve, China. Ecol. Inform. 11, 65–75, https://doi.org/10.1016/j.ecoinf.2012.05.006 (2012).
Blackwood, C. B. & Buyer, J. S. Evaluating the physical capture method of terminal restriction fragment length polymorphism for comparison of soil microbial communities. Soil. Biol. Biochem. 39, 590–599, https://doi.org/10.1016/j.soilbio.2006.09.010 (2007).
Avis, P. G., Dickie, I. A. & Mueller, G. M. A ‘dirty’ business: testing the limitations of terminal restriction fragment length polymorphism (TRFLP) analysis of soil fungi. Mol. Ecol. 15, 873–882, https://doi.org/10.1111/j.1365-294X.2005.02842.x (2006).
Dunbar, J., Ticknor, L. O. & Kuske, C. R. Phylogenetic specificity and reproducibility and new method for analysis of terminal restriction fragment profiles of 16S rRNA genes from bacterial communities. Appl. Environ. Microb. 67, 190 (2001).
Marx, M. C., Wood, M. & Jarvis, S. C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil. Biol. Biochem. 33, 1633–1640 (2001).
Saiyacork, K. R., Sinsabaugh, R. L. & Zak, D. R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil. Biol. Biochem. 34, 1309–1315 (2002).
Wallenius, K., Rita, H., Simpanen, S., Mikkonen, A. & Niemi, R. M. Sample storage for soil enzyme activity and bacterial community profiles. J. Microbiol. Meth. 81, 48–55, https://doi.org/10.1016/j.mimet.2010.01.021 (2010).
Joergensen, R. G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil. Biol. Biochem. 28, 25–31, https://doi.org/10.1016/0038-0717(95)00102-6 (1996).
Anderson, T. H. & Domsch, K. H. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil. Biol. Biochem. 22, 251–255 (1990).
Konopka, A., Oliver, L. & Jr, R. F. T. The Use of carbon substrate utilization patterns in environmental and ecological microbiology. Microb. Ecol. 35, 103–115 (1998).
Hussell, J. A. T. et al. Multiple carbon substrate utilization by bacteria at the sediment-water interface: seasonal patterns in a stratified eutrophic reservoir. Hydrobiologia 586, 43–56 (2007).
Legendre, P., Borcard, D. & Peres-Neto, P. R. Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecol. Monogr. 75, 435–450 (2005).
Acknowledgements
This work was supported by National Natural Science Foundation of China (nos 30860062, 31060082, 31660027), the Open Foundation of MOE Key Laboratory of Poyang Lake Environment and Resource (PYH2015-13) and Nanchang University Seed Grant for Biomedicine (9202-0210210807).
Author information
Authors and Affiliations
Contributions
Y.T.M. and J.Q.L. have done the main work of this paper, and prepared this manuscript; J.W. supplied some help in sampling process; Z.Y.K. and X.D. performed some data analysis and paper revise work; L.M.F. advised the writing skills and language; G.G. and L.W. conceived this study and offered financial support.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, Y., Li, J., Wu, J. et al. Bacterial and Fungal Community Composition and Functional Activity Associated with Lake Wetland Water Level Gradients. Sci Rep 8, 760 (2018). https://doi.org/10.1038/s41598-018-19153-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19153-z
- Springer Nature Limited
This article is cited by
-
A review of factors affecting the soil microbial community structure in wetlands
Environmental Science and Pollution Research (2024)
-
The home-field advantage of litter decomposition in lake wetlands and the community characteristics of bacterial and eukaryotic decomposers
Plant and Soil (2023)
-
First report on the bacterial community composition, diversity, and functions in Ramsar site of Central Himalayas, Nepal
Environmental Monitoring and Assessment (2023)
-
Dry-wet cycling area enhances soil ecosystem multifunctionality in the aquatic-terrestrial ecotones of the Caohai Lake in China
Environmental Science and Pollution Research (2023)
-
Distinct Assembly Mechanisms for Prokaryotic and Microeukaryotic Communities in the Water of Qinghai Lake
Journal of Earth Science (2023)