Abstract
The rice pathogenesis-related protein OsPR10a was scarcely expressed in OsCDPK1-silenced (Ri-1) rice, which was highly sensitive to pathogen infection. After inoculating the leaves with bacterial blight (Xanthomonas oryzae pv. oryzae; Xoo), we found that the expression of OsPR10a was up- and down-regulated in OEtr-1 (overexpression of the constitutively active truncated form of OsCDPK1) and Ri-1 rice plants, respectively. OsPR10a and OsCDPK1 showed corresponding expression patterns and were up-regulated in response to the jasmonic acid, salicylic acid and Xoo treatments, and OsPR1 and OsPR4 were significantly up-regulated in OEtr-1. These results suggest that OsCDPK1 may be an upstream regulator involved in rice innate immunity and conferred broad-spectrum of disease resistance. Following the Xoo inoculation, the OEtr-1 and Ri-1 seedlings showed enhanced and reduced disease resistance, respectively. The dihybrid rice Ri-1/OsPR10a-Ox not only bypassed the effect of OsCDPK1 silencing on the susceptibility to Xoo but also showed enhanced disease resistance and, consistent with Ri-1 phenotypes, increased plant height and grain size. Our results reveal that OsCDPK1 plays novel key roles in the cross-talk and mediation of the balance between stress response and development and provides a clue for improving grain yield and disease resistance simultaneously in rice.
Similar content being viewed by others
Introduction
In prokaryotic and eukaryotic cells, calcium ions (Ca2+) are intracellular second messengers that enable the sensing of a variety of environmental and developmental stimuli through temporal and spatial fluctuations or elevations in cytosolic Ca2+ concentrations1,2. In plants, calcium-dependent protein kinases (CDPKs)3,4,5 have been characterized as calcium sensors. CDPKs are considered Ser/Thr protein kinases and consist of the following four functional domains: the variable N-terminal domain, catalytic kinase domain, autoinhibitory region, and calmodulin-like regulatory domain6. CDPK activity was regulated by Ca2+, and its autoinhibitory domain can interact with the kinase domain in the absence of Ca2+, resulting in the inhibition of kinase activity1. Removing both the autoinhibitory region and the Ca2+ binding domains produced a constitutively active form of CDPK1,7. The CDPKs are encoded by multigene families in plants8,9 and play important physiological roles in response to diverse environmental stresses and developmental processes1,2,10,11,12,13.
In response to an extracellular pathogen attack, the cytosolic Ca2+ concentration increases as an early reaction in plant innate immunity14,15,16. The CDPKs have been suggested to function as mediators of Ca2+ signals, possibly instructing plants to initiate the defense response via specific protein phosphorylation events15,17,18. For example, the virus-induced gene silencing of NtCDPK2 and its homologue, NtCDPK3, delays and reduces the extent of necrotic symptoms in tobacco, indicating that NtCDPK2 plays a role in the hypersensitivity response to pathogens12. In Arabidopsis, the overexpression of the lipid-bodies and peroxisomal localized protein AtCPK1 results in the accumulation of salicylic acid (SA), which subsequently confers resistance to the pathogen Fusarium oxysporum, suggesting that lipid bodies have a function in plant innate immunity19.
In rice (Oryza sativa), only a few studies have investigated the role of the CDPKs in resistance to pathogen infection. For instance, overexpression of the constitutively active OsCPK10 in Arabidopsis and rice results in increased resistance to Pseudomonas syringae pv. tomato and the blast fungus Magnaporthe grisea, respectively20. The ectopic expression of the wheat powdery mildew resistance gene TaCPK2-A in japonica rice increased the expression of the pathogen resistance-related transcription factor OsWRKY45-1 and enhanced resistance to attack by bacterial blight Xoo 21. However, OsWRKY45-1 acts as a negative regulator of resistance to Xoo in the japonica rice ‘Nipponbare’22. The overexpression of OsCPK12 enhances tolerance to salt stress by reducing reactive oxygen species production but increases sensitivity to a blast fungus challenge23. Rice OsCPK18 was identified as an upstream kinase responsible for the phosphorylation and activation of OsMPK5, leading to the inhibition of the expression of defense-related genes (PR5, PR10, and chitinase) and negative regulation of blast fungus (Magnaporthe oryzae) resistance24. In addition, overexpression of OsCPK4 positively regulates salt and drought stress tolerance by reducing membrane lipid peroxidation25 and enhances resistance to M. oryzae infection by preventing fungal penetration26.
In our previous studies, we showed that OsCDPK1 positively regulates salt and drought tolerance but negatively affects seedling growth and seed development27. The overexpression of OsPR10a confers enhanced resistance to Xoo attack28. In the current study, we reveal that OsCDPK1 acts as a positive regulator of OsPR10a, indicating that OsCDPK1 is an upstream component of the defense signaling pathway. In addition, the dihybrid rice Ri-1/OsPR10a-Ox, which is derived from a cross between an OsPR10a overexpression line (OsPR10a-Ox) and an RNA interference knockdown of OsCDPK1 line (OsCDPK1-Ri; Ri-1), has a greater plant height, a larger seed size, and enhanced resistance to Xoo infection.
Results
Expression of OsPR10a is affected by OsCDPK1
The constitutively active mutants of CDPKs can bypass Ca2+ and stress signals and activate the expression of downstream responsive genes1,7. Therefore, we generated transgenic rice plants carrying a constitutively active truncated form of OsCDPK1 (OEtr-1) under the control of the maize ubiquitin promoter and performed functional studies27. The OEtr-1 transgenic rice seedlings showed a semi-dwarf phenotype, whereas the transformants subjected to the RNA interference gene knockdown (Ri-1) exhibited a slender-growth phenotype27. Two-dimensional gel electrophoresis was performed to isolate the proteins (or genes) regulated by OsCDPK1. Twenty up- or down-regulated candidate proteins were identified. Unexpectedly, among the differently regulated genes, OsPR10a (D38170), which encodes the pathogen resistance protein PBZ1, was highly expressed in WT seedlings, but only small amounts of the protein were detected in Ri-1 seedlings (Fig. 1). Therefore, we postulated that OsPR10a might be positively regulated by OsCDPK1. To test this hypothesis, the third leaf of three-week-old WT, OEtr-1 and Ri-1 seedlings were inoculated with a scissor-contaminated Xoo using the leaf tip-clipped method29 and then grown for 1 d. Total RNA extracted from the treated leaves was purified and subjected to northern blot analysis. In the controls, a small amount of OsPR10a mRNA could still be observed in OEtr-1, while it was almost undetectable in WT and Ri-1 (Supplementary Fig. S1a). After quantification of the hybridization signal by densitometer, the relative OsPR10a mRNA expression levels in the OEtr-1 and Ri-1 were 2.8- and 0.2-fold, respectively, when compared to the uninfected WT control (1.0-fold) (Supplementary Fig. S1b). In response to Xoo inoculation, the OsPR10a expression was strongly induced in OEtr-1 (30.8-fold), moderately induced in the WT (20.4-fold), and fewer induction in Ri-1 (11.2-fold), than that of the uninfected WT control (Supplementary Fig. S1). These results indicate that OsCDPK1 may mediate the defense signal to activate OsPR10a expression under Xoo attack.
Two-dimensional gel electrophoresis (2-DE) analysis of changes in the protein levels in the WT and Ri-1 seedlings. Total proteins were purified from 14-day-old seedlings, separated by 2-DE, and visualized by silver staining. The arrow indicates the OsPR10a protein spot that shows differences in the protein abundance between the WT (a) and Ri-1 (b) plants.
To verify the hierarchical relationship between OsCDPK1 and OsPR10a, we integrated the transgenes OEtr-1 and OsPR10a::GUS into a single genotype by crossing the OEtr-1 and OsPR10a::GUS lines to generate a dihybrid plant designated OEtr-1/OsPR10a::GUS. A PCR analysis of DNA isolated from calli induced from F2 seeds confirmed that the progeny harbored both transgenes. Positive calli (i.e., calli that harbored both transgenes) and calli derived from the OsPR10a::GUS line were established as suspension cell cultures. The cells were then transferred to fresh medium or 50 mL culture medium containing 10 μL Xoo (1.0 × 1010/mL) and cultured for an additional 1, 12, and 24 h. Before and after Xoo inoculation, the GUS staining and the quantitative GUS activity assay both detected in the OEtr-1/OsPR10a::GUS line was higher than that in the OsPR10a::GUS line (Fig. 2a and c). Similarly, when the leaves in three-week-old plants were either treated with Xoo (spray-inoculation) or wounding, or concurrent treatment by wounding and Xoo, all the treated leaves in OEtr-1/OsPR10a::GUS have showed stronger GUS activity than those leaves from the OsPR10a::GUS plants (Fig. 2b,d). Moreover, many GUS staining foci were distributed throughout the Xoo spray-inoculated leaves with or without wounding in both examined lines (Fig. 2b), demonstrating that the spray inoculation successfully achieved Xoo infection. Furthermore, an additional dihybrid plant, Ri-1/OsPR10a::GUS, was further analyzed using northern blot hybridization. Total RNA was extracted from the Xoo-infected leaves in 14-d-old OsPR10a::GUS, Ri-1/OsPR10a::GUS and OEtr-1/OsPR10a::GUS seedlings and subjected to northern blot analysis. The mRNA level of both OsPR10a and GUS was lower in Ri-1/OsPR10a::GUS but higher in OEtr-1/OsPR10a::GUS than it was in OsPR10a::GUS (Fig. 2e). These results suggest that OsCDPK1 is an upstream regulator of OsPR10a that positively affects its gene expression.
Histochemical staining of β-glucuronidase (GUS) activity in suspension-cultured cells and leaves from transgenic plants. (a) Suspension-cultured cells were inoculated with Xanthomonas oryzae pv. oryzae (Xoo) for 1, 12, and 24 h. Cells were harvested at the indicated time and stained with X-Gluc (5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid) for 2 h. (b) The third leaves of three-week-old seedlings were subjected to no treatment (control; C), spray-inoculation with Xoo (1.0 × 1010 CFU/mL) (Xoo), wounding with a sterilized razor blade (wou), or a concurrent treatment of wounding and spray-inoculation with Xoo (Wou + Xoo). One day post-inoculation, the treated leaves were cut, stained with X-Gluc for 12 h, and photographed. Fluorometric quantification of GUS activity in (c) suspension-cultured cells and (d) treated leaves using 4-MUG as the substrate. Different letters above the bars indicate significant differences as indicated by ANOVA (P < 0.05). The data are presented as the means ± SD (n = 12). (e) Northern blot analysis of OsPR10a and GUS gene expression in dihybrid plants following Xoo infection. Fourteen-day-old seedlings were wounded, spray-inoculated with Xoo (leaves were punctured with a needle before spraying) (1.0 × 1010 CFU/mL), and grown for 1 day. Total RNA from infected leaves was purified and subjected to northern blot hybridization using a probe prepared from an OsPR10a-specific region or GUS coding sequence. rRNAs served as the quantity control.
Expression of OsCDPK1 and OsPR10a is up-regulated in response to jasmonic acid, salicylic acid and Xoo treatments
The plant hormones jasmonic acid (JA) and salicylic acid (SA) play crucial roles in the defense signaling pathways. Therefore, we examined whether OsCDPK1 expression was induced by pathogen infection alone or also by JA or SA. The third leaf of three-week-old WT, OEtr-1 and Ri-1 seedlings was sprayed with JA (100 μM) or SA (100 μM) or the leaf tips were wounded and then spray-inoculated with Xoo (1.0 × 1010 CFU/mL), followed by growth for 1 d. Total RNA was obtained from the treated leaves and subjected to northern blot analysis. We observed that the mRNA level of OsCDPK1 was higher following the JA, SA, and Xoo treatments than it was in the control (Fig. 3a), indicating that the expression of OsCDPK1 was induced not only by Xoo but also by JA and SA. If OsCDPK1 acts as an upstream component of pathogen signaling and activates OsPR10a expression, the expression of OsPR10a should correspond to that of OsCDPK1 following the JA, SA and Xoo treatments. As shown in Fig. 3b, we also observed abundant expression of OsPR10a under JA, SA, and Xoo treatments. Similarly, slot-blot analysis reconfirmed the expression of both genes was induced by the JA and SA treatments and further increased following the combined exogenous application of JA (100 μM) and SA (100 μM) to the plants (Supplementary Fig. S4). These results suggested that OsCDPK1 may act as a mediator in the JA and SA signaling pathways, thereby enhancing OsPR10a gene expression.
Expression of OsCDPK1 and OsPR10a in response to salicylic acid (SA), jasmonic acid (JA) and Xanthomonas oryzae pv. oryzae (Xoo) treatments. The third leaves of three-week-old seedlings were wounded; spray-inoculated with Xoo (leaves were punctured with a needle before spraying) (1.0 × 1010 CFU/mL), JA (100 μM), or SA (100 μM); and grown for 1 d. Total RNA from the treated leaves was purified and subjected to a northern blot analysis using (a) OsCDPK1-specific or (b) OsPR10a-specific regions as probes.
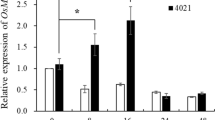
To further examine the possible roles of OsCDPK1 in the response to JA and SA signaling, the PR-related genes OsPR1 (encoding β-1,3-glucanase) and OsPR4 (encoding a member of the chitinase family), which are known as SA- and JA-signaling responsive marker genes29, respectively, and the genes OsLOX (encoding lipoxygenase) and OsPAL (encoding phenylalanine ammonia lyase), which are key enzymes in the JA and SA biosynthesis pathways30,31, were subjected to a real-time RT-PCR analysis. As shown in Fig. 4, compared with the levels observed in the WT plants, the relative expression levels of OsPR1, OsPR4, and OsPR10a were significantly up-regulated (P < 0.01) in the OEtr-1 lines but down-regulated in the Ri-1 plants, whereas the expression of OsLOX and OsPAL was unaffected. These results demonstrate that OsCDPK1 is involved in the innate immunity in rice and regulates a broad spectrum of PR-related genes involved in disease resistance.
Expression of pathogenesis-related genes in WT, Ri-1 and OEtr-1 seedlings inoculated with Xanthomonas oryzae pv. oryzae. The tip of the third leaf of the three-week-old seedlings was wounded with a needle and then spray-inoculated with Xoo (1.0 × 1010 CFU/mL). One day after the inoculation, total RNA was isolated from the treated leaves and subjected to a real-time RT-PCR analysis. Quantification of the relative expression levels of the selected genes normalized to the expression level of the internal control, i.e., OsActin. All reactions were analyzed in three replicates. Different letters above the bars indicate significant differences as indicated by ANOVA (P < 0.01). The data are presented as the mean ± SD of three independent repeats. OsPR10a: a ribonuclease-like pathogenesis-related gene; OsPR1: an antifungal protein family member; OsPR4: a chitinase gene; OsLOX: lipoxygenase gene; OsPAL: a phenylalanine ammonia lyase gene. Primer sets and gene accession numbers are listed in Supplementary Table S1.
In addition, we examined the expression of OsCDPK1 and OsPR10a in response to pathogen infection in germinating rice seeds. Transgenic seeds derived from the reporter lines OsCDPK1::GUS and OsPR10a::GUS 28 (Supplementary Fig. S5a) were germinated on ½ MS medium for 1–5 days and then stained to assess GUS activity. Compared with the germination in the healthy seeds, GUS was specifically expressed in the embryo and shoot, but not in the endosperm, in both transgenic lines (Supplementary Fig. S5b). Furthermore, we found that GUS staining was induced in the zone surrounding the brown spot lesions in the germinating seeds infected with an unidentified pathogen, but the staining was not visible around the spot lesions on the endosperm of the OsActin::GUS line (Supplementary Fig. S5c). These results demonstrate that OsCDPK1 and OsPR10a displayed similar gene expression patterns in the germinating rice seeds and were specifically induced by the pathogen infection.
Overexpression and RNA interference of OsCDPK1 enhanced and reduced pathogen resistance, respectively, in transgenic rice
The present data reveal that OsCDPK1 positive affects OsPR10a and that the expression of OsCDPK1 is induced by SA, JA, and Xoo treatments. Moreover, OsPR10a-overexpressing rice plants exhibit increased resistance to Xoo infection28. To determine whether OEtr-1 plants show increased resistance to pathogen attack, OEtr-1 and Ri-1 plants were inoculated with Xoo. A scissor contaminated with Xoo (1.0 × 1010 CFU/mL) was used to excise the apical 1-cm portion of the third leaf of three-week-old seedlings (Fig. 5a). At 10 DPI (days post-inoculation), the lesion area in the WT, OEtr-1 and Ri-1 leaves was 32 ± 6.1%, 18 ± 3.3%, and 46 ± 7.2%, respectively (Fig. 5b and c), revealing that the OEtr-1 plants had improved resistance to Xoo infection. To examine disease response in the whole plants, Xoo inoculation was performed as described in the Methods. Before Xoo inoculation, plants from the three tested lines were healthy, and the Ri-1 plants were taller while the OEtr-1 plants were shorter than WT plants (Supplementary Fig. S6a), this result is consistent with our previous finding in which OsCDPK1 was shown to act as a negative regulator of gibberellin (GA) biosynthesis27. At 10 DPI, the leaves of the Ri-1 and WT seedlings developed chlorotic or necrotic lesion symptoms, which developed earlier in the Ri-1 seedlings, but these lesions were barely detectable in the OEtr-1 seedlings (Supplementary Fig. S6a). At 15-20 DPI, an increase in the severity of the symptoms was observed in the Ri-1 seedlings, and up to 78–88% of all leaves became chlorotic or showed necrotic lesions with subsequently wilting and dying leaves, whereas only approximately 20–26% of the OEtr-1 seedlings displayed chlorotic symptoms (Supplementary Fig. S6a). At 20 DPI, the survival rate of the WT, OEtr-1, and Ri-1 seedlings was 21 ± 3.1%, 74 ± 10.3%, and 12 ± 3.2%, respectively (Supplementary Fig. S6b). These results indicated that OsCDPK1 enhances pathogen resistance in rice.
Ectopic expression of OsCDPK1 in rice and the dihybrid transgenic plant Ri-1/OsPR10a-Ox in response to Xanthomonas oryzae pv. oryzae infection. (a) and (d) Phenotypical comparison of three-week-old wild-type (WT) and transgenic seedlings. (b) and (e) The tip of the third leaf of three-week-old plants was excised with a razor blade contaminated with Xoo (1.0 × 1010 CFU/mL). Infected plants were incubated in a growth chamber for disease development. Photographs were obtained (b) 10 days or (e) 20 days after the inoculation. (c) and (f) Quantification of the lesion area. The experiments were repeated three times. Different letters above the bars indicate significant differences as indicated by ANOVA (P < 0.05). The data are presented as the means ± SD (n = 12).
Enhanced resistance to bacterial blight disease and seed size in the dihybrid transgenic rice Ri-1/OsPR10a-Ox
The OsCDPK1-silenced rice plants produce larger seeds and an increased crop yield27 but are more susceptible to pathogen infection (Fig. 5b and c; Supplementary Fig. S6). The present results demonstrate that OsCDPK1 acts as a positive regulator of OsPR10a. Therefore, we hypothesize that the overexpression of OsPR10a in the Ri-1 plants might lead to the development of a novel rice genotype with an improved crop yield and disease resistance. The Ri-1 line was crossed with OsPR10a-Ox7 28 (named OsPR10a-Ox in the present study) to generate the Ri-1/OsPR10a-Ox line. The genotypes of the F2 plants were examined by Southern blot hybridization using Hpt as a probe (Supplementary Fig. S7), which confirmed that the dihybrid plants harbored both transgenes. To evaluate the difference in disease resistance among the WT, OsPR10a-Ox, Ri-1, and dihybrid line Ri-1/OsPR10a-Ox, the plants were inoculated with Xoo by clipping the leaf tip as previously described. At 20 DPI, the leaves of the WT and Ri-1 plants had developed large lesions, but the leaves of the OsPR10a-Ox and Ri-1/OsPR10a-Ox lines had developed smaller lesions (Fig. 5e). The average lesion area in the WT, OsPR10a-Ox, Ri-1, and Ri-1/OsPR10a-Ox plants was 46 ± 10.3%, 25 ± 6.7%, 65 ± 11.4%, and 33 ± 5.2%, respectively (Fig. 5f). These results indicate that the dihybrid rice Ri-1/OsPR10a-Ox might also have improved disease resistance.
We also examined resistance to Xoo infection by performing a whole-plant experiment. Three-week-old seedlings were inoculated with Xoo as described in Supplementary Figure S8. Before Xoo infection, all plants showed healthy phenotypes, and the Ri-1 and Ri-1/OsPR10a-Ox plants were taller than the WT and OsPR10a-Ox plants (Supplementary Fig. S8a, upper panel). The Ri-1/OsPR10a-Ox plants inherited the slender-growth phenotype from the Ri-1 line27. At 20 DPI, severe disease symptoms were observed in the WT and Ri-1 plants, which was consistent with the results shown in Supplementary Figure S6, whereas only weak chlorosis was detected in the OsPR10a-Ox and Ri-1/OsPR10a-Ox seedlings (Supplementary Fig. S8a, middle panel). At 25 DPI, most WT (78%) and Ri-1 (86%) seedlings were necrotic and subsequently died, whereas only a few leaves were chlorotic in the OsPR10a-Ox and Ri-1/OsPR10a-Ox lines (Supplementary Fig. S8a, lower panel). Thus, the latter two lines exhibited strongly enhanced resistance to Xoo infection. The survival of the WT, OsPR10a-Ox, Ri-1, and Ri-1/OsPR10a-Ox seedlings at 25 DPI was 22 ± 6.4%, 68 ± 12.2%, 14 ± 5.5%, and 66 ± 16.4, respectively (Supplementary Fig. S8b). These results provide direct evidence indicating that the dihybrid rice Ri-1/OsPR10a-Ox can overcome the effect of OsCDPK1 silencing on the susceptibility to Xoo infection and achieve improved disease resistance. Furthermore, our previous studies demonstrated that the transgenic rice Ri-1 has enhanced seedling growth and produces larger seeds27, and no significant differences in seed size, seed set and seedling development were observed between the WT and OsPR10a-overexpressing rice plants28. The present results show that the dihybrid rice Ri-1/OsPR10a-Ox, which exhibits a phenotype similar to that of Ri-1, also has an increased plant height (Fig. 6a and b), grain size (Fig. 6c and d), and panicle length (Fig. 6e and f). The mature plant height of Ri-1/OsPR10a-Ox was 17.3% higher than that of the WT plants (Fig. 6b). Compared to the WT, the Ri-1/OsPR10a-Ox line showed an 8.3% increase in grain length (Fig. 6d) and a 15.8% increase in panicle length (Fig. 6f). These results demonstrate that the transgenic dihybrid rice Ri-1/OsPR10a-Ox has a potential to increase disease resistance and improve grain yield in rice.
Phenotypes of OsPR10a-Ox, Ri-1, the dihybrid Ri-1/OsPR10a-Ox, and wild-type (WT) rice. (a) Fourteen-day-old seedlings were transplanted individually into buckets. Mature plants (WT, OsPR10a-Ox, Ri-1, and Ri-1/OsPR10a-Ox) at the middle heading stage (98 days) were photographed. (b) Quantification of plant height. (c) Comparison of seed size between the WT and transgenic lines. (d) Quantification of grain length. (e) Panicle phenotypes of the WT and Ri-1/OsPR10a-Ox. Three panicles were collected from the WT and dihybrid plants and photographed. (f) Quantification of panicle length. (b, d and f) Different letters above the bars indicate significant differences as indicated by ANOVA (P < 0.05). The data are presented as the means ± SD (n = 20).
Discussion
The overexpression of OsPR10a in rice confers increased resistance to Xoo bacterial blight28. The OsCDPK1-silenced plants (Ri-1) produced larger seeds (Fig. 6), but the grain number was unaffected; therefore, these plants provided an improved crop yield27 but were more susceptible to the pathogen challenge (Fig. 5). In the current study, using a gene pyramiding approach to combine OsCDPK1 silencing and OsPR10a overexpression, we generated dihybrid plants that retained the higher crop yield trait of Ri-1 and not only overcame the sensitivity to pathogen infection but also showed enhanced disease resistance with a phenotype similar to that of OsPR10a-Ox.
Despite the importance of OsPR10a in the resistance to pathogen infection in rice, its regulatory pathway remains poorly understood. In the present study, we show that the protein kinase OsCDPK1 functions as a positive effector of OsPR10a in rice. Both loss-of-function and gain-of-function experiments were performed to support the role of OsCDPK1 in defense responses and the regulation of OsPR10a expression. These results support the hypothesis that OsCDPK1 acts as an upstream signal transducer in the rice defense response to a pathogen challenge. The plant hormone SA is known to play key roles in plant defense against biotrophic and hemi-biotrophic pathogens31, and JA is involved in resistance to necrotrophic pathogens and herbivorous insects31. The SA and JA signaling pathways interact antagonistically to regulate responses to different biotic stresses30,32,33,34,35. However, a few studies have reported that SA and JA may have a synergistic effect in defense signaling at low concentrations despite their seemingly antagonistic effects at high concentrations (i.e., SA ≥ 350 μM and JA ≥ 125 μM)36,37. OsPR10a (PBZ1) is induced by exogenous SA, JA, Xoo, and the blast fungus Magnaporthe grisea 28,38,39,40, suggesting that OsPR10a might confer broad-spectrum resistance to pathogens. These findings are consistent with the present results, and we showed that both OsCDPK1 and OsPR10a were synergistically up-regulated by the treatment with SA (100 μM) and/or JA (100 μM) or infection by the biotrophic pathogen Xoo (Fig. 3; Supplementary Fig. S4). In addition, we observed that the SA and JA signaling responsive genes OsPR1 and OsPR4 30, respectively, were both induced in OEtr-1 but repressed in Ri-1, and the expression of OsPAL and OsLOX, which are key enzymes in the SA and JA biosynthesis pathways, respectively31,32, did not significantly differ (P < 0.01) among the WT, OEtr-1, and Ri-1 (Fig. 4). These results suggest that OsCDPK1 might mediate the SA and JA signaling pathways, thereby inducing OsPR10a expression and resulting in broad-spectrum disease resistance in rice. Moreover, the GUS activity staining and its gene expression were considerable enhanced and reduced in the OEtr-1/OsPR10a::GUS and Ri-1/OsPR10a::GUS, respectively, in response to wounding and Xoo infection (Fig. 2), indicating that OsCDPK1 acts upstream of OsPR10a in rice defense signaling pathways upon pathogen attack and wounding. These results suggest that OsCDPK1 might phosphorylate its novel substrate thereby directly or indirectly regulates OsPR10a expression via an unknown transcription factor binding to the OsPR10a promoter. The ChIP (chromatin immunoprecipitation) cloning strategy therefore can be used to isolate this specific transcription factor to unravel the mechanism of OsCDPK1-mediated OsPR10a expression, to clarify the effect of OsCDPK1 on regulation of OsPR10a is through the direct or by indirect pathways. It is worth noting that OsPR10a was also expressed in response to Xoo infection in the Ri-1 plants (Supplementary Fig. S1), although at a lower level than that in the WT, suggesting that other signaling pathways may contribute to the regulation of OsPR10a expression following Xoo infection. Two rice transcription factors, i.e., OsWRKY6 and OsWRKY51, can bind to the OsPR10a promoter and activate its transcription in response to Xoo inoculation41,42. Moreover, overexpression of OsWRKY45–2 also induces OsPR10a expression in rice22. Collectively, these findings suggest that certain OsCDPK1-dependent and OsCDPK1-independent signaling pathways may coordinate the regulation of OsPR10a expression in response to Xoo attack.
In plants, the CDPKs play diverse roles in response to biotic and abiotic stresses and modulate various aspects of plant growth and development. Our results reveal that OsCDPK1 performs multiple functions in response to biotic and abiotic stresses and developmental regulation. The OsCDPK1-overexpressing rice (OEtr-1) shows enhanced tolerance to drought stress and negatively regulated GA biosynthesis, resulting in a semi-dwarf seedling phenotype27. Based on our current and previous studies, we propose a model in which OsCDPK1 plays vital roles in the interconnection of various signaling pathways to coordinate the physiological adaptive responses to biotic and abiotic stresses and adverse growing conditions (Fig. 7). The plant signaling pathways involved in the stress response and growth are generally antagonistic to each other, in which a few regulators play key roles in the cross-talk and mediation of the balance between stress response and development35. When plants are subjected to biotic and abiotic stresses, the stress-signaling networks induce an increase in the levels of certain phytohormones, such as SA, JA, abscisic acid (ABA), and ethylene, and subsequently induce the expression of stress-related genes. In contrast, these networks have a negative effect on plant growth-promoting hormones, such as GAs, auxin, and cytokinin, thus resulting in the attenuated expression of development-related genes43,44,45,46,47,48,49,50. For example, in Arabidopsis, the GA signaling repressor DELLA increases the sensitivity to biotrophic pathogens and resistance to necrotrophic pathogens by orchestrating the relative signaling strength of SA and JA51. DELLA competes with MYC2 (a transcriptional activator of JA signaling) for binding to JASMONATE-ZIM DOMAIN (JAZ; a key repressor of JA signaling), which results in the release of MYC2 and activates the JA signaling response52,53,54. These findings demonstrate that the JA signaling pathways may be compromised by GA through the degradation of DELLA. Therefore, DELLA might act as a key regulator of the crosstalk among the GA, SA, and JA signaling pathways. Moreover, SA signaling is induced by infection with virulent biotrophs, which simultaneously weakens ABA signaling, indicating that antagonistic interactions likely occur between the biotic and abiotic signaling pathways45,55. These results reveal that plants have developed multiple mechanisms to coordinate a variety of hormone signals to modulate the balance between biotic and abiotic stresses and growth responses.
Generate crops with stress tolerance and (or) resistance and a high yield using traditional breeding methods is challenging due to the antagonistic regulatory pathways involved in stress responses and developmental processes48. Thus, genetic modification strategies might be useful for achieving this aim, but an understanding of the essential genes involved in the antagonistic pathways in individual plants is essential. We have shown that OsCDPK1 enhances drought tolerance mediated by GF14c27 and increased resistance to Xoo infection by affecting OsPR10a expression (Figs 1, 2, 4, and 5). OsCDPK1 also confers a negative feedback loop to regulate GA biosynthesis by down-regulating GA20ox1 and GA3ox2 expression; therefore, OEtr-1 plants have semi-dwarf seedlings and smaller grain phenotypes27 (Fig. 5). We also observed that Ri-1 has an increased plant height and produces larger seeds but is more sensitive to Xoo. Using hybridization to combine the favorable traits of Ri-1 and OsPR10a-ox in a single genotype, we demonstrated that the dihybrid transgenic rice Ri-1/OsPR10a-ox, which bypasses the sensitivity to Xoo, not only shows enhanced resistance to Xoo but also increased plant height and grain size. These results provide insight that improves our understanding of the molecular mechanisms underlying the balance between growth and defense responses in plants, which may be beneficial for traditional breeding and biotechnological approaches or the recently proposed ‘molecular strengthening’ (MOST) strategy56 to simultaneously improve stress tolerance and (or) resistance and increase crop yield in rice.
Methods
Plant material and preparation of cell suspension cultures
Immature seeds of the rice cultivar ‘Tainung 67’ were used for callus induction as described previously27. After incubation for about 30–40 days, the calli derived from the scutellum were transferred to liquid MS57 complete medium (MS salts containing 3% sucrose and supplemented with 10 µM 2,4-dichlorophenoxyacetic acid) to establish a suspension cell culture.
Southern and northern and slot blot analyses
Genomic DNA or total RNA were isolated from three-week-old seedlings using urea extraction buffer or TRIzol reagent (Invitrogen, Carlsbad, CA, USA), respectively. DNA and RNA gel-blot analyses were conducted as described previously27. Ten micrograms of genomic DNA and total RNA were analyzed in 0.8% and 1.0% agarose gel, respectively, then transferred to a nylon membrane and hybridized with a digoxigenin-11-dUTP (DIG-11-dUTP) labelled probe. The blot was visualized using autoradiography with X-ray film.
Protein extraction and two-dimensional gel electrophoresis analysis
Total proteins were extracted from 14-day-old seedlings (wild type [WT] and Ri-1) in an extraction buffer and mixed with an equal volume of phenol (pH 7.5). The aqueous supernatant was precipitated with acetone, the protein pellet was washed with acetone and air-dried, and stored at −80 °C. For first-dimension analysis, the isolated protein was rehydrated with rehydration buffer and analyzed using immobilized pH gradient (IPG) strips with pH 4–7 in accordance with the manufacturer’s instructions (Bio-Rad, Richmond, CA, USA). The second dimension was carried out using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and the separated protein spots were visualized by silver staining. The candidate proteins were subjected to in-gel tryptic digestion and the samples were purified and subjected to liquid chromatography–tandem mass spectrometry as described previously28,58.
Primers
The sequence of all primers used for PCR and real time RT-PCR amplification are listed in Supplementary Table S1.
Construction of expression vectors
The plasmid constructs for generation of the transgenic plants OEtr-1 (overexpression of the constitutively active truncated form of OsCDPK1), Ri-1, OsPR10a-Ox and OsPR10a::GUS were constructed as described previously (Supplementary Fig. S5a)27,28. To construct the OsCDPK1::GUS expression vector, a 2.0-kb DNA fragment containing the promoter and 5′-untranslated region of OsCDPK1 (Supplementary Fig. S5a) was amplified by PCR using the primers OsCDPK1P-FP (5′-ATCCTGCAGTCTTATTAGGTAAGGCCTTG-3′) and OCDPK1P-RB (5′- ACTGGATCCAAGAACTCCTTATGCAAACC-3′). This DNA fragment was digested with PstI and BamHI, and cloned into the GUS expression vector pBX-2 as described previously59. The recombinant construct was then inserted into the pSMY1H binary vector57.
Plant transformation
Rice calli were transformed using Agrobacterium-mediated gene transformation as previously described57.
Real-time RT-PCR
The tips of the third leaf from three-week-old T2 seedlings were wounded with a needle and then spray-inoculated with Xoo (1.0 × 1010 CFU/mL). One day after the inoculation, the total RNA was isolated from the treated leaves using TRIzol reagent (Invitrogen), and the contaminated DNA was eliminated using a TURBO DNA-free kit (Ambion). Five micrograms of total RNA were used to synthesize first strand cDNA by M-MuLV reverse transcriptase (New England Biolabs) with oligo (dT) primer. Quantitative real time RT-PCR was performed with the Eco Real-Time PCR System (Illumina Inc., San Diego, CA) according to the manufacturer’s instructions. The gene-specific primer sets (Table S1) localized at the 3′-untranslated regions of each examined genes were used to evaluate the expression levels of OsPR1, OsPR4, OsPR10a, OsLOX and OsPAL in WT, OEtr-1 and Ri-1. The relative expression levels were normalized to the expression of the internal control, i.e., OsActin. All reactions were analyzed in three replicates.
Histochemical staining of GUS activity in rice cells and leaves
To stain the GUS activity in the cultured cells, the cell suspensions were cultured in liquid MS medium for 3 days, followed by inoculation with Xoo (1.0 × 108 CFU/50 mL). The cells were collected at 0 (before inoculation; control), 1, 12, and 24 h after the inoculation for the GUS staining. To assay GUS activity in the leaves, the third leaf of three-week-old seedlings was untreated (control), treated with a spray-inoculation with Xoo (1.0 × 1010 CFU/mL), wounded with a razor blade contaminated with Xoo and then sprayed with Xoo, or wounded with a sterilized razor blade. The leaves were incubated in a growth chamber at 28 °C with > 80% relative humidity under a 16-h light/8-h dark photoperiod for 1 d. The treated leaves were cut, stained with X-Gluc (5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid; Sigma-Aldrich, St Louis, MO, USA) for 12 h, and then photographed. The fluorometric quantification of GUS activity was conducted according to the manufacturer’s instructions.
Inoculation of rice seedlings with Xoo
To analyze the lesion area in the Xoo-infected leaves, the third leaf of three-week-old seedlings of the tested lines was inoculated with Xoo by excising the leaf tip with a scissor contaminated with Xoo (1.0 × 1010 CFU/mL)29. For the Xoo inoculation of whole plants, the tip of every leaf on three-week-old seedlings was penetrated at five different sites per leaf with a Xoo-contaminated needle and then spray-inoculated with Xoo (1.0 × 1010 CFU/mL) once daily for five days. The inoculated plants were incubated in a growth chamber at 28 °C, 90% relative humidity, under a 16 h light/8 h dark photoperiod for the development of the disease symptoms. The disease symptoms were evaluated by measuring the area of necrotic lesions on the leaves (expressed as a percentage of the total leaf area) or assessing the percentage survival of infected plants.
References
Harper, J. F., Breton, G. & Harmon, A. Decoding Ca(2+) signals through plant protein kinases. Annu Rev Plant Biol. 55, 263–288 (2004).
Sanders, D., Pelloux, J., Brownlee, C. & Harper, J. F. Calcium at the crossroads of signaling. Plant Cell. 14, Suppl: S401–417 (2002).
Ranty, B., Aldon, D. & Galaud, J. P. Plant calmodulins and calmodulin-related proteins. Plant Signal Behav. 1, 96–104 (2006).
DeFalco, T. A., Bender, K. W. & Snedden, W. A. Breaking the code: Ca2+ sensors in plant signalling. Biochem J. 425, 27–40 (2010).
Zeng, H. et al. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front Plant Sci. 6, 600, doi:10.3389 (2015).
Ludwig, A. A., Romeis, T. & Jones, J. D. CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot. 55, 181–188 (2004).
Sheen, J. Ca2+ -dependent protein kinases and stress signal transduction in plants. Science 274, 1900–1902 (1996).
Mori, I. C. et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4, e327 (2006).
Ray, S., Agarwal, P., Arora, R., Kapoor, S. & Tyagi, A. K. Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice (Oryza sativa L. ssp. indica). Mol Genet Genomics. 278, 493–505 (2007).
Abbasi, F., Onodera, H., Toki, S., Tanaka, H. & Komatsu, S. OsCDPK13, a calcium-dependent protein kinase gene from rice, is induced by cold and gibberellin in rice leaf sheath. Plant Mol Biol. 55, 541–552 (2004).
Chehab, E. W., Patharkar, O. R., Hegeman, A. D., Taybi, T. & Cushman, J. C. Autophosphorylation and subcellular localization dynamics of a salt- and water deficit-induced calcium-dependent protein kinase from ice plant. Plant Physiol. 135, 1430–1446 (2004).
Romeis, T., Ludwig, A. A., Martin, R. & Jones, J. D. Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 20, 5556–5567 (2001).
Wan, B., Lin, Y. & Mou, T. Expression of rice Ca(2+)-dependent protein kinases (CDPKs) genes under different environmental stresses. FEBS Lett. 581, 1179–1189 (2007).
Andolfo, G. & Ercolano, M. R. Plant innate immunity multicomponent model. Front Plant Sci. 6, 987, https://doi.org/10.3389/fpls.2015.00987 (2015).
Boudsocq, M. et al. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature. 464, 418–422 (2010).
Kushalappa, A. C., Yogendra, K. N. & Karre, S. Plant innate immune response: qualitative and quantitative resistance. Crit Rev Plant Sci. 35, 38–55 (2016).
Boudsocq, M. & Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 18, 30–40 (2013).
Gao, X., Cox, K. L. Jr & He, P. Functions of calcium-dependent protein kinases in plant innate immunity. Plants. 3, 160–176 (2014).
Coca, M. & San Segundo, B. AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J. 63, 526–540 (2010).
Fu, L., Yu, X. & An, C. Overexpression of constitutively active OsCPK10 increases Arabidopsis resistance against Pseudomonas syringae pv. tomato and rice resistance against Magnaporthe grisea. Plant Physiol Biochem. 73, 201–210 (2013).
Geng, S. et al. TaCPK2-A, a calcium-dependent protein kinase gene that is required for wheat powdery mildew resistance enhances bacterial blight resistance in transgenic rice. J Exp Bot. 64, 3125–3136 (2013).
Tao, Z. et al. A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 151, 936–948 (2009).
Asano, T. et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 69, 26–36 (2012).
Xie, K., Chen, J., Wang, Q. & Yang, Y. Direct phosphorylation and activation of a mitogen-activated protein kinase by a calcium-dependent protein kinase in rice. Plant Cell. 26, 3077–3089 (2014).
Campo, S. et al. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 165, 688–704 (2014).
Bundó, M. & Coca, M. Enhancing blast disease resistance by overexpression of the calcium-dependent protein kinase OsCPK4 in rice. Plant Biotechnol J. https://doi.org/10.1111/pbi.12500 (2015).
Ho, S. L. et al. Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant Mol Biol. 81, 347–361 (2013).
Huang, L. F. et al. Multiple patterns of regulation and overexpression of a ribonuclease-like pathogenesis-related protein gene, OsPR10a, conferring disease resistance in rice and Arabidopsis. PLoS ONE. 11, e0156414, doi:10.1371 (2016).
Kauffman, H. E., Reddym, A. P. K., Hsieh, S. P. V. & Merca, S. D. An improved technique for evaluating resistance to rice varieties of Xanthomonas oryzae. Plant Dis Rep. 57, 537–541 (1973).
Spoel, S. H. & Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol. 12, 89–100 (2012).
Loake, G. & Grant, M. Salicylic acid in plant defence-the players and protagonists. Curr Opin Plant Biol. 10, 466–472 (2007).
Wasternack, C. & Hause, B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 111, 1021–1058 (2013).
Bari, R. & Jones, J. D. Role of plant hormones in plant defence responses. Plant Mol Biol. 69, 473–488 (2009).
Smith, J. L., De Moraes, C. M. & Mescher, M. C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag Sci. 65, 497–503 (2009).
Verma, V., Ravindran, P. & Kumar, P. P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16, 86, https://doi.org/10.1186/s12870-016-0771-y (2016).
Mur, L. A. J., Kenton, P., Atzorn, R., Miersch, O. & Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 140, 249–262 (2006).
Thaler, J. S., Karban, R., Ullman, D. E., Boege, K. & Bostock, R. M. Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia. 131, 227–235 (2002).
Hwang, S. H., Lee, I. A., Yie, S. W. & Hwang, D. J. Identification of an OsPR10a promoter region responsive to salicylic acid. Planta. 227, 1141–1150 (2008).
Kim, S. T. et al. Proteomic analysis of differentially expressed proteins induced by rice blast fungus and elicitor in suspension-cultured rice cells. Proteomics. 3, 2368–2378 (2003).
Kim, S. T. et al. Proteomic analysis of pathogen-responsive proteins from rice leaves induced by rice blast fungus, Magnaporthe grisea. Proteomics. 4, 3569–3578 (2004).
Choi, C. et al. Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol. 208, 846–859 (2015).
Hwang, S. H. et al. OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 35, 1975–1985 (2016).
Ahuja, I., de Vos, R. C. H., Bones, A. M. & Hall, R. D. Plant molecular stress responses face climate change. Trends Plant Sci. 15, 664–674 (2010).
Bita, C. E. & Gerats, T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 4, 273 (2013).
Huang, D., Wu, W., Abrams, S. R. & Cutler, A. J. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J Exp Bot. 59, 2991–3007 (2008).
Kissoudis, C., van de Wiel, C., Visser, R. G. F. & van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front Plant Sci. 5, 207, https://doi.org/10.3389/fpls.2014.00207 (2014).
Larkindale, J. & Huang, B. Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol. 161, 405–413 (2004).
Li, W. et al. Overexpressing CYP71Z2 enhances resistance to bacterial blight by suppressing auxin biosynthesis in rice. PLoS One. 10, doi:e0119867 (2015).
López, M. A., Bannenberg, G. & Castresana, C. Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol. 11, 420–442 (2008).
O’Brien, J. A. & Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci. 4, 451 (2013).
Navarro, L. et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol. 18, 650–655 (2008).
Grant, M. R. & Jones, J. D. Hormone (dis)harmony moulds plant health and disease. Science. 324, 750–752 (2009).
Hou, X., Ding, L. M. & Yu, H. Crosstalk between GA and JA signaling mediates plant growth and defense. Plant Cell Rep. 32, 1067–1074 (2013).
Hou, X., Lee, L. Y., Xia, K., Yan, Y. & Yu, H. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell. 19, 884–894 (2014).
Kim, T. H. et al. Chemical genetics reveals negative regulation of abscisic acid signaling by a plant immune response pathway. Curr Biol. 21, 990–997 (2011).
Hu, S. & Lübberstedt, T. Getting the ‘MOST’ out of crop improvement. Trends Plant Sci. 20, 372–379 (2015).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15, 473–497 (1962).
Tsay, Y. G., Wang, Y. H., Chiu, C. M., Shen, B. J. & Lee, S. C. A strategy for identification and quantitation of phosphopeptides by liquid chromatography/tandem mass spectrometry. Anal Biochem. 287, 55–64 (2000).
Ho, S. L., Tong, W. F. & Yu, S. M. Multiple mode regulation of a cysteine proteinase gene expression in rice. Plant Physiol. 122, 57–66 (2000).
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of the Republic of China (MOST 105-2313-B-415-009- and MOST 106-2313-B-415-006-).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: S.L. Ho. Performed the experiments: S.L. He, C.H. Kuo, J.Z. Jiang and B.H. Chen. Analyzed the data and wrote the paper: S.L. Ho.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, SL., Jiang, JZ., Chen, BH. et al. Overexpression of a constitutively active truncated form of OsCDPK1 confers disease resistance by affecting OsPR10a expression in rice. Sci Rep 8, 403 (2018). https://doi.org/10.1038/s41598-017-18829-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18829-2
- Springer Nature Limited