Abstract
Meningococcal meningitis epidemics in the African meningitis belt consist of localised meningitis epidemics (LME) that reach attack proportions of 1% within a few weeks. A meningococcal serogroup A conjugate vaccine was introduced in meningitis belt countries from 2010 on, but LME due to other serogroups continue to occur. The mechanisms underlying LME are poorly understood, but an association with respiratory pathogens has been hypothesised. We analysed national routine surveillance data in high spatial resolution (health centre level) from 13 districts in Burkina Faso, 2004–2014. We defined LME as a weekly incidence rate of suspected meningitis ≥75 per 100,000 during ≥2 weeks; and high incidence episodes of respiratory tract infections (RTI) as the 5th quintile of monthly incidences. We included 10,334 health centre month observations during the meningitis season (January-May), including 85 with LME, and 1891 (1820) high-incidence episodes of upper (lower) RTI. In mixed effects logistic regression accounting for spatial structure, and controlling for dust conditions, relative air humidity and month, the occurrence of LME was strongly associated with high incidence episodes of upper (odds ratio 23.9, 95%-confidence interval 3.1–185.3), but not lower RTI. In the African meningitis belt, meningitis epidemics may be triggered by outbreaks of upper RTI.
Similar content being viewed by others
Introduction
The meningitis belt in sub-Saharan Africa has the highest incidence rate of meningococcal meningitis worldwide. This acute infection of the meninges occurs here with pronounced seasonality (seasonal hyperendemicity), sporadic localised epidemics and epidemic waves which emerge every 7–10 years1. The meningitis belt is characterized by six months of dry season and low annual precipitation amounts of 300–1100 mm2 during one single rainy season. During a localised meningitis epidemic (LME), the attack proportion can reach around 1% within a few weeks These LME usually do not concern all health centres of a district, but only a small subset3, 4. During epidemic waves, the frequency of LME is increased during 1–3 consecutive years. In any instance, case fatality is about 10% despite timely treatment and 20% of survivors have long-term psychomotor sequelae5.
The mechanisms behind the meningitis belt epidemiology are only partially understood. While the seasonal hyperendemicity is most likely related to the climatic conditions (low relative air humidity and dust-loaded wind)6, 7, the occurrence of epidemic waves could in part be explained by meningococcal strain evolutions and population immunity8,9,10. However, the factors leading to LME remain unknown. A hypothetical explanatory model proposed that a viral respiratory infection epidemic in a given population may act as an epidemic co-factor1, by rapidly increasing meningococcal transmission and acquisition in the nasopharynx. This would lead to a proportional increase in disease, on the background of high risk of invasive disease given carriage during the dry season11. If this hypothesis was correct, routine surveillance data of meningitis-belt countries would show an association between episodes of high incidence rates of acute respiratory infection and the occurrence of localised meningitis epidemics during the dry season. To explore this hypothesis, we conducted an ecological study based on routinely notified respiratory infections and bacterial meningitis from Burkina Faso.
Burkina Faso, which lies in the meningitis belt, introduced a meningococcal serogroup A conjugate vaccine by mass campaign into the 1- to 29-year-old population during 201012. This vaccine introduction appears to have eliminated serogroup A meningitis epidemics, while epidemics due to other serogroups (W, X, C) continue to occur9, 13. Burkina Faso also introduced Haemophilus influenzae type b and pneumococcal conjugate vaccine into the Expanded Program on Immunization in 2006 and 2013 respectively. These pathogens are, not involved in meningitis epidemics, although pneumococci substantially contribute to the seasonality of bacterial meningitis and cause outbreaks. Meningitis epidemics remain a major burden to the population and understanding the pathophysiological mechanism behind the phenomenon is needed to design appropriate and sustainable prevention strategies.
Methods
Data compilation
We used routine surveillance data at health centre resolution from 13 health districts in Burkina Faso, including weekly case counts of suspected acute bacterial meningitis3 and monthly case counts of clinically suspected upper (URTI) and lower (LRTI) respiratory tract infections. According to the national surveillance guidelines, URTI cases included otitis, severe sore throat and rhinopharyngitis; LRTI cases included pneumonia, bronchitis and bronchopneumonia. Two health regions (Hauts-Bassins and Nord) and two additional health districts (Boulsa in Centre-Nord region and Dédougou in Boucle du Mouhoun region), were selected for pre-existing research collaboration and history of occurrence of epidemic events. We contacted their district statistical offices to retrieve health centre level data for the period 2004 through 2014. For one of five districts in the Nord region, no data on respiratory infections could be retrieved for any year (Yako). Overall, 13 districts provided thus data (Fig. 1). Due to logistical constraints, 2004–2005 data were collected for three districts (Houndé, Orodara, Séguenéga) only, and 2013–2014 data for five districts (Boulsa, Dédougou, Houndé, Orodara, Séguenega), only. These districts are situated across the four regions. Ninety-five health centres in the Nord region were excluded as population size was unknown. Another 13 district years were excluded due to missing data on respiratory infections. The data used in this project had previously been collected by health authorities for the purpose of routine surveillance and disease control. The data consists of aggregated case counts without any individual-level information. As standard clinical procedures were not changed by routine surveillance and individual data were not recorded, no informed consent had been collected from patients. The present project of data analysis received authorisation from the Department of Disease Control (Direction de la lutte contre la maladie, DLM) at the Ministry of Health, Burkina Faso.
To validate the resulting database, we aggregated the observations into district weeks and compared them to the number of weekly cases per district routinely reported by the Ministry of Health to the World Health Organization. Based on over 4,000 district weeks, 62% of the health centre weeks showed a mismatch of ≤2 cases and 93% of ≤5 cases (median error: 0).
Definition of outcome and exposure variables
The incidence threshold recommended by WHO for epidemic response (10 per 100,000) is applied at the district level. By contrast, to identify individual localised epidemic events, we defined LME based on a threshold of weekly incidence rate at the health centre level, as previously reported4. The best performance in identifying epidemic annual incidences of ≥0.8% in individual health centre was obtained with a weekly threshold of ≥75 cases per 100,000 inhabitants during at least two consecutive weeks with at least 5 cases per week (sensitivity 98.5%, specificity 98.0%). Because URTI and LRTI data were available at monthly intervals only, months were categorized according to the identification of at least one week of LME. For a sensitivity analysis, we defined LME as a monthly meningitis incidence of 125 cases per 100,000. We assigned a predominant serogroup to each LME based on district level laboratory reports by the Ministry of Health. Because LE were identified only during January-May, we restricted the analyses to this period.
To avoid bias due to certain surveillance practices or population size estimation error, we excluded 722 health centre months which reported incidences of URTI or LRTI above the 95th percentile for >12 consecutive months. We categorised URTI and LRTI monthly incidences by quintiles across all health centre months, and considered episodes of URTI and LRTI above the 5th quintile (≥2.47 per 1000 inhabitants for URTI and ≥13.47 for LRTI) as high incidence episodes. Primary analyses took into account URTI, LRTI and LE observations during the same month. We created a secondary exposure variable to evaluate the association between LE during a given month and the level of URTI and LRTI during the month prior. We further differentiated months with first occurrence of LRTI or URTI incidences in the 5th quintile, from those where high incidences were already observed during the previous month. Finally, we conducted separate analyses grouping years with high serogroup A incidence (206–2008) and those with predominance of serogroups W and X (2010–2014). For these additional analyses, lower quintiles were collapsed to obtain sufficient statistical power.
Statistical analyses
As the outcome was epidemic/not epidemic and the data contained a spatial structure with monthly observations clustered within health centres, which lie within districts, we used a mixed-effects model for binary distribution specifying nested random effects for health centre and for districts with unstructured covariance (Stata command melogit). In the main analysis, a fixed effect for calendar year was introduced. In an alternative analysis, we included a crossed effects component with calendar year as a third random effect; this model required substantially greater computational effort, but yielded similar odds ratios (<10% difference). All analyses were conducted in Stata/IC14.1 (StataCorp. 2015).
Confounding variables
Potential confounders of the association between URTI or LRTI episodes and LME could be factors that increase transmission or disease susceptibility for meningococcal and respiratory disease pathogens locally and for a limited duration. We therefore included dust conditions, relative air humidity and calendar month into the multivariate models. For dust conditions, we used the MODIS deep-blue aerosol optical thickness (AOT) product at a daily time-step, which has been shown to be a good proxy for the dust load on the ground surface in Burkina Faso during the dry season14. The deep blue AOT, initially available at a 10 km-spatial resolution, were subsampled on a 1 km × 1 km grid, and pixels lying within the health centre coverage region were averaged. Values were further averaged on a monthly scale. We categorised monthly AOT values in quartiles across health centre months from January through to May. Data on monthly means of relative air humidity for the main city of each health region were extracted from the website tutiempo.net (http://tutiempo.net). No data were available for the Centre-Nord region (Boulsa district). We used three categories of relative air humidity <20%, 20–39.9% and ≥40%.
Data availability statement
The data that support the findings of this study are available from Direction de lutte contre la maladie (DLM), Ministry of Health of Burkina Faso, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data related to the present work are however available from the authors upon reasonable request and with permission of DLM, Ministry of Health of Burkina Faso. For this purpose, please contact the corresponding author JEM.
Results
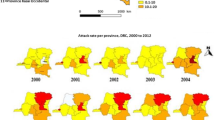
Among the 512 health centres included, which corresponded to 67,584 health centre months theoretically targeted, meningitis or population size data were not be retrieved for 33,477 health centre months (Fig. 1). Reasons included partial collection for most ancient and most recent years, missing population size (in particular for private clinics or regional reference hospitals) and missing meningitis data (in particular for health centre months prior to the creation of a new health centre). Meningitis incidence data were thus available from 427 health centres (34,107 health centre months), during which 15,420 suspected meningitis cases were reported. The typical incidence peak at the end of March was found in all districts, but declined over the observed time period (Fig. 2, and supplementary material S1 and S2a ). Particularly high incidences were observed during the epidemic wave from 2006 to 2008. We identified 72 LME, corresponding to 114 health centre months. Until 2008, all identified LME were related to serogroup A. After 2011, following serogroup A conjugate vaccine introduction, only serogroups W and X were found.
Overall, 401,110 URTI cases and 1,681,212 LRTI cases were reported during the observation period, with monthly incidence rates showing two seasonal peaks in October and February (supplementary material Figure S2b,c). Half (44% and 46%, respectively) of URTI and LRTI cases were reported from January through to May, even though incidences did not vary substantially across months during this period (Fig. 3). About half of the high URTI incidence episodes lasted for four months or longer. AOT showed the typical maximum during the month of March15 and relative air humidity increased from January towards the end of the dry season in May (Fig. 4).
For the period between January and May, we excluded health centre months with continuous peak URTI or LRTI incidences. We retained 10,334 health centre months for which data on meningitis and URTI or LRTI incidence were available (Fig. 1). These included 10,311 health centre months for URTI and 10,319 health centre months for LRTI; and 51 LME events, corresponding to 85 health centre months with LME (Table 1).
An LME was present during 0.8% of the health centre months considered, and this proportion increased from 0.2 to 1.8% across URTI quintiles (Table 2). The corresponding crude odds ratios (95% confidence interval (CI)) showed an increased risk of LME with increasing URTI incidence quintiles, with an OR of 10.10 (3.25–31.34) in the 5th quintile (Table 3) and a significant effect from the 3rd quintile onwards. After multiple adjustments, odds ratios were higher, however with wider confidence intervals. The crude odds ratio (95% CI) tended to be substantially but not significantly greater when a high incidence episode had been observed the month prior (12.23 (3.86–38.75)) compared to first-time episodes (6.07 (1.61–22.86)) (supplementary material Table S1). A weaker association was observed with a one month lag, linking LME to high URTI incidence episodes during the previous month (2.44, 1.07–5.59) (supplementary material Table S1). Strong associations were observed in analyses including the period between 2006 and 2008, where epidemic waves were due to serogroup A (5th vs 1st−4th quintile, crude OR 3.26, 95% confidence interval 1.62–6.57) or for the period from 2010 onwards, when only serogroups W and X were found (14.22, 0.91–223.18) (supplementary material Table S1). In the sensitivity analysis using a less specific but more sensitive LME definition of 125 monthly cases per 100,000, a similar increase of odds ratios across URTI quintiles was observed, with an OR of 4.89 (2.55, 9.38) for the fifth quintile.
An LME was present in 0.3 to 1.4% of health centre months across quintiles of LRTI (Table 2), however without any trend. The odds ratios (95% CI) varied from 0.41 (0.16–1.06) to 1.89 (0.97–3.69), without any trend (Table 4). A strong and significant association was observed from the fourth LRTI incidence quintile upwards only when adjusting for calendar year (5th quintile, 6.68 (1.94–23.07)) (Table 4). However, in analyses separating periods 2006–2008 and 2010–2014, the 5th quintile was significantly associated with LME occurrence, compared to other quintiles (supplementary material Table S1). The association between LME and the 5th LRTI quintile was weaker when the high incidence episode existed already during the month prior compared to first-time events (supplementary material Table S1).
Discussion
In this analysis of health centre surveillance data from Burkina Faso spanning a decade, we found that high incidence episodes of URTI were strongly associated with the occurrence of localised meningitis epidemics. The association with LRTI was inconsistent and only seen after adjusting for calendar year.
These findings strengthen the hypothesis that acute respiratory tract infections act as co-factors for the occurrence of localised meningitis epidemics in the meningitis belt1, 16. The trend of a gradually increasing risk of LME with increasing URTI incidence or duration, the persistence of the association with a time lag and the strength of the observed association may be considered as arguments for a causal relation.
At the individual level, (viral) inflammation of the nasopharygeal mucosa facilitates bacterial adhesion and colonization, including that of meningococci17, 18, which can result in increased transmission in the community. This effect is supported by associations between meningococcal carriage and clinical pharyngeal inflammation that had previously been described during meningitis epidemics19, 20, in contrast to absence of this association outside epidemics, as reported by a large multi-country carriage study21. The biological effect could be emphasized by behavioural changes induced by respiratory infections, such as sneezing and coughing, which can further facilitate meningococcal transmission. At the population level, such accelerated transmission is supported by the over10 times increase in meningococcal carriage prevalence during epidemic situations, as observed by most studies during meningitis epidemics11, 20. According to the hypothetical model cited above1, the surge in transmission causes the epidemic increase in meningitis incidence, mediated by a high case-carrier ratio during the dry season11. The high risk of invasive disease given carriage, in turn could be related to the damage of the mucosal barrier function during periods of low humidity and high dust load.
Viral especially influenza infections lead to immune depression, which facilitates the development of invasive bacterial disease, including meningococcal disease22. Studies during meningitis epidemics in the meningitis belt or outbreaks in Europe have reported strong associations between influenza infection or flu-like symptoms and meningitis15, 23, 24. While we believe that accelerated bacterial transmission should be the main mechanism behind the hypothesised link between RTI and meningitis epidemcis, this effect could further accelerate the emergence of an LME. In our analysis, an association between LRTI and LME was seen only after adjustment for calendar year. This adjustment considerably increased odds ratios for both URTI and LRTI, suggesting negative confounding. Given the limited number of LME included overall, caution is required when interpreting the year-adjusted results. The difference in odds ratio between URTI and LRTI may mean that inflammation of the nasopharynx is required to obtain the epidemiogenic effect in question; or that the incriminated pathogen rarely causes LRTI. However, given the limited quality of the available RTI data, we cannot conclude on the absence of an effect from LRTI.
The limited number of LME did not allow serogroup-specific analysis, but a strong association between URTI and LME was seen during epidemic waves due to serogroup A and epidemics due to serogroups W or X. The recent epidemic wave due to a new meningococcal serogroup C strain in Nigeria and Niger9 was constituted of localised epidemics that were spread across the epidemic regions without any systematic pattern25. In the light of the present findings, the epidemic emergence could be interpreted as a coincidence between the introduction of a new meningococcal strain and the occurrence of respiratory pathogen outbreaks.
URTI usually show mild clinical symptoms and do not lead to medical consultation, especially in the low resource settings of Burkina Faso. In consequence, our URTI incidences based on consultations are probably largely underestimated. Furthermore, URTI and LRTI reporting may have been neglected during LME due to the strain on the health care system. Both would have led to an underestimated association between URTI or LRTI and LME.
No laboratory information was available for any of the reported respiratory infections. One case-control study during a meningitis epidemic in Chad reported higher prevalence of mycoplasmal and viral infection among cases compared to controls16. Investigating the nature of the respiratory pathogen (a specific pathogen or a generic effect of various pathogens) will require clinical studies during LME. In theory, meningococcal colonisation itself could cause URTI symptoms; however, if so, they would be mild and not motivate seeking medical care.
Our study has several limitations. First, we may not have adjusted for all relevant confounding factors. Although the ecological level is appropriate in analysing risk factors for epidemic occurrence, no conclusion on a causal link can be made from this type of study, as several behavioural and biological aspects, which are only available from individual level studies (eg, crowded living conditions, smoking, complement deficiencies), may interplay26. Although we had collected information on social events, cultural context and vaccination campaigns with polysaccharide meningococcal vaccines, the exhaustiveness of the data did not allow inclusion in the present analysis.
The routine nature of the data limits the validity of our results. Misclassification between URTI and LRTI is likely, as clinical symptoms overlap. The reporting practice may have evolved over the observed period, in particular for LRTI, targeted for pandemic flu preparedness during that decade. Also, reporting practice probably varied between health regions and districts. Although these effects should have been taken into account by the mixed effects logistic regression models, reporting artefacts may occur. Finally, our analysis assumes that changes in RTI reporting incidence are parallel to changes in the true RTI incidence in the general population, which may not be the case, given social and behavioural variations or seasonality of other diseases.
We have selected aerosol load and relative air humidity to represent climatic influences that could explain the observed association, and we acknowledge that other factors, such as temperature or wind speed could be considered in future analyses investigating the hypothesis.
Our study shows high incidence rates of acute respiratory infections in Burkina Faso, with seasonal peaks. A study in Benin found that an increase of consultations for LRTI occurs during the cold dry season27. Further evaluation of the specific burden of respiratory diseases in the meningitis belt and their association with climatic factors is needed.
In conclusion, this study adds evidence to the hypothesis that URTI outbreaks during the dry season function as co-factors for localised meningitis epidemics. Depending on the aetiology, this may open opportunities for the development of serogroup-unspecific preventive interventions at the population level, including vaccination against the involved pathogen. Given the continuing difficulties in preventing and controlling epidemics of all meningococcal serogroups, any additional preventive intervention may be beneficial. Beyond this, the described association represent a striking example of the interactions between climate, bacteria and viruses that threaten human health.
References
Mueller, J. E. & Gessner, B. D. A hypothetical explanatory model for meningococcal meningitis in the African meningitis belt. Int J Infect Dis. 14, e553–9 (2010).
Ibrahim, B., Karambiri, H., Polcher, J., Yacouba, H. & Ribstein, P. Changes in rainfall regime over Burkina Faso under the climate change conditions simulated by 5 regional climate models. Clim Dyn. 42, 1363–81 (2013).
World Health Organization. Control of epidemic meningococcal disease: WHO practical guidelines. http://www.who.int/csr/resources/publications/meningitis/WHO_EMC_BAC_98_3_EN/en/index.html (1998).
Tall, H. et al. Definition and characterization of localised meningitis epidemics in Burkina Faso: a longitudinal retrospective study. BMC Infect Dis. 12, 2 (2012).
Chang, Q., Tzeng, Y. L. & Stephens, D. S. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 4, 237–45 (2012).
Yaka, P. et al. Relationships between climate and year-to-year variability in meningitis outbreaks: a case study in Burkina Faso and Niger. Int J Health Geographics. 7, 34 (2008).
Martiny, N. & Chiapello, I. Assessments for the impact of mineral dust on the meningitis incidence in West Africa. Atmospheric Environment. 70, 245–53 (2013).
Irving, T. J. et al. Modelling meningococcal meningitis in the African meningitis belt. Epidemiol Infect. 140, 897–905 (2012).
Kretz, C. B. et al. Whole-genome characterization of epidemic Neisseria meningitidis serogroup C and resurgence of serogroup W, Niger, 2015. Emerg Infect Dis. 22, 1762–8 (2016).
Caugant, D. A. et al. Molecular characterization of invasive meningococcal isolates from countries in the African meningitis belt before introduction of a serogroup A conjugate vaccine. PLoS ONE. 7, e46019 (2012).
Koutangni, T., Maïnassara, H. B. & Mueller, J. E. Incidence, carriage and case-carrier ratios for meningococcal meningitis in the African meningitis belt: a systematic review and meta-analysis. PLoS ONE. 10, e0116725 (2015).
Diomandé, F. V. et al. Public health impact after the introduction of PsA-TT: the first 4 years. Clin Infect Dis. 61(Suppl), S467–72 (2015).
Xie, O., Pollard, A. J., Mueller, J. E. & Norheim, G. Emergence of serogroup, X meningococcal disease in Africa: need for a vaccine. Vaccine. 31, 2852–61 (2013).
Deroubaix, A., Martiny, N., Chiapello, I. & Marticorena, B. Suitability of OMI aerosol index to reflect mineral dust surface conditions: preliminary application for studying the link with meningitis epidemics in the Sahel. Remote Sensing Environment. 133, 116–27 (2013).
Marticorena, B. et al. Temporal variability of mineral dust concentrations over West Africa: analyses of a pluriannual monitoring from the AMMA Sahelian Dust Transect. Atmos Chem Phys. 10, 8899–915 (2010).
Moore, P. Respiratory viruses and mycoplasma as cofactors for epidemic group A meningococcal meningitis. JAMA. 264, 1271–5 (1990).
Avadhanula, V. et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 80, 1629–36 (2006).
Fainstein, V., Musher, D. & Cate, T. Bacterial adherence to pharyngeal cells during viral infection. J Infect Dis. 141, 172–6 (1980).
Mueller, J. E. et al. Association of respiratory tract infection symptoms and air humidity with meningococcal carriage in Burkina Faso. Trop Med Int Health. 13, 1543–52 (2008).
Raghunathan, P. L. et al. Predictors of immunity after a major serogroup W-135 meningococcal disease epidemic, Burkina Faso, 2002. J Infect Dis. 193, 607–16 (2006).
The MenAfriVac Consortium. The diversity of meningococcal carriage across the African meningitis belt and the impact of vaccination with a group A meningococcal conjugate vaccine. J Infect Dis. 212, 1298–1307 (2015).
Cartwright, K. et al. Influenza A and meningococcal disease. Lancet. 338, 554–7 (1991).
Mueller, J. E. et al. Study of a localized meningococcal meningitis epidemic in Burkina Faso: incidence, carriage, and immunity. J Infect Dis. 204, 1787–95 (2011).
Makras, P., Alexiou‐Daniel, S., Antoniadis, A. & Hatzigeorgiou, D. Outbreak of meningococcal disease after an influenza B epidemic at a Hellenic air force recruit training center. Clin Infect Dis. 33, e48–50 (2001).
Maïnassara, H. B. et al. Evaluation of response strategies against epidemics due to Neisseria meningitidis C in Niger. Trop Med Internat Health. 22, 196–204 (2017).
Agier, L. et al. Towards understanding the epidemiology of Neisseria meningitidis in the African meningitis belt: a multi-disciplinary overview. Int J Infect Dis. 54, 103–12 (2016).
De Longueville, F., Hountondji, Y. & Henry, S. Relations potentielles entre infections respiratoires aiguës basses et conditions météorologiques au Bénin [Potential relations between lower respiratory tract infections and meteorological conditions in Benin]. Environ Risque Sante. 12, 139–50 (2013).
Acknowledgements
We acknowledge the work of the Burkina Faso health centre nurses who reported cases in parallel to clinical care over one decade. We thank regional and district level health officers and statisticians for their collaboration in the data compilation. Compilation of the health centre level data was supported by the World Health Organization. This work was conducted with support from the TELEDM project located at Centre de Recherches de Climatologie (CRC)/BIOGEOSCIENCES (University of Burgundy) and funded by Centre national d’études spatiales (CNES). It was conducted in the frame of a Masters internship of the ENS, École normale Supérieure, Paris (MW).
Author information
Authors and Affiliations
Contributions
J.E.M. and H.T. designed the work. J.E.M., M.W., S.P., H.T., N.M. implemented data compilation and preparation. J.E.M. and Y.M. conducted data analyses. All authors contributed to interpretation of the results and have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mueller, J.E., Woringer, M., Porgho, S. et al. The association between respiratory tract infection incidence and localised meningitis epidemics: an analysis of high-resolution surveillance data from Burkina Faso. Sci Rep 7, 11570 (2017). https://doi.org/10.1038/s41598-017-11889-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11889-4
- Springer Nature Limited
This article is cited by
-
High-spatial resolution epidemic surveillance of bacterial meningitis in the African meningitis belt in Burkina Faso
Scientific Reports (2022)
-
Risk factors for meningococcal disease in children and adolescents: a systematic review and META-analysis
European Journal of Pediatrics (2020)