Abstract
Two Solanaceae invasive plant species (Physalis angulata L. and P. philadelphica Lam. var. immaculata Waterfall) infest several arable crops and natural habitats in Southeastern Anatolia region, Turkey. However, almost no information is available regarding germination biology of both species. We performed several experiments to infer the effects of environmental factors on seed germination and seedling emergence of different populations of both species collected from various locations with different elevations and habitat characteristics. Seed dormancy level of all populations was decreased with increasing age of the seeds. Seed dormancy of freshly harvested and aged seeds of all populations was effectively released by running tap water. Germination was slightly affected by photoperiods, which suggests that seeds are slightly photoblastic. All seeds germinated under wide range of temperature (15–40 °C), pH (4–10), osmotic potential (0 to −1.2 MPa) and salinity (0–400 mM sodium chloride) levels. The germination ability of both plant species under wide range of environmental conditions suggests further invasion potential towards non-infested areas in the country. Increasing seed burial depth significantly reduced the seedling emergence, and seeds buried below 4 cm of soil surface were unable to emerge. In arable lands, soil inversion to maximum depth of emergence (i.e., 6 cm) followed by conservational tillage could be utilized as a viable management option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Two invasive plant species of Solanaceae family, i.e., Physalis angulata L. (cutleaf groundcherry) and P. philadelphica Lam. var. immaculata Waterfall (Mexican groundcherry) are distributed in several parts of the world. Although both plant species are used as food crops and have many medicinal benefits, these have also been reported as noxious/invasive weeds of cotton, corn and soybean in several parts of the world1,2,3. P. angulata was firstly reported in Turkey during 20004, whereas first record of P. philadelphica dates back to 20025. Both species are listed as “invasive” in Turkey6,7, co-occur in the distribution range and infest different field crops including cotton, maize, cucumber, tomato, pepper and olive orchards in Southeastern Anatolia (SEA) region of the country8. The SEA region have an arid to semi-arid climate with hot summers9,10. The evaporative demands of crops are met by irrigation in the region. Inadequate infrastructure of drainage11 and excessive use of irrigation water coupled with high evaporation have raised soil salinity in the region11. Farooq et al.8 indicated irrigation as primary, and soil pH, and electrical conductivity as secondary invasion drivers of both species in the region.
P. angulata and P. philadelphica produce enormous amounts of seeds even under adverse environmental conditions10, which are readily deposited to soil seed bank12,13. Management of these plant species, due to their annual nature and enormous seed production capability is difficult without the use of herbicides12,14. However, recorded lower control of some herbicides1 indicates the evolution of herbicide resistance. Thus, adoption of alternative sustainable management practices is necessary. Germination biology is important to develop management strategies against weeds and invasive plant species15. Germination under wide range of environmental conditions increases the chances of successful invasion16,17,18. The knowledge of germination biology could facilitate management of invasive plant species either by suppressing or stimulating the germination when seedlings can be managed successfully19. The differences in germination and emergence patterns of different populations are also of great significance for inferring the further range expansion at regional scales18.
Variations in seed traits among and within populations are among the key factors responsible for establishment and persistence of invasive plant species20,21,22. Seed traits are governed by many factors including genetic variation and environmental conditions prevailing during seed development22,23. These factors collectively determine the persistence (dormancy), germination and dispersal or mortality23,24,25,26 of seeds produced by a plant species. The seeds of different individuals within a population of the same species show variations in seed germination biology, including dormancy and receptiveness of dormancy-breaking factors27. Moreover, seeds collected from different sites, years, altitude gradients and habitats also exhibit differences in seed germination biology due to inherent environmental conditions23,28,29,30.
Seed germination in natural and cultivated habitats is significantly influenced by several environmental factors such as temperature, light, moisture, pH, salinity and seed burial depth19,24,31,32. Temperature influences the germination by regulating the enzyme activities and promote/inhibit the hormone synthesis that promote dormancy/germination33. Light requirement of weeds/invasive plant species determine their emergence patterns with reference to seed burial depth24,33. For example, seeds requiring light for germination will not emerge when buried deep in the soil24. Besides, seeds present in the soil seed bank at various depths exhibit different germination response with respect to variation in moisture, temperature, light, EC and pH.
Both Physalis species are widely distributed in arid and semi-arid regions of Turkey and started to incur losses in crop production1,8, however no information is available on their germination biology. Both plant species are naturally found in tropical parts of the world34 and a little information is present for germination requirements of P. angulata 13. However, the species in Turkey are observed in various habitats of different climatic zones (especially arid and semi-arid regions) with different elevation gradients6,7. Germination biology of these species could give valuable insights on their possible adaptations strategies for persisting in arid and semi-arid regions, could facilitate to assess their further spread potential and help in devising successful management strategies.
The current study was conducted to infer the dormancy patterns, effects of temperature, light, water stress, pH and salt stress on germination, and effect of seed burial depth on seedling emergence of different populations of P. angulata and P. philadelphica. The germination behavior will give theoretical and practical information about their possible adaptation strategies in arid and semi-arid region, range expansion potential and management. It was hypothesized that; i) populations arising from higher altitude will show variations in dormancy and optimum light and temperature requirements for germination compared to lower altitudes, ii) both co-occurring plant species will probably show similar germination behavior under similar environmental conditions, iii) increasing pH, water stress and salt stress will decrease germination and iv) increasing seed burial depth will decrease seedling emergence.
Results
Seed age significantly affected the seed dormancy level of different populations of both plant species (Table S1). Freshly harvested seeds of all populations of both plant species were highly dormant. Seed dormancy was released with increasing age of seeds as freshly harvested seeds had 85.22% seed dormancy, while it was reduced to 15.33% in 12 months old seeds (Fig. 1). The seed dormancy of fresh and aged seeds of both plant species was effectively released by running tap water. Seed germination was progressively increased by running tap water with increasing age of the seeds (Fig. 2a). The interactive effect of plant species and their populations on final germination percentage of seeds kept under running tap water was also significant (Table S2). Seeds collected from higher elevation gradient had slightly higher seed dormancy level compared to the seeds collected from lower elevation gradients (Fig. 2b).
Effect of seed age (A) and invasive plant species × populations’ interactions (B) on seed dormancy release (seeds kept under running tap water for 24 hours) of different populations of Physalis angulata and P. philadelphica arising from various elevation gradients and habitats. Any two means sharing different letters are significantly different from each other. The vertical bars represent standard errors of means.
Seed germination was significantly influenced by photoperiods, plant species × photoperiods and plant species × population’s × photoperiods’ interactions (Table S3). The seeds of both plant species incubated in 12 h light and 12 h dark period exhibited the highest germination, while continuous dark and continuous light treatments had lower final germination percentage for all populations of tested plant species (Fig. 3). Similarly, the seeds of populations collected from higher elevation gradient had lesser final germination percentage compared to those collected from lower elevations (Fig. 3).
Germination was significantly (p ≤ 0.01) affected by individual and interactive effects of plant species, populations and experimental treatments (i.e., different temperatures, pH, water stress and salt stress levels (Tables S4–S7). Similarly, seedling emergence was also significantly influenced by individual and interactive effects of plant species, populations and seed burial depths (Table S8). P. philadelphica seeds were able to germinate over wide range of temperature regimes (>50% germination under 15 to 40 °C) compared with P. angulata (>50% germination under 25–40 °C) (Fig. 4). P. angulata had higher temperature requirement (optimum temperature for germination) for maximum germination (33.03, 33.50 and 32.44 °C for populations collected from 755, 527 and 120 m altitude, respectively). Contrastingly, P. philadelphica required lower temperature for maximum germination (28.55, 28.76 and 27.91 °C for populations collected from 755, 527 and 120 m altitudes, respectively) (Fig. 4).
Effect of different constant temperature regimes on final germination percentage of different populations of two invasive Physalis species collected from varying elevation gradients and habitats. The lines represent three parametric Gaussian model fitted to the final germination data at 30 days after start of the experiment. The vertical bars represent standard errors of means.
Seeds of tested species were capable of germinating under a broad range of pH (5 to 10). However, highly acidic or alkaline pH considerably reduced seed germination (Fig. 5). Populations collected from higher altitudes required pH near to neutral for peak germination (6.98 and 7.50 for P. angulata and 7.16 and 7.29 for P. philadelphica populations collected from 755 and 527 m, respectively). Interestingly peak germination in lower altitude (120 m) populations was observed under slightly alkaline pH (7.93 and 7.98 for P. angulata and P. philadelphica, respectively) (Fig. 5).
Effect of different osmotic potentials (water stress) on final germination of different populations of two invasive Physalis species collected from different elevation gradients and habitats. The lines represent three parametric sigmoidal function fitted to model the final germination data at 30 days after start of the experiment. The vertical bars represent standard errors of means.
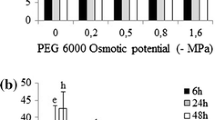
P. philadelphica seeds germinated under broad range of osmotic potentials (>50% germination under −0.59 to −1.03 MPa). However, germination of P. angulata seeds was reduced under higher (>50% germination under −0.51 to −0.82 MPa) osmotic potentials (Fig. 6). Populations arising from higher altitudes were lesser tolerant to water stress (osmotic potential required to inhibit 50% of maximum germination was low) compared to those collected from low altitude (Fig. 6). Similar to water stress, P. philadelphica seeds proved more tolerant to salinity (higher salinity levels were required to retard 50% of the maximum germination) compared with P. angulata (Fig. 7). Similarly, lower altitude populations were more tolerant to higher salinity levels compared with populations collected from higher altitudes (Fig. 7).
Effect of different NaCl concentrations (salt stress) on final germination percentage of different populations of two invasive Physalis species collected from different elevation gradients and habitats. The lines represent three parametric sigmoidal function fitted to model the final germination data at 30 days after start of experiment. The vertical bars represent standard errors of means.
Effect of varying pH levels on final germination percentage of different populations of two invasive Physalis species collected from different elevation gradients and habitats. The lines represent three parametric Gaussian model fitted to the final germination data at 30 days after start of experiment. The vertical bars represent standard errors of means.
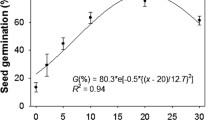
Seedling emergence was initially increased up to 2 cm (maximum seedling emergence was recorded at this depth) and sudden decline in seedling emergence was recorded for both plant species (Fig. 8). More than 80% of seedlings of tested species were unable to emerge when seeds were buried below 6 cm soil depth (Fig. 8).
Effect of seed burial depth on seedling emergence percentage of different populations of two invasive Physalis species collected from different elevation gradients and habitats. The lines represent three parametric Gaussian model fitted to the final germination data at 40 days after start of experiment. The vertical bars represent standard errors of means.
Discussion
Dormancy and germination are considered as important adaptive traits for successful establishment of invasive plant species. Higher dormancy helps to persist in soil seed bank for longer periods, whereas germination under wide range of environmental conditions enables the plant species to adapt diverse ecological conditions. Therefore, higher germination and seedling recruitment have been recognized among the major factors promoting naturalization success of invasive plant species18,23,35.
In the current study, freshly harvested seeds of different populations of P. angulata and P. philadelphica exhibited equal level of dormancy (unlike as hypothesized), although collected from different altitudinal gradients and habitats. Seed traits, such as dormancy are highly dependent on the environmental conditions faced by the maternal plants prior to seed filling and ripening22. The species tested in the current study are probably in their naturalization phase as these have been recently observed in the country4,5. Thus the tested species invest all resources on seedling recruitment as it promotes establishment and subsequent spread of invasive plant species18,35. Since both species have recently been reported in the country, long term climatic conditions have probably not influenced the dormancy traits6,7. Although several studies indicated that different populations of same species might show different levels of seed dormancy23,27, some contrasting reports also revealed that different populations of some species have equal levels of dormancy and seed germination36.
Seed dormancy level of all populations of both species was linearly decreased with increasing seed age. Running tap water effectively released dormancy of both freshly harvested and aged seeds of all populations of both species. The seeds probably exhibit dormancy due to toxic substances present on the seeds immediately after harvest and running tap water flushed these substances, thus released dormancy. Irrigation is frequently practiced for agricultural production in SEA region, therefore irrigation water is thought to be sufficient for releasing seed dormancy under natural conditions in the currently invaded areas.
Light is inherent requirement for germination of some weed species, while others do not require light at all for germination31,32,37,38. Seeds of different populations of both species proved slightly photoblastic. It has also been reported earlier that P. angulata have no strict light requirements for seed germination13. The slightly photoblastic nature of the seeds indicates that seedlings could emerge from different seed burial depths which explains the distribution of both species in diverse habitats in the country6,7,8.
Temperature influences germination by regulating enzyme activities and promotes/inhibits the hormone synthesis required to promote dormancy/germination33,39. Different populations of the tested Physalis species responded differently to various constant temperatures (Fig. 4). P. angulata germinated under 25 to 40 °C with highest germination under 35 °C. Whereas, P. philadelphica not only germinated under wide range of temperatures (15 to 40 °C), but also exhibited maximum germination under 25–30 °C. The results are controversial to findings of Bell and Oliver13, who reported that P. angulata seeds failed to germinate under constant temperatures. The differences in temperature requirements are owed to their differential adaptation potential. The adaptations for germination under diverse environmental conditions are of great ecological significance indicating the potential of invasive plant species to invade more areas. Several studies have been conducted to determine the optimum temperature requirements of different weeds and invasive plant species24,25,26,31,32,37,38. The findings on the germination adaptations of both species to persist under arid and semi-arid regions are novel.
The distinct temperature requirements of different populations are also quite interesting. Both species share the same origin and currently distributed in the same region in Turkey6,7, however, undergone differential adaptations for temperature requirements. The results indicate that P. philadelphica is able to withstand harsher environmental conditions compared with P. angulata. The results also suggest that P. philadelphica have higher range expansion potential to even warmest regions of the country, while P. angulata could be limited to only slightly warmer areas.
Seeds of both plant species were able to germinate under wide range of water and salinity stresses. The populations collected form lower altitude and agricultural habitats proved more resistant to higher levels of water and salinity stress compared to those from higher altitudes. The germination potential of different populations indicates that they have distinct advantage of range expansion to even marginal habitats. A large portion of Turkey have arid and semi-arid climate along with relatively high salinity9,11, which is suspected to be under the invasion risk. Higher germination under elevated levels of water and salinity stress is probably related to higher tolerance of low altitude populations to low rainfall (Table 1) and high salinity levels11 compared to high altitudinal populations. However, both plants species, especially P. philadelphica, not only germinate under higher water stress and salinity levels, but also capable of growing and producing enormous amounts of seeds2,10,13. Therefore, the plant species are expected to create severe economic losses and ecological problems in the region as the invasion further expands to the non-infested areas.
Irrigation, salinity and fine texture of soils have been reported as main invasion drivers of both plant species in the region8. Due to inadequate drainage infrastructure and excessive use of irrigation water, soils are poorly drained and hold water for long time which naturally releases seed dormancy. Hence suitable irrigation systems (such as drip irrigation) which do not provide moisture over all field could be used to control the germination of both species. Both species could outcompete natural vegetation due to the high tolerance to salinity and drought10, thus effective management practices are inevitable to combat with them.
Different populations of both species germinated under wide range of pH indicating that they have adapted to diverse range of soil environments. Maximum germination under alkaline pH indicates that agricultural populations of both species have adapted to existing pH in the region and could invade areas of higher soil pH as suggested in the earlier studies for other weeds and invasive plant species33,38,40. Excessive use of fertilizers and irrigation water in the region raise EC and soil pH11, therefore alternative irrigation systems and sustainable use of fertilizers warrant stronger justification for suppressing both species. Like in temperature, water and salinity stress experiments, P. philadelphica seeds had higher germination rate under wider range of pH compared to P. angulata. This also supports the above findings that P. philadelphica could be more problematic in future.
Seed burial depth initially increased the seedling emergence up to 2 cm and then a sharp decline was observed for deeper seed burial depths. The poor soil and seed contact and low water imbibition are the possible reasons of low emergence observed in the seeds placed on soil surface24. Seeds proved slightly photoblastic and it was expected to emerge even from deeper soil layers. However, emergence was severely retarded beyond 2 cm depth (Fig. 8). Lower emergence of several weed species due to the higher burial depth have been reported24,38,41,42. As seeds proved slightly photoblastic and are of smaller size, lack of energy to push the seedling from increased burial depth is probably the reason of low emergence in the current study42,43.
Seeds of P. philadelphica progressively lose vigor and germination ability at a rate of 9% annually44. It seems that soil inversion by tillage to a maximum depth of emergence could be used as an efficient management tool to combat with both species. However, mold board plow (conventional tillage) turns over topsoil (bringing buried weed seeds to the top), which is frequently practiced in the region for preparing seedbed especially for cotton crop. Cotton is the most infested crop in the SEA8, therefore seeds buried can again come to soil surface with tillage in subsequent growing seasons. The results of seed burial experiments provided two distinct directions for management of the species. The first option is to bury the seeds deep enough in soil and practice conservational tillage in the subsequent years. Conservational tillage along with effective management of emerging seedlings will deplete the soil seeds banks in the long run. The second option is to practice only shallow tillage and manage the emerging seedlings through integrated weed management approach.
Conclusion
The results of the current study suggest that both plant species are in naturalization phase and exhibit no differences for seed dormancy trait. However, both species have undergone extensive adaptations for germination to persist under wide range of environmental conditions. The country has a large portion with arid and semi-arid climate along with high salinity, therefore both plant species could expand their range to even these marginal habitats. P. philadelphica is expected to invade more areas compared with P. angulata in future. Soil inversion to bury the seeds to a maximum depth of emergence followed by conservational tillage practices and managing emerging seeds could be used as a viable management tool to reduce the densities and soil seed bank of both species in long run. Similarly, adoption of an alternative irrigation system could simply suppress the germination of both species as well. The results of the current study necessitate the studies on developing alternative, effective management strategies against both weeds under different tillage and irrigation systems.
Methods
Seed collection
The seeds were collected from different populations of P. philadelphica and P. angulata distributed at various elevation gradients and habitats in South (Mersin) and South eastern Anatolia (Adiyaman and Batman provinces) regions of Turkey. The mature berries of both species were harvested and brought to laboratory, dried, cleaned and used in the experiments. The information related to habitat and prevailing environmental conditions at the seed collection sites are provided in Table 1.
General procedure for germination
Freshly harvested seeds (except dormancy experiment) were used in all experiments and the dormancy was released by keeping seeds under running tap water for 24 hours. The seeds were then placed on two layers of moistened Whatman no.1 filter paper in 9 cm Petri dishes. The filter paper layers were initially moistened with 4 ml deionized water or respective treatment solution and then moistened according to requirements. In all experiments, 5 replicates of 50 seeds placed in Petri dishes were incubated at 30 °C, 12 h photoperiod and 60% relative humidity. Experiments were conducted in completely randomized design and place of Petri dishes was changed every day. The germinated seeds were counted daily (except photoperiod experiment to exclude the effect of light on seed germination) for 30 days taking radical protrusion (2 mm visible) as criterion. Germinated seeds were removed from the Petri dishes. The germination for photoperiod experiment was observed at the end of experiment. Germination percentage was computed 30 days after initiation of the experiments and used in analysis. Experiments were repeated over time (two experimental runs for each type of experiment) for validation of results.
Experiment 1: Seed dormancy level and seed dormancy release
Freshly collected seeds of all populations of both species were dormant (see results section for details). Therefore, effect of seed age and running tap water on seed dormancy release was tested. Seeds of different age (fresh, 3, 6 and 12 months old) were incubated in light to infer the levels of seed dormancy. For running tap water treatment, seeds were wrapped in a thin cloth and kept under running tap water for 24 hours. The seeds were then surface dried and germination test was completed as described above.
Experiment 2: Effect of photoperiod on seed germination
Germination of freshly harvested seeds all populations of both species was observed under three different photoperiods (continuous dark, continuous light and 12 hours alternating light and dark) to determine the effect of light on germination. The light was provided by cool, white fluorescent lamps, at 350 µEm−2 s−1 intensity, whereas the Petri dishes were wrapped in four layers of aluminum foil for creating complete dark.
Experiment 3: Effect of temperature on seed germination
To determine the effects of temperature on germination, freshly harvested seeds of different populations were germinated under a wide range of constant temperatures (10, 15, 20, 25, 30, 35, 40 and 45 °C). The temperature regimes were constant (no day and night variation) throughout the experiment duration. The higher temperature regimes were included in the experiment because of high temperature prevailing in South eastern Anatolia region, Turkey9 during the growth season of both plant species.
Experiment 4: Effect of water stress on seed germination
Freshly harvested seeds of all populations of both plant species were incubated with osmotic potentials of −0.2, −0.4, −0.8, −1.0, −1.2 and −1.4 MPa along with a control treatment (0 osmotic potential, only distilled water) to test the effect of water stress on germination. The osmotic potential solutions were prepared by dissolving polyethylene glycol 8000 in distilled water as described earlier45.
Experiment 5: Effect of salt stress on seed germination
To test the effect of salt stress on germination of different populations of both Physalis species, freshly harvested seeds were incubated in sodium chloride (NaCl) solution of different concentrations (50, 100, 150, 200, 400 and 600 mM). The experiment also had a control treatment (only distilled water) for comparison.
Experiment 6: Effect of pH on seed germination
To assess the effects of pH on germination of both species, freshly harvested seeds of different populations were germinated under wide range of pH (4.0, 5.0 and 6.0 for acidic medium), (7.0 as neutral medium) and (8.0, 9.0, 10.0 and 11.0 as alkaline medium). The solutions were prepared as described in previous study46.
Experiment 7: Effect of seed burial depth on seedling emergence
A greenhouse experiment was conducted to test the effect of seed burial depth on seedling emergence of both plant species by placing 50 seeds on the soil surface (0 cm burial depth) or in the soil filled in 15 cm diameter plastic pots at various depths. Seeds were buried at various depths (1, 2, 4, 6, 8, 10 and 12 cm) by covering the seeds with soil to achieve the respective seed burial depth. A seed was considered as “emerged” when cotyledon was easily visible. The pots were initially irrigated with overhead sprinkler, while sub-irrigation was applied at later stages of experiment. The experiment was carried out for 40 days starting from the day of seed burial.
Statistical analyses
The collected data were analyzed in four steps. In the first step, the difference between experimental runs were tested using Paired T test. No significant differences were observed in experimental runs, therefore data of both experimental runs were combined for further processing. The data of each type of experiment were subjected to analysis of variance (ANOVA). Three-way ANOVA was used to test the difference between plant species, populations and treatments (temperature, photoperiod, water stress, salinity stress, pH and seed burial depth). Finally, least significant difference test was used as post-hoc to separate the means of dormancy and photoperiod experiments. While a three-parameter sigmoid model was fitted to final germination percentage values obtained from water and salt stress experiments. The model was
In above model; G is the cumulative percentage germination at time x, Gmax is the maximum germination (%), T50 is osmotic potential or salinity level required for 50% inhibition of maximum germination, and Grate indicates the slope.
Similarly, the final germination percentage values resulting from temperature, pH and seed burial experiments were modelled with a three-parameter Gaussian model. The model was:
The Gaussian model gives a “bell curved” graph. In the above model, “a” corresponds to the height of the curve’s peak (maximum germination or emergence); “b” is the position of center of the peak (temperature, pH or depth of seed burial to achieve maximum germination or seedling emergence); and “c” is the width of the “bell”.
References
Arslan, M., Uremis, I. & Uludag, A. Determining bio-herbicidal potential of Rapeseed, radish and turnip extracts on germination inhibition of cutleaf ground-cherry (Physalis angulata L.) seeds. Journal of Agronomy 4, 134–137 (2005).
Travlos, I. S. Invasiveness of cut-leaf ground-cherry (Physalis angulata L.) populations and impact of soil water and nutrient availability. Chilean Journal of Agricultural Research 72, 358 (2012).
Travlos, I., Travlos, S., Economou, G. & Lyberopoulou, S. The weed Physalis angulata in western Greece. in Proceedings of 16th Conference of the Greek Weed Science Society, Karditsa. 1–2 (2010).
Kaya, M., Yıldırım, A. & Uygur, F. N. A New Record for the Flora of Turkey Physalis angulata L.(Solanaceae). Turkish Journal of Botany 24, 299–302 (2000).
Bükün, B., Uygur, F. N., Uygur, S., Türkmen, N. & Düzenli, A. A new record for the Flora of Turkey: Physalis philadelphica Lam. var. immaculata Waterf.(Solanaceae). Turkish Journal of Botany 26, 405–407 (2002).
Ozaslan, C., Bukun, B., Ozcan, S. & Onen, H. Physalis angulata. In Invasive Plants Catalogue of Turkey Vol. 1(ed Huseyin Onen) 424–432 Ministery of Agriculture, (2015).
Ozaslan, C., Bukun, B., Ozcan, S. & Onen, H. Physalis philadelphica. in Invasive Plants Catalogue of TurkeyVol. 1(ed Huseyin Onen) 433–440 Ministery of Agriculture, (2015).
Farooq, S., Onen, H., Ozcan, S. & Ozaslan, C. Invasion status of Physalis spp in Diyarbakir and Vicinities. in International Diyarbakir Symposium. 149. (2016).
Sensoy, S., Demircan, M., Ulupinar, Y. & Balta, İ. Climate of Turkey. Climate of Turkey. 2007. Devlet Meteoroloji İşleri Genel Müdürlüğü, 13 Feb. 2009 http://www.dmi.gov.tr/index.aspx (2008).
Ozaslan, C. et al. Invasion Potential of Two Tropical Physalis Species in Arid and Semi-Arid Climates: Effect of Water-Salinity Stress and Soil Types on Growth and Fecundity. Plos One 11(10), e0164369 (2016).
Kendirli, B., Cakmak, B. & Ucar, Y. Salinity in the Southeastern Anatolia Project (GAP), Turkey: issues and options. Irrigation and Drainage 54, 115–122 (2005).
Price, A., Monks, C. & Kelton, J. Cutleaf Groundcherry (Physalis angulata) Density, Biomass and Seed Production in Peanut (Arachis hypogaea L.) Following Regrowth Due to Inadequate Control. Peanut. Science 40, 120–126 (2013).
Bell, V. & Oliver, L. Germination, control, and competition of cutleaf groundcherry (Physalis angulata) in soybeans (Glycine max). Weed Science, 133–138 (1979).
Bukun, В. Determination of economic threshold level and critical period of groundcherry species (Physalis spp.) in cotton growing area in Harran plain, Ph. D. Thesis, University of Cukurova, Adana, Turkey (2001).
Brownsey, R. N., Kyser, G. B. & Di Tomaso, J. M. Seed and germination biology of Dittrichia graveolens (Stinkwort). Invasive Plant Science and Management 6, 371–380 (2013).
Moravcova, L., Pyšek, P., Pergl, J., Perglova, I. & Jarošík, V. Seasonal pattern of germination and seed longevity in the invasive species Heracleum mantegazzianum. Preslia 78, 287–301 (2006).
Skálová, H., Moravcová, L. & Pyšek, P. Germination dynamics and seedling frost resistance of invasive and native Impatiens species reflect local climatic conditions. Perspectives in Plant Ecology, Evolution and Systematics 13, 173–180 (2011).
Udo, N., Tarayre, M. & Atlan, A. Evolution of germination strategy in the invasive species Ulex europaeus. Journal of Plant Ecology, rtw032 (2016).
Chauhan, B. S. & Johnson, D. E. The role of seed ecology in improving weed management strategies in the tropics. Advances in Agronomy 105, 221–262 (2010).
Daehler, C. C. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Review of Ecology, Evolution, and Systematics 34, 183–211 (2003).
Li, Y.-P. & Feng, Y.-L. Differences in seed morphometric and germination traits of crofton weed (Eupatorium adenophorum) from different elevations. Weed Science 57, 26–30 (2009).
Eslami, S. Comparative germination and emergence ecology of two populations of common lambsquarters (Chenopodium album) from Iran and Denmark. Weed science 59, 90–97 (2011).
Fernández-Pascual, E., Jiménez-Alfaro, B., Caujapé-Castells, J., Jaén-Molina, R. & Díaz, T. E. A local dormancy cline is related to the seed maturation environment, population genetic composition and climate. Annals of botany, mct154 (2013).
Chauhan, B. S. Germination biology of Hibiscus tridactylites in Australia and the implications for weed management. Scientific reports 6 (2016).
Chauhan, B. S. & Johnson, D. E. Germination ecology of southern crabgrass (Digitaria ciliaris) and India crabgrass (Digitaria longiflora): two important weeds of rice in tropics. Weed Science 56, 722–728 (2008).
Chauhan, B. S. & Johnson, D. E. Germination ecology of Chinese sprangletop (Leptochloa chinensis) in the Philippines. Weed Science 56, 820–825 (2008).
Bewley, J. D., Black, M. & Halmer, P. The encyclopedia of seeds: science, technology and uses. CABI (2006).
Herranz, J. M., Copete, M. A., Ferrandis, P. & Copete, E. Intermediate complex morphophysiological dormancy in the endemic Iberian Aconitum napellus subsp. castellanum (Ranunculaceae). Seed Science Research 20, 109–121 (2010).
Wagmann, K. et al. Seed dormancy distribution: explanatory ecological factors. Annals of botany, mcs194 (2012).
Cavieres, L. A. & Arroyo, M. T. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae)–altitudinal variation in the Mediterranean Andes of central Chile. Plant Ecology 149, 1–8 (2000).
Chauhan, B. S., Gill, G. & Preston, C. Factors affecting seed germination of threehorn bedstraw (Galium tricornutum) in Australia. Weed Science 54, 471–477 (2006).
Chauhan, B. S., Gill, G. & Preston, C. Factors affecting seed germination of little mallow (Malva parviflora) in southern Australia. Weed Science 54, 1045–1050 (2006).
Awan, T. H., Chauhan, B. S. & Cruz, P. C. S. Influence of environmental factors on the germination of Urena lobata L. and its response to herbicides. Plos One 9, e90305 (2014).
Bermejo, J. E. H. & León, J. Neglected crops: 1492 from a different perspective. Food & Agriculture Org., (1994).
Mandák, B. Germination requirements of invasive and non-invasive Atriplex species: a comparative study. Flora-Morphology, Distribution, Functional Ecology of Plants 198, 45–54 (2003).
Opeña, J. L., Chauhan, B. S. & Baltazar, A. M. Seed germination ecology of Echinochloa glabrescens and its implication for management in rice (Oryza sativa L.). Plos One 9, e92261 (2014).
Chauhan, B. & Johnson, D. Ecological studies on Echinochloa crus-galli and the implications for weed management in direct-seeded rice. Crop Prot 30, 1385–1391 (2011).
Chauhan, B. S. & Johnson, D. E. Seed germination ecology of junglerice (Echinochloa colona): a major weed of rice. Weed Science 57, 235–240 (2009).
Baskin, J. M. & Baskin, C. C. A classification system for seed dormancy. Seed science research 14, 1–16 (2004).
Javaid, M. & Tanveer, A. Germination ecology of Emex spinosa and Emex australis, invasive weeds of winter crops. Weed research 54, 565–575 (2014).
Tang, W., Xu, X., Shen, G. & Chen, J. Effect of environmental factors on germination and emergence of aryloxyphenoxy propanoate herbicide-resistant and-susceptible Asia minor bluegrass (Polypogon fugax). Weed Science 63, 669–675 (2015).
Mahmood, A. H. et al. Influence of various environmental factors on seed germination and seedling emergence of a noxious environmental weed: green galenia (Galenia pubescens). Weed Science 64, 486–494 (2016).
Milberg, P., Andersson, L. & Thompson, K. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Science Research 10, 99–104 (2000).
Pichardo-González, J. M., Ayala-Garay, Ó. J., González-Hernández, V. A. & Flores-Ortiz, C. M. Fatty acids and physiological quality of tomatillo (Physalis philadelphica Lam.) seed during natural ageing. Chilean journal of agricultural research 74, 391–396 (2014).
Michel, B. E. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant physiology 72, 66–70 (1983).
Chauhan, B. S., Gill, G. & Preston, C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Science 54, 854–860 (2006).
Acknowledgements
The current study was funded by the Scientific and Technological Council of Turkey (TUBITAK) with a Grant Number of TOVAG 113 O 790 as a part of the COST Action (TD-1209 - European Information System for Alien Species). Shahid Farooq extends thanks to TUBITAK for supporting PhD studies through BIDEB-2215 program.
Author information
Authors and Affiliations
Contributions
H.O. and S.F. conceived and designed the experiments. C.O., S.O. and S.F. performed the experiment. C.O. provided materials and reagents. S.F. and H.O. analyzed the data and wrote the first draft of the manuscript. H.G., B.B. provided input to the final draft. H.O. supervises the study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ozaslan, C., Farooq, S., Onen, H. et al. Germination Biology of Two Invasive Physalis Species and Implications for Their Management in Arid and Semi-arid Regions. Sci Rep 7, 16960 (2017). https://doi.org/10.1038/s41598-017-17169-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17169-5
- Springer Nature Limited
This article is cited by
-
Comprehensive analysis and implications of Veronica persica germination and growth traits in their invasion ecology
Scientific Reports (2024)
-
Reproductive biology and hybridization of Physalis L. species
Brazilian Journal of Botany (2022)
-
Growth and ecological characteristics of Physalis angulata invasive weed species in several invaded communities
Biologia (2022)