Abstract
Persistent and stable respiratory activity across behavioral states is key to homeostasis. Extrasynaptic δ-subunit containing GABAA receptors (δGABAARs) mediate tonic inhibition and regulate network activity. However, the influence of δGABAARs on respiratory rhythm and motor outputs is unknown. We manipulated extra-synaptic GABAA receptor function in the preBötzinger Complex (preBötC), a site central to the generation of inspiratory motor activity in mammals. Activation of preBötC δGABAARs in anesthetized rats and wild-type mice decreased breathing rate. In δGABAAR knockout (Gabrd −/−) mice, however, δGABAARs activation had no effect on breathing rate. We then found that during active wakefulness associated with behaviors and movements, diaphragm activation was higher in the Gabrd −/− compared to wild-type mice, but not in other states. These findings identify that δGABAARs modulate the respiratory network, which is critical to understand how δGABAARs change breathing in pathological conditions affecting extra-synaptic GABAA receptor function such as exposure to anesthetics and neurosteroids.
Similar content being viewed by others
Introduction
Rhythmic breathing is generated by a complex neural network in the brainstem1 that combines excitatory and inhibitory circuits. The respiratory network produces an intricate respiratory pattern with three phases: inspiration, post-inspiration, and late expiration. Inspiratory motor output is driven by a population of neurons that forms the preBötzinger Complex (preBötC)2,3,4, whereas expiratory activity is regulated by the Bötzinger Complex5, a structure with direct projections from and to the preBötC, and the retrotrapezoid nucleus6. In the preBötC, excitatory neurons drive inspiratory output2, whereas inhibitory neurons coordinate the generation of inspiratory and expiratory phases7. Optogenetically targeted excitation of glycinergic neurons in the preBötC shape respiratory pattern by inducing prolonged apnea, but rhythm persists even after substantial inhibition of glycinergic cells8. The functional role of GABAergic neurons in the preBötC is, however, not clear. Expression of the GABA-synthesizing enzyme glutamate decarboxylase shows that GABAergic neurons constitute a large part of inhibitory preBötC neurons9. In brainstem sections in vitro, blocking GABAergic neurotransmission increased rhythm variability, but did not otherwise change the frequency of rhythmic bursts10. In vivo, application of the GABAA receptor agonist muscimol at the preBötC abolished rhythmic activity5, whereas the GABAA receptor antagonist bicuculline did not change rhythmic breathing, but suppressed the Breuer-Hering inspiratory inhibitory reflex11. In rabbits, GABAA receptor blockade in the preBötC decreased respiratory rate, but did not completely blocked it12. These studies suggest that the endogenous role of GABAA receptors in the preBötC is to shape respiratory motor output, for instance, during voluntary control of breathing11, but this hypothesis has not yet been tested.
GABAA receptors mediate fast synaptic inhibition, whereas GABA can also activate highly-sensitive extra-synaptic GABAA receptors and produce tonic inhibition13. Extrasynaptic δ-subunit containing GABAA receptors (δGABAARs) mediate tonic inhibition and regulate network excitability14 and outputs15. δGABAARs also function to dampen network excitability and prevent excessive excitation16. In the context of endogenously active rhythmic networks, such as the respiratory network, these observations suggest that extra-synaptic δGABAARs may also play a significant role in modulating respiratory activity. However, the role of extra-synaptic δGABAARs in modulating respiratory rhythm has never been tested. Here, we propose that δGABAARs provide a level of tonic inhibition in the respiratory network to modulate rhythmic breathing. To test these concepts, we pharmacologically manipulated the preBötC of rats and mice, the latter including wild-type mice and mice lacking δGABAARs17. Our data identify for the first time that δGABAARs function to modulate respiratory network activity.
Methods
Experimental animals
All procedures were performed in accordance with the recommendations of the Canadian Council on Animal Care, and were approved by the University of Toronto Animal Care Committee. Twelve adult male Wistar rats (255–350 g, Charles River, Saint-Constant, Quebec, Canada), and nineteen adult mice lacking δGABAARs (Gabrd −/−) plus nineteen wild-type littermate controls were used for physiological recordings (body weight: 30.4–37.0 g). The generation of Gabrd −/− mice has been described previously18. Mice and rats were housed with free access to food and water under a 12-hour light:dark cycle (lights on at 7 am).
Microperfusion and recordings in anesthetized rats
In anesthetized rats, we used reverse-microdialysis to unilaterally microperfuse selected agents into the preBötC. The experimental procedures were as described previously19,20. Briefly, we recorded activities of the diaphragm and tongue muscles in isoflurane-anesthetized (2–2.5% inspired), tracheotomised and spontaneously breathing (50% inspired oxygen, balance nitrogen) rats. Diaphragm muscle activity was recorded using stainless steel bipolar electrodes positioned and sutured onto the right crural diaphragm. Tongue muscle activity was recorded using two stainless steel needles inserted via a per-oral approach. The electromyogram signals were amplified (CWE Inc., Ardmore, Pennsylvania, USA), band-pass filtered (100–1000 Hz), integrated, and digitized at a sampling rate of 1000 Hz using an acquisition system and Spike software version 6 (both from Cambridge Electronic Design Limited, Cambridge, UK). Rats were kept warm with a heating pad during the experiments. Using a dorsal approach, a microdialysis probe (CX-I-12-01, Eicom, Kyoto, Japan) of 200 µm diameter and 1 mm diffusing membrane length was inserted into the preBötC using a stereotaxic frame and micromanipulator (ASI Instruments, Warren, Michigan, USA). The probe was placed 12.2 mm posterior, 2.0 mm lateral, and 10.5 mm ventral to bregma according to a standard rat brain atlas21 and our previous experience with this model19,20. Baseline (aCSF) data for breathing rate and diaphragm amplitude were averaged over a 30-min period preceding the start of THIP infusion. THIP data were averaged for a 30-min period starting 30 min after THIP infusion was initiated. Only experiments where the probe was located within 2 mm from the center of the preBötC were analysed.
Anatomical location of the microdialysis probes in rats
As described previously we used three criteria to target the preBötC, to position the probe, and to confirm its location19,20: (i) When the probe was inserted into the medulla, tongue muscle activity decreased ~30% as it reached the vicinity of the preBötC. (ii) Microperfusion of the µ-opioid receptor agonist DAMGO (5 µM) decreased breathing rate by approximately 50%19. (iii) Post-mortem histology confirmed the probe location in the preBötC using anatomical markers such as the semi-compact division of nucleus ambiguus and the caudal part of the facial nucleus. We used the caudal part of the facial nucleus as a reference to identify the brain section located 11.6 mm posterior to bregma according to Paxinos atlas21. Once the coronal section where the probe was located was identified, we used the nucleus ambiguus to then determine the medial-lateral and dorsal ventral coordinates. Once the location of the probe site was determined, we determined 3-dimensional coordinates (anterior-posterior, medial-lateral, and dorsal-ventral) using bregma as reference. We used these three anatomical and functional criteria, and experience from our previous studies, to confirm that the probes were positioned in the region of the preBötC. On rare occasions (~1/20 experiments), the probes damaged the preBötC and respiratory rhythm was irregular and unstable. In such an event, we discontinued the experiment.
Microperfusions
Fresh artificial cerebrospinal fluid (aCSF) was made for each experiment with the following composition (in mM): NaCl (125), KCl (3), KH2PO4 (1), CaCl2 (2), MgSO4 (1), NaHCO3 (25), and glucose (30); pH was adjusted to 7.4 by bubbling CO2 into aCSF. A microdialysis probe was perfused with aCSF and baseline levels of the physiological variables were recorded for at least 30 min followed by 120 min of recordings during microperfusion of the selected agents. We applied the δGABAAR-preferring agonist 4,5,6,7- tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride (THIP), the GABAA receptor antagonist bicuculline and the µ-opioid receptor antagonist DAMGO to the preBötC. All drugs were obtained from Tocris (Minneapolis, Minnesota, USA).
Correlation between the distance from perfusion sites to the preBötC and the resultant effect on respiratory activity
We calculated the correlation to relate the location of the intervention sites with the resultant effect on respiratory activity as previously described19. The rationale for these correlations is that for a locus of effect of THIP at the preBötC, the latency for the drug to diffuse through the tissue and to progressively change respiratory activity will vary as a function of the distance of the probe from the preBötC. We first determined the locations of the intervention (perfusion) sites using the criteria described above. For each animal, we also calculated the distances from the corresponding perfusion site to the center of the preBötC (coordinates: anterior-posterior = −12.2 mm, dorsal-ventral = −10.5 mm, medial-lateral = −2.0 mm). We then calculated the latencies to a 10% change in respiratory rate in response to THIP, and correlated these distances with the latencies or the magnitudes of THIP effects on respiratory rate.
Microperfusion and recordings in anesthetized mice
In anesthetized (isoflurane, 1.5–2%), spontaneously breathing (50% oxygen, balance nitrogen) wild-type and Gabrd −/− mice, we also used reverse microdialysis to microperfuse agents into the preBötC. We recorded diaphragm muscle activity using a similar approach to the rats and as previously described for mice22. The mice were also kept warm with a heating pad. We inserted the microdialysis probe into the medulla 6.7 mm posterior, 1.2 mm lateral, and 5.7 mm ventral to bregma according to the standard brain atlas23 and prior experience24. For wild-type or Gabrd −/− mice, baseline levels were recorded for at least 30 min followed by 120 min of recordings during microperfusion of the selected agents. Baseline (aCSF) mean data were calculated for the 30-min preceding the start of THIP infusion. THIP mean data were calculated between 30–60 min after the start of THIP infusion. The anatomical and functional criteria defined in rats were also used for the experiments in mice. We used the caudal part of the facial nucleus to identify the brain section 6.5 mm posterior to bregma as shown in the mouse Paxinos atlas23. All experiments with probe sites located within 1 mm were used to calculate mean data.
Implantations for chronic interventions
To identify the role of δGABAARs in regulating respiratory and motor activities in freely behaving wild-type and Gabrd −/− mice, we recorded electroencephalogram (EEG) and postural (neck) muscle electrodes to identify sleep-wake states, and diaphragm muscle electrodes for respiratory muscle recordings. One week prior to the behavioural experiments, sterile surgery was performed under isoflurane anaesthesia to implant the mice with the EEG, neck, and diaphragm muscle electrodes. To record diaphragm activity, two wires were sutured onto the costal diaphragm via an abdominal approach. The mouse was placed in the prone position in the stereotaxic apparatus (Model SAS-4100) with blunt ear bars, and three holes were drilled into the skull for the placement of the EEG electrodes. Two stainless steel screws (size 0–80 × 1/16, Plastic One Inc., Roanoake, VA, USA) were placed approximately 1 mm to the right and 1 mm anterior to bregma, and 1 mm to the left and 2 mm posterior to bregma for EEG activity; the third electrode used as a common reference was placed 2 mm to the left and 2 mm anterior to bregma. Insulated multi-stranded stainless steel wires were also sutured on the dorsal neck muscles to record the electromyogram. Post-surgical care consisted of a subcutaneous injection of an anti-inflammatory drug (ketoprofen, 2 mg/kg) and an analgesic (buprenorphine, 1 mg/kg).
Recordings in freely behaving mice
Mice recovered for one week prior to the experiments. On the first and second experimental days, mice were connected to the recording apparatus and placed in a large open-topped Plexiglas bowl filled with fresh bedding, food and water. Mice were left in the chamber for about 6 hours each day to habituate the animal to its environment. On the third day, experiments were performed to record electrophysiological signals while the mice were awake and asleep, and able to move freely. The Plexiglas bowl was placed on a rotating turntable (Raturn, BASi, West Lafayette, IN, United States) that automatically adjusts its position when the mouse physically moves; this response of the turntable avoids entanglements of the recording cable. The movement of the turntable were also recorded as a DC voltage. EEG, neck, and diaphragm muscle activities were recorded for a period of 3 hours from 10am to 1 pm. Data were amplified, filtered, moving-time averaged, sampled and analyzed as described previously19.
Identification of sleep-wake states
For every 10-second epoch, sleep-wake states were visually identified as active wakefulness (AW), quiet wakefulness (QW), non-rapid-eye-movement (NREM), and rapid-eye-movement (REM) sleep. Prevailing sleep/wake states were identified according to standard criteria25. EEG frequencies were also calculated in the following frequency bands: δ (1–4 Hz), θ (4–7.5 Hz), α (7.5–13.5 Hz), β1 (13.5–20 Hz), β2 (20–30 Hz). AW was characterized by low δ frequencies, high neck muscle activity and was often accompanied by body movements. QW was characterized by low δ frequencies, and low neck muscle activity without body movements. NREM sleep was characterized by high δ frequencies, high EEG amplitude and low neck muscle activity. REM sleep was characterized by low δ frequencies, high θ frequencies and low neck muscle activity.
Behavioural assessments and movements
To determine whether the animal was moving in its environment, we used a combination of video analysis, activation of the rotating table, and activation of postural neck muscle. Specifically, we separated periods of active wakefulness into two distinct periods for subsequent comparison with each other and with quiet wakefulness: (i) Periods of active wakefulness with body movements (i.e. MOV). These periods were recorded during the automatic adjustments of the turntable under the recording chamber as the mouse physically moved, and were verified by video recordings. (ii) Active wakefulness without such movements (i.e. NO-MOV). Such periods of NO-MOV occurred during specific behaviors while stationary, such as sniffing or grooming, and were also identified from the video recordings. (iii) Periods of quiet wakefulness as identified by low δ EEG frequencies, low neck muscle activity and no body movements.
Diaphragm and neck muscle measurements
Breathing rate was defined as the number of diaphragmatic breaths per minute. Diaphragm amplitude was defined as the amplitude of integrated diaphragm of a breath by subtracting the lower diaphragm signal to the peak diaphragm signal. To determine synchronization of postural neck muscle and diaphragm muscle activities, we computed the cross-correlation function using MATLAB Signal Processing Toolbox (function xcorr MATLAB, R12, Mathworks). This function is a measure of similarity of two waveforms. A value of 1 means that the two signals are identical, whereas a value of 0 means that they are completely different. Cross-correlation function was calculated for each 10 s epoch and associated with a specific state. Breathing rates, diaphragm amplitude and neck muscle activities were also measured for each 10-second epoch.
Statistical analysis
In the figures, group data are illustrated as the mean ± standard error of the mean (S.E.M.). Before analysis of variance tests are performed, all data are first tested for normality with the Shapiro-Wilk test and for equality of variances with the Brown-Forsythe test. For the studies in rats, we used 1-way ANOVAs with the repeated factor being the treatment with aCSF or drug of choice. For the studies in mice, we tested for group differences using 2-way ANOVAs, with one factor being genotype (i.e. wild-type or Gabrd −/−) and the second repeated factor being drug treatment (i.e. aCSF or drug of choice). If the ANOVA was statistically significant, an all pairwise multiple comparison procedure (Holm-Sidak tests) was then used to determine significant differences between conditions (e.g., aCSF versus drug). In studies with small groups of animals (n = 6), the non-parametric Mann Whitney rank sum test was used to test significance. All statistical tests were two-tailed with the level of significance set at P < 0.05. All tests were performed with SigmaPlot version 11 (Systat Software Inc, San Jose, CA, USA).
Results
Activation of δGABAARs at the preBötC reduces rhythmic respiratory activity
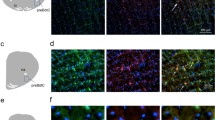
Tonic inhibition mediated by extrasynaptic GABAA receptors regulates the magnitude and frequency of oscillations in neuronal networks by adjusting the excitability of the network13. Here we tested this concept in the respiratory network and determined the contribution of δGABAARs to rhythmic breathing. To activate δGABAARs, we first applied the δGABAAR-preferring agonist THIP to the preBötC of anesthetized rats while simultaneously recording diaphragm and tongue muscle activities. Microperfusion of THIP (20 µM) into the preBötC (Fig. 1a–c), at a concentration known to activate δGABAARs26, decreased breathing rate and tongue muscle activity, but did not affect diaphragm amplitude (Fig. 1b,d). The GABAA receptor antagonist bicuculline partially reversed the reduction induced by THIP (Fig. 1b,d). Only experiments where the microdialysis probe was located within 2 mm from the center of the preBötC were analysed. According to this criteria, 3 out of 12 animals were excluded. Group mean data from 9 animals show that THIP significantly decreased breathing rate by 23.2 ± 2.1% (n = 9, P < 0.001, Fig. 1e) but not diaphragm amplitude (P = 0.122, Fig. 1f). Tongue muscle activity was not significantly changed (P = 0.210, Fig. 1g).
Activation of δGABAARs with the δGABAAR-preferring agonist THIP slows rhythmic breathing. In the anesthetized rats, THIP was microperfused in the preBötzinger Complex while diaphragm and tongue muscles were recorded (a). THIP (20 µM) substantially decreased breathing rate (b,d), an effect that was partially reversed by addition of the GABAA receptor antagonist bicuculline (100 µM). Microperfusion was done in the preBötzinger Complex region (c). Mean data indicate that THIP significantly reduced breathing rate (e) but not diaphragm muscle (f) and tongue muscle activities (g). Data are indicated as mean ± SEM. *Indicates mean data significantly different from aCSF condition with P < 0.05). Dia, diaphragm muscle. aCSF, artificial cerebro-spinal fluid. THIP, 4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride. BIC, bicuculline.
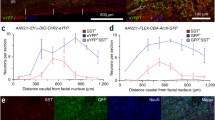
To identify whether the preBötC is sensitive to the δGABAAR-preferring agonist THIP, we determined the relationship between the proximity of the perfusion sites (Fig. 2a) to the preBötC and the latency at which THIP reduced breathing rate19 (Fig. 2b). The reasoning is that when THIP is microperfused close to the preBötC, then the latency for the agent to decrease breathing rate will be short, whereas when the microperfusion is further away from the preBötC the latency will be longer. We calculated the correlations of the distances (Fig. 2a) from perfusion sites (Fig. 2c) to the center of the preBötC with: (i) the latencies for THIP to decrease breathing rate by 10%, and (ii) the magnitudes of the reductions in breathing rate (Fig. 2b). We identified significant correlations between the distances and (i) the latencies of the breathing rate reductions (R = 0.705, P = 0.010, n = 12, Fig. 2d) as well as (ii) the magnitudes of the reductions in breathing rate (R = 0.746, P = 0.005, Fig. 2e). These results show that when THIP is microperfused close to the preBötC it quickly and markedly reduced breathing rate, with the responses being slower and of lesser magnitude with interventions performed further away from the preBötC.
WCMicroperfusions of THIP in the medulla were performed and the location of the perfusion sites were determined by histology (a). The distance from the perfusion sites to the center of the preBötzinger was calculated for each animal. Using diaphragm recordings, the duration from the start of microperfusion to a 10% decrease of breathing rate was calculated. Red indicates the latency to diffuse through the tissue and decreased breathing rate by 10%. Blue indicates percentage of baseline rate due to THIP after 30 min of drug perfusion (b). Diagrams showing brainstem sections where the microdialysis membranes (indicated by purple bars) were positioned for each experiment (c). Positive and significant correlation between latencies and distances from perfusion sites to the preBötC (c, n = 12) and between percentages of baseline breathing rate and distances from perfusion sites to the center of the preBötC (d, n = 12). Dia, diaphragm muscle. THIP, 4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride.
δGABAARs inhibit rhythmic breathing in wild-type but not Gabrd −/− mice
Although THIP is δGABAAR-preferring agonist27, we further verified that THIP selectively targets δGABAARs by using Gabrd −/− mice that lack these receptors. We microperfused THIP into the preBötC region of anesthetized wild-type and Gabrd −/− mice (Fig. 3a). Example (Fig. 3b) and group data (Fig. 3c) show that microperfusion of THIP (20 µM) into the preBötC region decreased breathing rate in the wild-type but not Gabrd −/− mice. Two-way repeated measures ANOVA showed that THIP significantly reduced breathing rate, but that the effect depended on genotype (P = 0.032, n = 8 per group). Post-hoc analyses identified a decrease in breathing rate of 23.2 ± 2.6% in the wild-type mice (P < 0.001, Fig. 3c), but no changes in Gabrd −/− mice (P = 0.155, Fig. 3c). Responses were specific to breathing rate as there were no THIP-induced changes in diaphragm amplitude in the wild-type or Gabrd −/− mice (P = 0.130, Fig. 3d). To determine whether the preBötC region was sensitive to other manipulations not involving δGABAARs, i.e., that the absence of effects in the Gabrd −/− mice was not due to unresponsive neurons or incorrectly positioned probes, we perfused the µ-opioid receptor agonist DAMGO (10 µM) into the preBötC region19 of Gabrd −/− mice. DAMGO activates µ-opioid receptors and decreased breathing rate by 41.9 ± 3.6% (P = 0.016, ANOVA on ranks, Fig. 3e), but did not change diaphragm amplitude (P = 0.486, Fig. 3f), therefore showing that microperfusion of DAMGO was performed in the responsive preBötC region and had the capacity to inhibit breathing rate by activating µ-opioid receptors as previously demonstrated22.
THIP decreases breathing rate by acting on δGABAAR. In anesthetized mice, THIP was microperfused in the preBötzinger Complex while diaphragm muscle was recorded (a). THIP (20 µM) substantially decreased breathing rate. In Gabrd −/− mice, THIP did not affect breathing rate (c). Post-mortem histology showed that microperfusion was performed in the vicinity of the preBötC in both wild-type and Gabrd −/− (b) mice. Comparison of wild-type and Gabrd −/− (c) showed that THIP significantly reduced breathing rate in wild-type, but not in Gabrd −/− mice. THIP had no effect on diaphragm muscle amplitude both in wild-type and Gabrd −/− mice (d). As positive control, the µ-opioid receptor agonist DAMGO (10 µM) was microperfused in Gabrd −/− mice to determine whether the probe was correctly positioned. DAMGO consistently decreased breathing rate (e) to the level found with THIP in wild-type mice, but did not change diaphragm amplitude (f). Data are indicated as mean ± SEM. *Indicates mean data significantly different from corresponding aCSF condition with P < 0.05. Dia, diaphragm muscle. aCSF, artificial cerebro-spinal fluid. THIP, 4,5,6,7- Tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride.
Activation of δGABAARs following systemic injection of THIP decreases rhythmic breathing
To determine whether general activation of δGABAARs would also affect respiratory activity, we systemically administered THIP in anesthetized mice while recording diaphragm muscle activity (Fig. 4a,b). Example (Fig. 4b) and group data (Fig. 4c) identified that the systemic injection of THIP (8 mg/kg) decreased breathing rate in the wild-type but not Gabrd −/− mice. Two-way repeated measures ANOVA showed that THIP significantly reduced breathing rate, but that the effect depended on genotype (P = 0.037, n = 6 per group). Post-hoc analyses identified a decrease in breathing rate of 19.9 ± 2.7% in the wild-type mice (P = 0.015, Fig. 4c). In Gabrd −/− mice, however, THIP did not change breathing rate (P = 0.627, Fig. 4c). Due to the low number of experiments (n = 6 per group), we performed additional Mann-Whitney Rank Sum non-parametric tests and showed that breathing rate was decreased by THIP in wild-type (P = 0.041), but not in Gabrd −/− mice (P = 0.485). The data also showed that there was no effect of genotype on the response of diaphragm amplitude to systemic injection of THIP (both P > 0.688, two-way repeated measures ANOVAs, Fig. 4d). A Mann-Whitney test also showed no significant differences between the two groups (P = 0.394). These results further support the idea that THIP reduces rhythmic breathing without affecting motor amplitudes by specifically activating δGABAARs.
Systemic activation of δGABAAR decreases rhythmic breathing in anesthetized wild-type, but not in Gabrd −/− mice. Diaphragm muscle activity was recorded during intramuscular injection of THIP in wild-type (n = 6) and Gabrd −/− (n = 6) mice (a). THIP (8 mg/kg) significantly decreased breathing rate in wild-type but not in Gabrd −/− mice (b,c). THIP did not change diaphragm muscle amplitude (d). Dia, diaphragm muscle activity. *Indicates mean significantly different from the corresponding baseline mean with P < 0.05. Data are indicated as mean ± SEM.
State-dependent regulation of respiratory network activity by δGABAARs
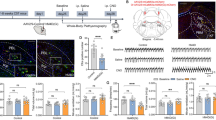
Under anesthesia, the frequency of diaphragm muscle activations corresponds to the frequency of breathing, because rhythmic breathing is autonomously generated by the respiratory network and is not influenced by non-respiratory or behavioural activations28,29. To determine whether the frequency of diaphragm muscle activations is influenced by δGABAARs in freely-behaving animals, we measured respiratory muscle activities in non-anesthetized wild-type and Gabrd −/− mice (Fig. 5a,b). We first found that the effect of genotype on the frequency of diaphragm muscle activations depended upon the prevailing sleep-wake state (P = 0.039). During active wakefulness, Gabrd −/− mice exhibited an increased frequency of diaphragm muscle activations compared to wild-type mice (P = 0.005, Fig. 5c,d), but no differences were observed in quiet wakefulness, NREM and REM sleep (P > 0.235, Fig. 5d). The averaged amplitude of diaphragm activity was not statistically different between the wild-type and Gabrd −/− mice (P = 0.587) and there was no effect of genotype that depended upon sleep-wake state (P = 0.639, Fig. 5d). These data suggest that a δGABAAR mechanism controls the rhythmic components of respiratory network activity rather than the magnitude of the oscillatory motor output.
δGABAAR regulate diaphragm muscle activity during active wakefulness but not in quiet wakefulness and sleep. In freely behaving adult mice, diaphragm rhythmic activity (numbers of activations per minute) was increased in Gabrd −/− compared to wild-type mice (a–c) in active wakefulness (AW), but not in quiet wakefulness (QW), non-rapid eye movement (NREM) sleep or rapid eye movement (REM) sleep. There was no differences in diaphragm amplitude between wild-type and Gabrd −/− mice at each states (d). Data are indicated as mean ± SEM. *Indicates mean data significantly different with P < 0.05. Dia, diaphragm muscle. Neck, neck muscle. EEG, electroencephalography.
We then determined whether the lack of δGABAARs in Gabrd −/− mice simply increases overall activity, i.e. the animal is more active. We found that Gabrd −/− mice exhibited similar amounts of time spent in each sleep-wake state compared to wild-type mice (P = 0.384, n = 7 per group, Fig. 6a-d). Gabrd −/− mice also exhibited a similar overall EEG frequency spectrum (Fig. 6e) compared to wild-type mice. There was no main effect of genotype on EEG frequencies in the δ, θ, and β2 frequency bands (each P > 0.156, n = 7 per group, Fig. 6f), whereas the effect in the β1 band was at the threshold for significance (P = 0.050), and α activity was significantly lower in the Gabrd −/− mice (P = 0.038). There was no effect of genotype on EEG activity in any frequency band that depended upon the prevailing sleep-wake state (all P > 0.440). Overall, these data show that, in the Gabrd −/− mice, the increased frequency of diaphragm muscle activations in active wakefulness (Fig. 5c) was due to effects of altered δGABAAR-mediated inhibition during active behaviors.
Sleep architecture and cortical changes in Gabrd −/− compared to wild-type mice. Spectrogram, electroencephalography activity in wild-type (a) and Gabrd −/− mice (b). Gabrd −/− mice (c) showed the same amount of time spent at each sleep-wake state than Gabrd −/− mice (d). Electrocortical band powers (e) were quantified for wild-type and Gabrd −/− mice for each sleep-wake state. Gabrd −/− mice showed similar power bands than wild-type mice (f). Data are presented as mean ± S.E.M. AW, active wakefulness, QW, quiet wakefulness, NREM, non-rapid-eye movement sleep. REM, rapid-eye movement sleep. EEG, electroencephalography.
Behaviour-dependent modulation of respiratory network activity by δGABAARs
The above data showed that reduced δGABAAR-mediated tonic inhibition led to increased diaphragmatic rhythmic activity (Fig. 5a). We then performed additional analyses of these signals in wakefulness to further stratify the effects by behaviors (Fig. 7a,b). The effect of genotype on the frequency of diaphragm muscle activations depended upon the type of behavior exhibited during wakefulness (P = 0.003, n = 7 in each group, 2-way repeated measures ANOVA). Post-hoc analysis identified that during MOV, the frequency of diaphragm activations (i.e., the combination of behavioral and respiratory activations) was significantly higher in the Gabrd −/− mice compared to the wild-type mice (P = 0.006, Fig. 7c). This effect was specific to MOV because this difference between the Gabrd −/− mice and the wild-types did not occur during NO-MOV (P = 0.801) or quiet wakefulness (P = 0.147). In contrast, there was no effect of genotype on diaphragm amplitude that depended upon the type of behavior exhibited during wakefulness (P = 106). We also observed that neck muscle activity was lower in the Gabrd −/− mice compared to wild-type mice. These additional analyses, however, identified that this genotype effect on neck muscle activity was confined to periods of MOV (P = 0.001, Fig. 7e ), and not NO-MOV or quiet wakefulness (both P > 0.147). These data further suggest that a δGABAAR-mediated mechanism modulates the rhythmic components of respiratory network activity rather than the magnitude of the oscillatory motor output.
Synchronization of diaphragm and neck muscle activities during active behaviours in the absence of δGABAAR. In wild-type mice during movements and active wakefulness, diaphragm and neck muscle were not synchronized (a), whereas they were highly synchronized in Gabrd −/− mice (b). Diaphragm muscle activity was significantly higher in Gabrd −/− than in wild-type mice during movements (MOV) but not during active wakefulness without movements (NO-MOV) and quiet wakefulness (n = 7, c). Diaphragm amplitude did not differ between wild-type and Gabrd −/− mice (d). Cross-correlation indexes between diaphragm and neck muscle activities were significantly higher in Gabrd −/− during movements and active wakefulness without movements (e). Neck muscle amplitude was higher in wild-type than in Gabrd −/− mice during movements (f) *indicate values significantly different between wild-type and Gabrd −/− mice with P < 0.05. MOV, active wakefulness with movements. NO-MOV, active wakefulness without movements. QW, quiet wakefulness. Dia, diaphragm muscle activity. Neck, neck muscle activity. Dia x Neck, cross-correlation between diaphragm and neck muscle activities. Gabrd −/−, δ-subunit knockout mice.
δGABAARs and respiratory activity during active wakefulness associated with movements
To identify whether the increased in diaphragm activation observed above is due to behavioural control of respiratory circuits, we tested the degree of coupling between diaphragm muscle and postural motor activities, two motor systems controlled by different neural circuits, across the behavioural states, and tested for an influence of δGABAAR-mediated modulation of respiratory network activity. Accordingly, using cross correlation, we quantified the degree to which the diaphragm and postural (neck) muscles are synchronized across MOV, NO-MOV and quiet wakefulness in the wild-type and Gabrd −/− mice. In the Gabrd −/− mice, the diaphragm and neck muscle activities showed distinct activations with multiple periods of coordination and synchronization during periods of MOV compared to the wild-type mice, (Fig. 7a,b). The group data confirmed that the effect of genotype on the synchronization of diaphragm and neck muscle activations depended upon the type of behavior exhibited during wakefulness (P = 0.006, n = 7 in each group, 2-way repeated measures ANOVA). Post-hoc analyses showed that diaphragm and neck muscle activities were highly synchronized during MOV in the Gabrd −/− mice but were not in the wild-type mice (P = 0.006, Fig. 7e). In NO-MOV, the diaphragm and neck muscle activations were also synchronized in Gabrd −/− but less so in wild-type mice (P = 0.029, Fig. 7f). Together, these results indicate that the absence of δGABAAR-mediated tonic inhibition leads to more synchronization between diaphragm and postural (neck) motor activities during periods of active wakefulness associated with movements.
Discussion
Post-synaptic inhibition, such as GABAA receptor-mediated inhibition, is important to modulate rhythm in many neural circuits. Synaptic GABAA receptors are implicated in respiratory control and are present throughout the respiratory network30, but the role of extra-synaptic GABAA receptors is unknown. Here, we studied whether extrasynaptic δGABAARs provide a level of tonic inhibition in the respiratory network to modulate diaphragm rhythmic activity and to dampen rhythmicity during active motor behaviours. Using a combination of pharmacological and genetic approaches, we found that δGABAARs in the preBötC region function to decrease respiratory network activity. We then identified that the absence of δGABAARs in the Gabrd −/− mice increases respiratory network activity and motor activities during wakefulness when compared to wild-type mice. In Gabrd −/− mice, diaphragm activity is also synchronized to locomotor activity, an effect less pronounced in wild-type mice. The presence of an abnormal number of behavioral components in diaphragm muscle activity observed in Gabrd −/− mice suggests that δGABAARs may dampen the influence of non-respiratory behaviors on respiratory network activity to maintain breathing.
GABA and the respiratory network
In the ventrolateral medulla of adult rats, glutamic acid decarboxylase immunoreactivity, a marker of GABAergic cells, has been identified in post-inspiratory and inspiratory neurons (Okazaki et al., 2001). In the preBötzinger Complex, GABAA receptors are present in inspiratory neurons and non-respiratory neurons7, suggesting that GABA in the preBötC may modulate inspiratory activity but also non-respiratory activity. GABAA receptor stimulation at the preBötC can depress breathing whereas antagonism has little or no effect9,10, suggestive of a GABAA receptor-mediated inhibition that shapes respiratory motor outputs rather than generate respiratory rhythm per se 11. Although GABAA receptors mainly exert their effects by inducing fast post-synaptic hyperpolarization, GABA also activates extra-synaptic GABAA receptors located outside the synapse and generates tonic inhibition to reduce network excitability13. Our data indicate that the δGABAAR-preferring agonist THIP perfused in the ventrolateral medulla decreased breathing rate, at least in great part, by acting at the preBötC level. To confirm the site of action of THIP and to demonstrate that THIP modulates neurons in the vicinity of the preBötC, we showed that when THIP was perfused close to the preBötC, it induced a faster and more pronounced decrease in breathing rate than when it is perfused away from it. These relationships were supported by correlations between the latencies or magnitudes of THIP effect and distances from perfusion site to the preBötC as previously described and validated19,20,24. Although THIP is a δGABAAR-preferring agonist27, we further verified that THIP selectively targets δGABAARs by using Gabrd −/− mice that lack these receptors. The absence of response to THIP in Gabrd −/− mice further identified that selective activation of δGABAARs underlie inhibition of respiratory network activity by THIP. The combination of these two approaches support the idea that δGABAARs in the region of the preBötC have the capacity to modulate respiratory network activity.
Respiratory network excitability
The respiratory network, and at its core the preBötC, form a dynamic network endogenously generating rhythmic motor activity. To spontaneously generate respiratory motor rhythm, the network needs a sufficient level of excitability31. In vitro, when the respiratory network is isolated and network excitability is reduced, potassium concentration is typically raised to increase excitability and so generate rhythmic activity. In addition, respiratory network excitability is modulated by G-protein-coupled receptors such as µ-opioid receptors, where their activation at the preBötC inhibits breathing rate19, an effect not observed in mice lacking functional G-protein-inwardly rectifying potassium (GIRK) channels22. Inhibition through GIRK channels likely involves a network level modulation32. Such large scale effect of neurotransmitters is known as volume transmission and δGABAARs reduce network excitability through this mechanism33. Volume transmission, as opposed to wiring transmission, modulate network excitability via neurotransmitters diffusing into the extracellular space and then by activating extra-synaptic receptors14. Although this type of neurotransmission is slower than wiring transmission, it is sufficiently fast to modulate the respiratory network and ongoing motor systems27. Volume transmission and GABAergic inhibition through δGABAARs may therefore be a potential mechanism modulating network excitability. Here, we identify, for the first time, the functional role of δGABAARs, which are uniquely expressed in the extrasynaptic space, in the preBötC region and the capacity of these receptors to modulate respiratory network activity during active behavior.
Respiratory network behaviour and extra-synaptic GABAA receptors
In states of reduced arousal, i.e. anesthesia, when behaviour is suppressed and the respiratory network is autonomous, activation of δGABAARs at the preBötC significantly reduced respiratory network activity. Although these data demonstrate that δGABAARs in the preBötC region have the capacity to modulate respiratory network activity, the role of δGABAARs in response to endogenous GABA release cannot be assessed due to the lack of a selective δGABAAR antagonist. To identify the endogenous role of δGABAARs in modulating respiratory network activity, we compared diaphragm muscle activity (a surrogate of respiratory network activity) between wild-type and Gabrd −/− mice at various states of arousal and during behaviours. Although δGABAARs did not play a significant role in modulating respiratory network activity during sleep and quiet wakefulness, rhythmic activity of the diaphragm muscle was higher in Gabrd −/− than in wild-type mice in active wakefulness, especially during movements. Gabrd −/− mice also showed synchronized diaphragm and neck muscle activities during movements, pointing out that, without δGABAARs, non-respiratory behaviours greatly influenced respiratory network activity. Although the mechanisms underlying the role of δGABAARs in inhibiting respiratory network activity only during movements have not been yet identified, our data suggest that δGABAARs play an endogenous role in the modulation of respiratory network activity, especially during active non-respiratory behaviors.
Regulation of network excitability by tonic inhibition
Extra-synaptic δGABAARs play a significant role in modulating respiratory activity during conscious behaviors in vivo when the levels of excitation independent of breathing per se is high28,29. Excitatory inputs from descending motor pathways may be inhibited by δGABAARs to maintain the essential autonomic function of breathing while non-respiratory behaviours are performed. The notion of tonic inhibition regulating network excitability has been demonstrated in thalamo-cortical networks26, in the hippocampus34, and in the cerebellum35. Our findings identify that δGABAARs modulate rhythmic breathing, and dampen behavioral influences on rhythmic motor activity during active behaviors in vivo. These results identify a previously unrecognized role of δGABAARs in modulating the endogenously active respiratory network in vivo, the persistence and stability of which is critical to homeostasis. Identification of the role of extrasynaptic GABAA receptors provides a better understanding of how breathing is affected by changes in extrasynaptic GABAA receptor expression and function under pathological conditions such inflammation36 and exposure to anesthetics and neurosteroids13,18.
References
Feldman, J. L. & Kam, K. Facing the challenge of mammalian neural microcircuits: taking a few breaths may help. J Physiol 593, 3–23 (2015).
Smith, J. C., Ellenberger, H. H., Ballanyi, K., Richter, D. W. & Feldman, J. L. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729 (1991).
Gray, P. A., Janczewski, W. A., Mellen, N., McCrimmon, D. R. & Feldman, J. L. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat.Neurosci. 4, 927–930 (2001).
Tan, W. et al. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat.Neurosci. 11, 538–540 (2008).
Marchenko, V. et al. Perturbations of Respiratory Rhythm and Pattern by Disrupting Synaptic Inhibition within Pre-Botzinger and Botzinger Complexes. eNeuro 3 (2016).
Feldman, J. L., Del Negro, C. A. & Gray, P. A. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75, 423–452 (2013).
Kuwana, S. et al. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Botzinger complex. Eur J Neurosci 23, 667–674 (2006).
Sherman, D., Worrell, J. W., Cui, Y. & Feldman, J. L. Optogenetic perturbation of preBotzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci 18, 408–414 (2015).
Koizumi, H. et al. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Botzinger complex respiratory microcircuits. J Neurosci 33, 2994–3009 (2013).
Ritter, B. & Zhang, W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci 12, 2975–2984 (2000).
Janczewski, W. A., Tashima, A., Hsu, P., Cui, Y. & Feldman, J. L. Role of inhibition in respiratory pattern generation. J Neurosci 33, 5454–5465 (2013).
Bongianni, F., Mutolo, D., Cinelli, E. & Pantaleo, T. Respiratory responses induced by blockades of GABA and glycine receptors within the Botzinger complex and the pre-Botzinger complex of the rabbit. Brain Res 1344, 134–147 (2010).
Walker, M. C. & Semyanov, A. Regulation of excitability by extrasynaptic GABA(A) receptors. Results and problems in cell differentiation 44, 29–48 (2008).
Fuxe, K. et al. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Frontiers in physiology 3, 136 (2012).
Vida, I., Bartos, M. & Jonas, P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49, 107–117 (2006).
Lee, V. & Maguire, J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Frontiers in neural circuits 8, 3 (2014).
Nusser, Z., Sieghart, W. & Somogyi, P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18, 1693–1703 (1998).
Mihalek, R. M. et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA 96, 12905–12910 (1999).
Montandon, G. et al. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci 31, 1292–1301 (2011).
Montandon, G. & Horner, R. L. State-dependent contribution of the hyperpolarization-activated Na+/K+ and persistent Na+ currents to respiratory rhythmogenesis in vivo. J Neurosci 33, 8716–8728 (2013).
Paxinos, G. & Watson, C. Paxinos and Watson’s The rat Brain in Stereotaxic Coordinates. Vol. Ed. 7 (Elevier Academic Press, 2014).
Montandon, G. et al. G-protein-gated Inwardly Rectifying Potassium Channels Modulate Respiratory Depression by Opioids. Anesthesiology 124, 641–650 (2016).
Paxinos, G. F., K. The Mouse Brain in Stereotaxic Coordinates. 4th edn, (Academic Press, 2012).
Montandon, G., Liu, H. & Horner, R. L. Contribution of the respiratory network to rhythm and motor output revealed by modulation of GIRK channels, somatostatin and neurokinin-1 receptors. Scientific reports 6, 32707 (2016).
Mesbah-Oskui, L. & Horner, R. L. Enhanced Thalamic Spillover Inhibition during Non-rapid-eye-movement Sleep Triggers an Electrocortical Signature of Anesthetic Hypnosis. Anesthesiology, (2016).
Mesbah-Oskui, L., Orser, B. A. & Horner, R. L. Thalamic delta-subunit containing GABAA receptors promote electrocortical signatures of deep non-REM sleep but do not mediate the effects of etomidate at the thalamus in vivo. J Neurosci 34, 12253–12266 (2014).
Mortensen, M., Ebert, B., Wafford, K. & Smart, T. G. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol 588, 1251–1268 (2010).
Orem, J. Respiratory neurons and sleep. Principles and practice of sleep medicine. Edited by Kryger, M. H., Roth, T., and Dement, W. C. Saunders, Philadelphia, 177–193, (1994).
Phillipson, E. A. & Bowes, G. In Handbook of Physiology - The respiratory system II 649–989 (1986).
McCrimmon, D., Dekin, M. & Mitchell, G. Glutamate, GABA, and serotonin in ventilatory control. Lung biology in health and disease 79, 151–218 (1995).
Funk, G. D. & Greer, J. J. The rhythmic, transverse medullary slice preparation in respiratory neurobiology: contributions and caveats. Respir Physiol Neurobiol 186, 236–253 (2013).
Luscher, C. & Slesinger, P. A. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11, 301–315 (2010).
Agnati, L. F., Guidolin, D., Guescini, M., Genedani, S. & Fuxe, K. Understanding wiring and volume transmission. Brain research reviews 64, 137–159 (2010).
Rombo, D. M., Ribeiro, J. A. & Sebastiao, A. M. Hippocampal GABAergic transmission: a new target for adenosine control of excitability. J Neurochem, (2016).
Duguid, I., Branco, T., London, M., Chadderton, P. & Hausser, M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci 32, 11132–11143 (2012).
Avramescu, S. et al. Inflammation Increases Neuronal Sensitivity to General Anesthetics. Anesthesiology 124, 417–427 (2016).
Acknowledgements
This work was supported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563 to R.L.H.) and the National Sanitarium Association Innovative Research Program (awarded to R.L.H.). G.M. was supported by a Parker B. Francis Fellowship. H.W. was supported by joint funding from the Department of Science and Technology, Yunnan Province, China, and Kunming Medical School, Yunnan Province, China. R.L.H. is supported by a Tier 1 Canada Research Chair in Sleep and Respiratory Neurobiology. M.T.V was supported by Sleep and Biological Rhythms Toronto, a Canadian Institutes of Health Research-funded research and training program at the University of Toronto (to R.L.H.), as well as a Queen Elizabeth II Graduate Scholarship in Science and Technology.
Author information
Authors and Affiliations
Contributions
G.M., B.A.O., and R.L.H. wrote and reviewed the manuscript. G.M., H.L., H.W., and M.T.W. performed experiments, collected, and analyzed data. G.M. prepared all figures.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montandon, G., Wu, H., Liu, H. et al. δ-Subunit Containing GABAA Receptors Modulate Respiratory Networks. Sci Rep 7, 18105 (2017). https://doi.org/10.1038/s41598-017-17379-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17379-x
- Springer Nature Limited
This article is cited by
-
Measurement and State-Dependent Modulation of Hypoglossal Motor Excitability and Responsivity In-Vivo
Scientific Reports (2020)