Abstract
A facile, highly efficient approach to obtain molybdenum trioxide (MoO3)-doped tungsten trioxide (WO3) is reported. An annealing process was used to transform ammonium tetrathiotungstate [(NH4)2WS4] to WO3 in the presence of oxygen. Ammonium tetrathiomolybdate [(NH4)2MoS4] was used as a dopant to improve the film for use in an electrochromic (EC) cell. (NH4)2MoS4 at different concentrations (10, 20, 30, and 40 mM) was added to the (NH4)2WS4 precursor by sonication and the samples were annealed at 500 °C in air. Raman, X-ray diffraction, and X-ray photoelectron spectroscopy measurements confirmed that the (NH4)2WS4 precursor decomposed to WO3 and the (NH4)2MoS4–(NH4)2WS4 precursor was transformed to MoO3-doped WO3 after annealing at 500 °C. It is shown that the MoO3-doped WO3 film is more uniform and porous than pure WO3, confirming the doping quality and the privileges of the proposed method. The optimal MoO3-doped WO3 used as an EC layer exhibited a high coloration efficiency of 128.1 cm2/C, which is larger than that of pure WO3 (74.5 cm2/C). Therefore, MoO3-doped WO3 synthesized by the reported method is a promising candidate for high-efficiency and low-cost smart windows.
Similar content being viewed by others
Introduction
Electrochromic (EC) materials have attracted much attention owing to their potential applications in smart windows, antiglare mirrors, data storage devices, displays, sunroofs, and sunglasses. Various materials can be used as an EC layer, including inorganic metal oxides and organic conducting polymers1,2,3,4,5,6,7,8.
Conducting polymers provide benefits such as multiple colors, a fast switching time, and flexibility, but their disadvantages, including relatively nonuniform films, low material stability, and a limited range of colors severely limit their practical applications9,10. On the other hand, tungsten trioxide (WO3) is a well-known metal oxide owing to its excellent EC performance. WO3 with different structures has been prepared by techniques such as hydrothermal process, chemical vapor deposition, thermal evaporation, and sputtering11,12,13,14,15. However, these approaches have drawbacks that restrict the commercial application of WO3 EC films, including complicated preparation, high energy consumption, expensive equipment, or the use of toxic and dangerous reagents16.
Molybdenum oxide is one of the important semiconducting metal oxides and can be used in various applications, including photovoltaic cells, organic light-emitting diodes, gas sensors, hydrogen evolution systems, transistors, and EC devices17,18,19,20,21,22. However, the coloration efficiency of pure molybdenum oxide used in EC devices is not high. For example, Patil et al. found that MoO3 used as an EC layer had a coloration efficiency of 34 cm2/C23. A combination of tungsten and molybdenum oxide has been used in EC devices recently. For instance, Mahdavi et al. investigated the effect of molybdenum in a WO3 thin film prepared by RF magnetron sputtering and obtained a coloration efficiency of 42.5 cm2/C24. Kharade et al. synthesized MoO3 mixed with WO3 using a hybrid physicochemical method and achieved a high coloration efficiency of 121.56 cm2/C25. However, the complicated synthesis method with high cost is a drawback. Consequently, it is urgently necessary to develop immediate, effective, and facile methods to synthesize tungsten oxide films with enhanced EC performance.
In this study, we report a facile, low-cost method of producing EC thin films based on WO3 for smart window applications. In our previous work, we investigated the (NH4)2WS4 precursor annealed at 350 °C as a hole transport layer in an organic solar cell26. In this work, we investigate the use of different annealing temperatures to obtain WO3, and then we add the (NH4)2MoS4 precursor as a dopant to the (NH4)2WS4 precursor at different concentrations (10, 20, 30, and 40 mM) to obtain optimal EC films. A spin-coating method with an annealing process was applied to obtain EC films with excellent features such as high EC energy efficiency, high coloration efficiency, low cost, excellent chemical stability, fast switching speed, and good adhesion to the substrate.
Results and Discussion
The Raman spectra of the (NH4)2WS4 films annealed at different temperatures are shown in Fig. 1(a). The Raman peak of the S–W–S stretching mode is weakened for the amorphous phase as the temperature is increased, whereas the peaks corresponding to WO3 phases (O–W–O bending and stretching) are strengthened27. Therefore, the WO3 phases are completely decomposed after annealing at 500 °C, indicating a monoclinic crystal system. Figure 1(b) shows the Raman spectra of the MoO3-doped WO3 film. The peak at 675 cm−1 is ascribed to the coordinated oxygen in Mo crystal structure and stretching mode, which confirms that (NH4)2MoS4 was transformed to a MoO3 crystal, as indicated by the edge-shared oxygen28. Moreover, the Raman peaks at 272 cm−1 is assigned to O=Mo=O wagging modes28. Fig. 1(c) shows the XRD patterns of (NH4)2WS4 thin films annealed at different temperatures. The pristine WS4 shows broad WS2 peaks (2θ = 15–35°) related to weak crystallinity at annealing temperatures below 400 °C. The broad peak intensity decreases after annealing at 500 °C, and the peaks corresponding to WO3 structure are observed as well. These peaks are quite similar to those reported for monoclinic WO3 29. Fig. 1(d) confirms the nanocrystallinity of the MoO3-doped WO3 films. The molybdenum is incorporated into the film, producing a new phase with orthorhombic crystal structure which is similar to the previously reported structures24,30. Moreover, the XRD results of the MoO3-doped WO3 did not show the peaks related to metallic Mo or MoO3, suggesting the well-diffusion of molybdenum atoms into WO3 crystal structure and substitution of Mo in W sites24.
The XPS spectra of (NH4)2WS4 films annealed at different temperatures are shown in Fig. 2(a). As the temperature increases to 500 °C, the S 2 s and N 1 s peaks related to the (NH4)2WS4 precursor disappear, suggesting the complete decomposition of the (NH4)2WS4 precursor into WO3. WO3 appears owing to the presence of O2 in the air26. High-resolution views of the W 4 f and O 1 s peaks are shown in Fig. 2(b). The peak appears at 33.5 eV for the (NH4)2WS4 film annealed at 200 °C, and the peaks at 35.4, 36.3, and 38 eV are ascribed to W4+, W5+, and W6+ 26. The peak of W5+ is related to oxygen vacancy31. As the annealing temperature increases, the peak located at 33.5 eV vanish, confirming the transformation of WS2 to WO3. The observed shifts of the peaks in the O 1 s and W 4 f spectra toward lower binding energy can be attributed to the emission of photoelectrons from the higher to lower oxidation states of W32. For the XPS data in the O 1 s region, the peak density related to the oxide phase near 531 eV increases as the annealing temperature increases beyond 300 °C (Fig. 2(b))33. The XPS survey scan of the MoO3-doped WO3 film (Fig. 2(c)) shows additional peaks that are related to Mo 3d and Mo 3p. The XPS peak positions of Mo 3d3/2 and Mo 3d1/2 are 233 and 236.2 eV, respectively (inset of Fig. 2(c)), which are attributed to pair of orbital spinning of MoO3 34. The two observed peaks of W 4f7/2 and W 4f5/2 appear at 35.9 and 38 eV, respectively (Fig. 2(d)). Figure 2(e) shows the high-resolution XPS O 1 s spectrum, in which the oxygen O 1 s peaks are observed at 530.5 and 539.2 eV. The spectra indicate the presence of W, Mo, and O in the as-prepared MoO3-doped WO3 EC film with oxidation states of +6, +6, and −2, respectively25. All of XPS results and observed peaks are confirmed by previously reported works25,26,27,28,29,30,31,32,33,34,35.
(a) XPS survey spectra of (NH4)2WS4 films annealed at different temperatures, (b) high-resolution W 4 f and O 1 s spectra of (NH4)2WS4 films annealed at different temperatures, (c) XPS survey spectra of (NH4)2WS4/(NH4)2MoS4 (30 mM) film annealed at 500 °C with high-resolution Mo 3d spectrum (inset), (d) W 4 f and (e) O 1 s spectra of (NH4)2WS4/(NH4)2MoS4 (30 mM) film annealed at 500 °C.
The atomic ratios of (NH4)2WS4 films annealed at different temperatures are shown in Fig. 3(a). Those of sulfur (S 2 s) and nitrogen (N 1 s) approach zero as the annealing temperature is increased to 400 °C. The pie chart in Fig. 3(b) indicates that the oxygen content of the MoO3-doped WO3 is higher than that of other atoms owing to the annealing process and the presence of oxygen in both the MoO3 and WO3 structures. It is calculated that the level of doping was 0.76% (30 mM (NH4)2MoS4 into (NH4)2WS4 precursor). These results not only support the formation of an oxide surface layer on the sulfide (NH4)2WS4 backbone, but also represent the functionalization of MoO3 on the WO3 structure. As shown in the inset of Fig. 3(c), the work function of the pure WO3 thin film increases as the annealing temperature increased to 500 °C. The work function of WO3 at 500 °C is 4.71 eV (inset of Fig. 3(c)). The increase of work function is attributed to the formation of WO3 26. In addition, the green curve shown in Fig. 3(c) indicates that the work function of MoO3-doped WO3 at 500 °C is 5.02 eV, which is higher than that pure WO3. These data suggest better performance of electrochromic in MoO3-doped WO3 device by facilitating charge transfer. Figure 3(d) shows the valance band maxima (VBMs) of the WO3 and MoO3-doped WO3, which decrease with increasing annealing temperature. However, MoO3 doping method increases the VBM owing to changes in the O 1 s states and promoting the transition of intervalance within metal ions34,36. These data suggest the enhanced transition of electron in MoO3-doped WO3 film, resulting in increased coloration efficiency.
(a) Atomic ratios of (NH4)2WS4 films annealed at different temperatures, (b) atomic content of (NH4)2WS4/(NH4)2MoS4 (30 mM) film annealed at 500 °C, (c) UPS spectra of (NH4)2WS4/(NH4)2MoS4 (30 mM) film annealed at 500 °C and (inset) work function of (NH4)2WS4 films annealed at different temperatures, (d) ultraviolet photoelectron spectra of (NH4)2WS4 films annealed at different temperatures and (NH4)2WS4/(NH4)2MoS4 (30 mM) film annealed at 500 °C.
Figure 4 shows FESEM and HRTEM images of the WO3 and MoO3-doped WO3. Both samples have porous and compact surfaces. The observed cracks are attributed to the annealing process (Fig. 4(a) and (b)). Moreover, the MoO3-doped WO3 exhibits a more uniform than pure WO3 (Fig. 4(c)). In addition, porosity measurement was carried out by using MATLAB software whose method was previously reported37. In order to measure the porosity, the FESEM images of WO3 and MoO3-doped WO3 were converted to binary image (see Figure S1) and then the percentage of porosity was calculated by following formula:
where P is the porosity percent, n is the number of white pixels, and N is the total number of white and black pixels. The percentage of porosities were obtained 58.2 and 75.6% in WO3 and MoO3-doped WO3 film, respectively. The lattice fringes in the HRTEM images demonstrate that the WO3 films are crystallized, confirming the XRD results. In addition, the estimated lattice spacings of 0.302 and 0.364 nm are assigned to the monoclinic d-spacing of the (020) plane of WO3 and the orthorhombic (002) plane of MoO3 (Fig. 4(d) and (e)). Figure 4(f) shows the elemental distributions of W, O, and Mo for the MoO3-doped WO3 thin film. MoO3 atoms not only grew on the WO3 surface, but are also diffused into the film. These data indicate that the method has great potential for efficient doping.
According to the AFM images (Fig. 5), the measured roughness of the WO3 and MoO3-doped WO3 is 6.5 and 2.4 nm, respectively. Therefore, the uniformity of film is increased without aggregation caused by doping. The AFM images confirm that the porosity of the film is greater after doping. The higher porosity is expected to improve the EC performance by enhancing the diffusion constant of the intercalating ions through the pore interface38. In addition, higher roughness and porosity of film can improve the distribution of electrical field during redox process resulting in enhanced electron transfer and ion-insertion, leading to high EC performance24,34,39,40.
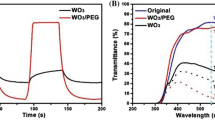
Figure 6(a) shows the configuration of the EC cell. The transmittance spectra (wavelength 400–900 nm) of WO3 and MoO3-doped WO3 in the colored and bleached states were measured (Fig. 6(b)). The color was changed to dark blue when a DC voltage of −2.5 V was applied across the ITO. After the voltage was changed to +2.5 V, the EC cell returned to the transparent state. The mechanism is thought to be the oxidation and reduction process. Li+ ions are inserted into the EC film, leading to reduction of W6+ to W5+ and the increase in the cathodic current change the color of the film. The change from the colored state to the transparent state is ascribed to oxidation (W5+ to W6+) due to the changing in redox state of the tungsten ions and the number of electrons (charge) inserted into EC film24. In the doped EC film, Mo is involved in the reduction/oxidation process (Mo6+ to Mo5+ and Mo5+ to W6+), which causes to the enhanced transition of intervalency and electron transition within ions34. Optical modulation is one of the most important parameters in EC devices and can be defined as ΔT = Tb − Tc, where Tb and Tc are the transmittance in the bleached and colored states, respectively, at a particular wavelength41. The difference in transmission (ΔT at 675 nm) between the bleached and colored states in WO3 and MoO3-doped WO3 was enhanced from ΔT1 = 35% to ΔT2 = 49%. This improvement is attributed to a change in the crystal structure of WO3 caused by substitution of Mo at W sites and charge transfer between the Mo5+ and W6+ sites24,42. Fig. 6(c) shows the stability of the WO3 and MoO3-doped WO3 in the colored state for several weeks after the voltage is removed. MoO3-doped WO3 exhibited better memory behaviour in air, in which it relatively retained the colored state very well even after 4 weeks. On the other hand, some parts of the pure WO3 EC film became transparent as time passed. Therefore, the memory behaviour of MoO3-doped WO3 is better than that of pure WO3 because of the increase in the diffusion coefficient (D) of Li+ ions in MoO3-doped WO3 during the intercalation process24. Diffusion coefficient during intercalation process can be calculated by Randles−Sevcik equation24,43:
where ip is the anodic peak current density, n is the number of electrons transferred during redox process, C0 is the concentration of active ions in the electrolyte, ν is the scan rate, and A is the area of the EC film24. The diffusion coefficient was obtained 1.23 × 10−11 and 9.42 × 10−11 for the WO3 and MoO3-doped WO3 EC film, respectively. Figure 6(d) and (e) shows the current-voltage (CV) curves of pure WO3 and MoO3-doped WO3 thin films, which were measured in a 1 M aqueous solution at a scan rate of 50 mV/s. In the cathodic process, the current of pure WO3 is higher than that of the MoO3-doped WO3 thin film. The MoO3-doped WO3 has a higher conductivity than pure WO3 because more defect states are created owing to integration of the two metal oxides, decreasing the energy required to extract the intercalated Li+ ions after MoO3 doping24,25. In addition, the cycling stability of both thin films after 300 cycle steps revealed that the current in the MoO3-doped WO3 did not change and remained constant compared to that of the pure WO3 EC film. Moreover, the CV curves not only indicate a well-crystallized WO3 structure in both films, but also confirm the XRD and TEM results.
The coloration efficiency (CE), which is an important parameter for EC devices, was calculated as follows23,24,30:
where ΔOD is the change in optical density, Q is the charge density, Tb is the transmittance of the film in the bleached state, and Tc is the transmittance of the film in the colored state23,24,41. The color efficiency of various EC layers and various MoO3 concentrations (λ = 675 nm) is presented in Fig. 6(f). The CE values of the MoO3-doped WO3 are higher than that of pure WO3 (74.5 cm2/C). To determine the optimal concentration of MoO3 in WO3, the CE was measured for different MoO3 concentrations (10, 20, 30, and 40 mM) in WO3. As shown in Fig. 6(f), a much higher CE (128.1 cm2/C) was obtained for the MoO3-doped WO3 with a MoO3 concentration of 30 mM. This result indicates that the optimal amount of molybdenum has a crucial role in obtaining high efficiency. Furthermore, the response times of the colored and bleached states for MoO3-doped WO3 are found to be 3.6 and 4.5 s, respectively, whereas the response times of the colored and bleached states for pure as-prepared WO3 are 8 and 9.5 s, respectively. Enhanced EC properties is caused by improvement in extra electron intervalance transfer W6+ and Mo6+ active sites. In addition, molybdenum after intercalation of Li+ ions are more near to the sensitivity of human’s vision. The disorder could be increased by random distribution of molybdenum, resulting in the betterment EC properties25. Therefore, the performance was improved by MoO3 doping. Table 1 compares our results with the reported values through various materials and methods.
Conclusions
WO3 doped with MoO3 was prepared by a facile and low-cost method involving solution and annealing processes. The results indicated that the (NH4)2MoS4/(NH4)2WS4 precursor decomposed to MoO3-doped WO3 when the film was annealed at 500 °C in air. The N 1 s and S 2 s emission in the XPS spectrum of the (NH4)2WS4 precursor annealed at 500 °C, as well as weakening of the S–W–S bond, increased O–W–O bond peaks in the Raman spectra, and the appearance of peaks in the XRD spectra, suggested full decomposition to monoclinic crystalline WO3. In addition, (NH4)2MoS4 was added to the (NH4)2WS4 precursor at various concentrations as a dopant, and the resulting films were then annealed at 500 °C to transform them to MoO3-doped WO3. The XRD and Raman spectroscopy results confirmed the decomposition to nanocrystalline MoO3-doped WO3. Moreover, the morphology of the as-prepared films was observed using FESEM and AFM, which showed that the MoO3-doped WO3 was more uniform and porous than pure WO3, suggesting high EC performance. Furthermore, the high doping capability with good distribution of MoO3 into WO3 was confirmed by HRTEM images. As a result, enhanced EC performance was obtained when the MoO3-doped (30 mM) WO3 was used as an EC layer. The coloration efficiency was high (CE = 128.1 cm2/C), and the response time was rapid (tc = 3 s, tb = 4.5 s). These values are much higher than those of pure WO3 (CE = 74.5 cm2/C, tc = 8 s, tb = 9.5 s). In conclusion, the MoO3-doped WO3 prepared by the annealing–solution process is a remarkable candidate for use in high-efficiency, low-cost smart windows that can be efficiently commercialized.
Method
Preparation of thin film of MoO3-doped WO3
Figure 7 illustrates the synthesis of the WO3 and MoO3-doped WO3 thin films and fabrication of the EC cell. Indium tin oxide (ITO) substrates were ultrasonically cleaned sequentially with DI water, isopropanol, and acetone, and then dried; they were then treated by ultraviolet ozone for 20 min and maintained there until the start of the spin-coating process. After drying, PtCl4 dispersed in isopropanol was coated on one piece of the ITO conductive glass by spin-coating, and the ITO was dried on a hotplate at 250 °C to evaporate the solvent and chlorine; this sample was used as a counter electrode. Next, (NH4)2WS4 (200 mg) was dissolved in 1 ml of N,N-dimethylformamide, resulting in the formation of a yellowish tungsten sol. Then, a homogenous thin film was prepared by spin-coating the as-prepared solution onto the ITO substrate at 4000 rpm for 60 s. After drying in air, the coated substrates were annealed in a furnace at different temperatures (200, 300, 400, and 500 °C) for 2 h. A transparent, colorless WO3 thin film was obtained at 500 °C. To form the MoO3-doped WO3 thin films, (NH4)2MoS4 was added to the (NH4)2WS4 solution separately (at different concentrations, 10, 20, 30, and 40 mM, to determine the optimal amount of doping) to form a homogenous solution. Then, this procedure was repeated to synthesize MoO3-doped WO3 thin films at 500 °C.
Fabrication of the EC cell
The EC device structure for the MoO3-doped WO3 thin films was glass/ITO/MoO3-doped WO3/LiClO4 + propylene carbonate (PC)/Pt/ITO/glass. The ITO substrate coated with the MoO3-doped WO3 thin film acts as a working electrode, and the Pt/ITO-coated conducting glass substrate acts as a counter electrode; the electrodes are assembled to fabricate a sandwich-type EC device. The liquid electrolyte, 1 M lithium perchlorate (LiClO4)/PC, was injected into the device through a small hole, which was then sealed with Resibond epoxy glue.
Characterizations
X-ray photoelectron spectroscopy (XPS) was performed using an ESCA-3000 (VG Scientific Ltd., England) instrument analyzer under a vacuum better than 1 × 10−5 mbar using Mg Kα radiation (1250 eV) and a constant pass energy of 50 eV. The composition of the thin film samples was determined by X-ray diffraction (XRD) analysis (Bruker AXS Model D8 Advance X-ray diffractometer) with a Cu Kα target having a wavelength 0.1542 nm. Raman spectra (LabRAM HR, Horiba Jobin Yvon, Japan) were obtained at an excitation wavelength of 514 nm. Field-emission scanning electron microscopy (FESEM, Zeiss 300 VP) images were taken at an acceleration voltage of 50 kV. Transmission electron microscopy (TEM) was performed with a JEOL-2100F (Japan) instrument. Contact-mode atomic force microscopy (AFM, XE-100/PSIA) was used to determine the roughness and porosity of the thin films. Cyclic voltammetry (CV) and electrochemical measurements were performed in a quartz electrochemical cell connected to a potentiostat (Ivium 5612, Netherlands). WO3 or MoO3-doped WO3 was used as the working electrode, and a Ag/AgCl electrode and platinum (Pt) wire were applied as the reference and counter electrodes, respectively. The transmittance spectra were measured by a UV–vis spectrophotometer (V-670). The coloration/bleaching switching characteristics of the EC films were recorded as the changes in the transmittance at a wavelength of 675 nm under alternating application of a potential of ±2.5 V for 60 s for each state. For the XPS, high-resolution TEM (HRTEM), FESEM, XRD, Raman, and AFM measurements of the MoO3-doped WO3, samples fabricated using 30 mM of (NH4)2MoS4 in the (NH4)2WS4 precursor were used.
References
Runnerstrom, E. L. et al. Nanostructured electrochromic smart windows: traditional materials and NIR-selective plasmonic nanocrystals. Chem. Commun. 50, 10555–10572, https://doi.org/10.1039/c4cc03109a (2014).
Tajima, K., Hotta, H., Yamada, Y., Okada, M. & Yoshimura, K. Electrochromic switchable mirror glass with controllable reflectance. Appl. Phys. Lett. 100, doi:Artn 09190610.1063/1.3691256 (2012).
Wang, K. et al. Integrated energy storage and electrochromic function in one flexible device: an energy storage smart window. Energ. Environ. Sci. 5, 8384–8389, https://doi.org/10.1039/c2ee21643d (2012).
Cheng, C. P. et al. Performance improvement of electrochromic display devices employing micro-size precipitates of tungsten oxide. Appl. Phys. A. 116, 1553–1559, https://doi.org/10.1007/s00339-014-8371-x (2014).
Ma, C. et al. Smart sunglasses based on electrochromic polymers. Polym. Eng. Sci. 48, 2224–2228 (2008).
Lampert, C. M. Chromogenic smart materials. Mater. Today 7, 28–35, https://doi.org/10.1016/S1369-7021(04)00123-3 (2004).
Xiong, S. et al. Organic/inorganic electrochromic nanocomposites with various interfacial interactions: A review. Mater. Sci. Eng. B 221, 41–53, https://doi.org/10.1016/j.mseb.2017.03.017 (2017).
Sassi, M. et al. Organic electrochromic polymers: state-of-the-art neutral tint multichromophoric polymers for high-contrast see-through electrochromic devices. Adv. Funct. Mater. 26, 5239–5239, https://doi.org/10.1002/adfm.201601819 (2016).
Yuksel, R. et al. Silver nanowire/conducting polymer nanocomposite electrochromic supercapacitor electrodes. J. Electrochem. Soc. 164, A721–A727, https://doi.org/10.1149/2.0791704jes (2017).
Liang, Y., Strohecker, D., Lynch, V. M., Holliday, B. J. & Jones, R. A. A thiophene-containing conductive metallopolymer using an Fe (II) Bis (terpyridine) core for electrochromic materials. ACS Appl. Mater. Interfaces 8, doi:https://doi.org/10.1021/acsami.6b11657 (2016).
Su, X. et al. Synthesis of uniform WO3 square nanoplates via an organic acid-assisted hydrothermal process. Mater. Lett. 64, 1232–1234, https://doi.org/10.1016/j.matlet.2010.02.063 (2010).
Marsen, B. et al. Progress in sputtered tungsten trioxide for photoelectrode applications. Int. J. Hydrogen Energy 32, 3110–3115, https://doi.org/10.1016/j.ijhydene.2006.01.022 (2007).
Gogova, D. et al. Structural and optical properties of CVD thin tungsten oxide films. Physical Status Solidi A. 176, 969–984, doi:10.1002/(SICI)1521-396X(199912)176:2<969::AID-PSSA969>3.0.CO;2-9 (1999).
Ponzoni, A. et al. Nanostructured WO3 deposited by modified thermal evaporation for gas-sensing applications. Thin Solid Films 490, 81–85, https://doi.org/10.1016/j.tsf.2005.04.031 (2005).
Leftheriotis, G. et al. Structural and electrochemical properties of opaque sol–gel deposited WO3 layers. Appl. Surf. Sci. 218, 276–281, https://doi.org/10.1016/S0169-4332(03)00616-0 (2003).
Xie, Z. et al. Fast fabrication of a WO3· 2H2O thin film with improved electrochromic properties. J. Mater. Chem. 22, 19904–19910, https://doi.org/10.1039/C2JM33622G (2012).
Kwon, K. C. et al. Wafer-scale transferable molybdenum disulfide thin-film catalysts for photoelectrochemical hydrogen production. Energ. Environ. Sci. 9, 2240–2248, https://doi.org/10.1039/C6EE00144K (2016).
Kim, Y. H. et al. Ultrasensitive reversible oxygen sensing by using liquid-exfoliated MoS2 nanoparticles. J. Mater. Chem. A 4, 6070–6076, https://doi.org/10.1039/C6TA01277A (2016).
Nguyen, T. P. et al. MoS2 nanosheets exfoliated by sonication and their application in organic photovoltaic cells. Sci Adv. Mater. 7, 700–705, https://doi.org/10.1166/sam.2015.1891 (2015).
Van, L. Q. et al. The use of UV/ozone-treated MoS2 nanosheets for extended air stability in organic photovoltaic cells. Phys. Chem. Chem. Phys. 16, 13123–13128, https://doi.org/10.1039/c4cp01598c (2014).
Kim, S. et al. High-mobility and low-power thin-film transistors based on multilayer MoS2 crystals. Nat. Commun. 3, 1011, https://doi.org/10.1038/ncomms2018 (2012).
Jeon, P. J. et al. Low power consumption complementary inverters with n-MoS2 and p-WSe2 dichalcogenide nanosheets on glass for logic and light-emitting diode circuits. ACS Appl. Mater. Inter. 7, 22333–22340, https://doi.org/10.1021/acsami.5b06027 (2015).
Patil, R. S. et al. Electrosynthesis of electrochromic molybdenum oxide thin films with rod-like features. Int. J. Electrochem. Sci 3, 259–265 (2008).
Madhavi, V., Kumar, P. J., Kondaiah, P., Hussain, O. M. & Uthanna, S. Effect of molybdenum doping on the electrochromic properties of tungsten oxide thin films by RF magnetron sputtering. Ionics 20, 1737–1745, https://doi.org/10.1007/s11581-014-1073-8 (2014).
Kharade, R. R. et al. Hybrid physicochemical synthesis and electrochromic performance of WO3/MoO3 thin films. Electroanal 26, 2388–2397, https://doi.org/10.1002/elan.201400239 (2014).
Le, Q. V. et al. (NH4)2WS4 precursor as a hole-injection layer in organic optoelectronic devices. Chem Eng J 284, 285–293, https://doi.org/10.1016/j.cej.2015.08.142 (2016).
Xu, L. J., Yin, M. L. & Liu, S. Z. Agx@WO3 core-shell nanostructure for LSP enhanced chemical sensors. Sci Rep-Uk 4, doi:ARTN 674510.1038/srep06745 (2014).
Sharma, R. K. & Reddy, G. B. Synthesis and characterization of alpha-MoO3 microspheres packed with nanoflakes. J. Phys. D: Appl. Phys. 47, https://doi.org/10.1088/0022-3727/47/6/065305 (2014).
Balzer, R. et al. Synthesis and structure-activity relationship of a WO3 catalyst for the total oxidation of BTX. J. Brazil. Chem. Soc. 25, 2026–2031, https://doi.org/10.5935/0103-5053.20140187 (2014).
Zhang, H., Li, Y., Duan, G., Liu, G. & Cai, W. Tungsten oxide nanostructures based on laser ablation in water and a hydrothermal route. Cryst. Eng. Comm. 16, 2491–2498, https://doi.org/10.1039/C3CE42320D (2014).
Li, Y., Tang, Z., Zhang, J. & Zhang, Z. Defect engineering of air-treated WO3 and Its enhanced visible-light-driven photocatalytic and electrochemical performance. J. Phys. Chem. C 120, 9750–9763, https://doi.org/10.1021/acs.jpcc.6b00457 (2016).
Vuong, N. M., Kim, D. & Kim, H. Porous Au-embedded WO3 nanowire structure for efficient detection of CH4 and H2S. Sci Rep-Uk 5, https://doi.org/10.1038/srep11040 (2015).
Ryu, W.-H. et al. Heterogeneous WSx/WO3 thorn-bush nanofiber electrodes for sodium-ion batteries. ACS Nano 10, 3257–3266, https://doi.org/10.1021/acsnano.5b06538 (2016).
Dong, W. et al. Bifunctional MoO3–WO3/Ag/MoO3–WO3 films for efficient ITO–free electrochromic devices. ACS Appl. Mater. Inter. 8, 33842–33847, https://doi.org/10.1021/acsami.6b12346 (2016).
Dalavi, D. S. et al. Efficient electrochromic performance of nanoparticulate WO3 thin films. J. Mater. Chem. C 1, 3722–3728, https://doi.org/10.1039/C3TC30378K (2013).
White, R. T., Thibau, E. S. & Lu, Z.-H. Interface structure of MoO3 on organic semiconductors. Sci Rep-Uk 6, https://doi.org/10.1038/srep21109 (2016).
Entekhabi, E., Nazarpak, M. H., Moztarzadeh, F. & Sadeghi, A. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater. Sci. Eng. C 69, 380–387, https://doi.org/10.1016/j.msec.2016.06.078 (2016).
Balaji, S. et al. Porous orthorhombic tungsten oxide thin films: synthesis, characterization, and application in electrochromic and photochromic devices. J. Mater. Chem. 21, 3940–3948, https://doi.org/10.1039/C0JM03773G (2011).
Luo, W., Fu, X. K. & Ma, L. H. The research on the high quality TiO2, MoO3-doped WO3 electrochromic film. Chinese. Chem. Lett. 18, 883–886, https://doi.org/10.1016/j.cclet.2007.05.003 (2007).
Zhang, J., Tu, J., Xia, X., Qiao, Y. & Lu, Y. An all-solid-state electrochromic device based on NiO/WO3 complementary structure and solid hybrid polyelectrolyte. Sol. Energ. Mat. Sol. C 93, 1840–1845, https://doi.org/10.1016/j.solmat.2009.06.025 (2009).
Granqvist, C. G. Handbook of inorganic electrochromic materials. (Elsevier, 1995).
Yao, J., Loo, B., Hashimoto, K. & Fujishima, A. Photochromic characteristics of mixed WO3–MoO3 thin films in alcohol vapors. Berichte der Bunsengesellschaft für physikalische Chemie 95, 554–556, https://doi.org/10.1002/bbpc.19910950502 (1991).
Nicholson, R. S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 37, 1351–1355, https://doi.org/10.1021/ac60230a016 (1965).
Paipitak, K. et al. Characterization of sol-gel derived ti-doped tungsten oxide electrochromic thin films. Energy. procedia. 9, 446–451, https://doi.org/10.1016/j.egypro.2011.09.050 (2011).
Wei, H. et al. Electropolymerized polyaniline stabilized tungsten oxide nanocomposite films: electrochromic behavior and electrochemical energy storage. J. Phys. Chem. C 116, 25052–25064, https://doi.org/10.1021/jp3090777 (2012).
Lin, F. et al. Low-temperature ozone exposure technique to modulate the stoichiometry of WOx nanorods and optimize the electrochromic performance. Nanotechnology 23, 255601, https://doi.org/10.1088/0957-4484/23/25/255601 (2012).
Chang, X., Sun, S., Dong, L., Hu, X. & Yin, Y. Tungsten oxide nanowires grown on graphene oxide sheets as high-performance electrochromic material. Electrochim. Acta 129, 40–46, https://doi.org/10.1016/j.electacta.2014.02.065 (2014).
Acknowledgements
This research was supported in part by National Research Foundation of Korea (NRF) grants provided by the Korean government (MSIP) (Nos 2015K1A3A1A59073839, 2017H1D8A1030599, 2017K1A3A1A67014432) and in part by Korea Agency for Infrastructure Technology Advancement grant funded by Ministry of Land, Infrastructure and Transport (17IFIP-B133622-01).
Author information
Authors and Affiliations
Contributions
A.H. and Q.V.L. contributed equally to this work. A.H. and Q.V.L. performed the synthesized materials and most of characterization. T.P.N., K.S.C., and W.S. performed device characterization. J.K.K. analyzed materials’ characterization. H.W.J. and S.Y.K. supervised the experiments and contributed to manuscript preparation. H.W.J. and S.Y.K. initiated and directed the research. S.Y.K. conceived the idea and designed the experiments. All authors were involved in writing the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hasani, A., Le, Q.V., Nguyen, T.P. et al. Facile Solution Synthesis of Tungsten Trioxide Doped with Nanocrystalline Molybdenum Trioxide for Electrochromic Devices. Sci Rep 7, 13258 (2017). https://doi.org/10.1038/s41598-017-13341-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13341-z
- Springer Nature Limited
This article is cited by
-
Photochromic property of ternary transition metal oxide nanocomposite prepared with co-solvated deep eutectic mixtures
Research on Chemical Intermediates (2021)
-
XRD peak profile and optical properties analysis of Ag-doped h-MoO3 nanorods synthesized via hydrothermal method
Journal of Materials Science: Materials in Electronics (2020)
-
Influence of the annealing temperature on the formation of Mo17O47 and MoO3 nanoparticles and their Photocatalytic performances for the degradation of MB dye
Journal of Materials Science: Materials in Electronics (2020)