Abstract
Chaperonin and cochaperonin, represented by E. coli GroEL and GroES, are essential molecular chaperones for protein folding. The double-ring assembly of GroEL is required to function with GroES, and a single-ring GroEL variant GroELSR forms a stable complex with GroES, arresting the chaperoning reaction cycle. GroES I25 interacts with GroEL; however, mutations of I25 abolish GroES-GroEL interaction due to the seven-fold mutational amplification in heptameric GroES. To weaken GroELSR-GroES interaction in a controlled manner, we used groES 7, a gene linking seven copies of groES, to incorporate I25 mutations in selected GroES modules in GroES7. We generated GroES7 variants with different numbers of GroESI25A or GroESI25D modules and different arrangements of the mutated modules, and biochemically characterized their interactions with GroELSR. GroES7 variants with two mutated modules participated in GroELSR–mediated protein folding in vitro. GroES7 variants with two or three mutated modules collaborated with GroELSR to perform chaperone function in vivo: three GroES7 variants functioned with GroELSR under both normal and heat-shock conditions. Our studies on functional single-ring bacterial chaperonin systems are informative to the single-ring human mitochondrial chaperonin mtHsp60-mtHsp10, and will provide insights into how the double-ring bacterial system has evolved to the single-ring mtHsp60-mtHsp10.
Similar content being viewed by others
Introduction
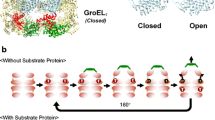
Molecular chaperone Hsp60 and its cochaperone Hsp10, also called chaperonin and cochaperonin, are highly conserved among the three domains of life1, and they are essential for cellular viability by mediating folding of cellular proteins. Hsp60 is the only molecular chaperone that is required for cell growth under normal and stressful conditions2. The E. coli GroEL and GroES have served as the paradigm for detailed mechanistic understandings of the chaperonin system3,4,5,6,7. GroEL consists of two heptameric rings stacked back-to-back, to form two functionally correlated folding cavities8. Each GroEL monomer consists of three domains. The apical domain, located at the opening of the folding cavity, binds the misfolded protein substrate and the cochaperonin GroES. The equatorial domain, located at the bottom of the folding cavity, binds ATP and forms inter- and intra-ring interactions. The intermediate domain connects the apical and equatorial domains and transmits signals between the two domains. GroES consists of one heptameric ring9, 10, and binds to the end of one GroEL ring to form an enclosed chamber for the folding of the substrate protein11. Three GroES residues I25/V26/L27 from a loop, termed the GroES mobile loop, interact with residues from the GroEL apical domain via hydrophobic interaction. The GroEL-interfacing tri-peptide sequence is highly conserved in the cochaperonin family1, suggesting the conserved chaperonin-cochaperonin interface. In a chaperonin-mediated folding reaction, the misfolded substrate protein is captured into the folding cavity via the apical domain. ATP binding to the substrate-loaded GroEL ring causes a series of large conformational changes, priming the ring for GroES binding, and binding of GroES sequesters the bound substrate into the newly formed enclosed folding chamber, initiating the folding process of the substrate. Binding of ATP to the GroEL ring opposite to the GroES-bound ring and the subsequent ATP hydrolysis dissociate GroES from GroEL, and release the folding substrate.
The above trans-ring allosteric effect of ATP binding/hydrolysis on GroES dissociation and substrate release is essential, and the two-ring assembly of GroEL is required for the GroEL-GroES chaperone function. Interestingly, human mitochondrial mtHsp60 exists as a single heptameric ring12, 13. mtHsp60 interacts with its cochaperonin mtHsp10 only transiently14, and as such dissociation of mtHsp10 and release of folding substrate from mtHsp60 do not require the trans-ring allostery driven by the ATP binding/hydrolysis as seen in the double ring GroEL-GroES system (above). However, the model that mtHsp60-mtHsp10 functions as a single ring14, 15 has been challenged. It is proposed that although mtHsp60 exists as a single heptameric ring and interacts with heptameric mtHsp10, in the course of chaperone reaction cycle, two mtHsp60-mtHsp10 complexes associate via mtHsp60 equatorial domains to form a football shape (mtHsp60-mtHsp10)2 16. An mtHsp60 mutant bound with mtHsp10 was crystalized in the football conformation17, 18, however, the structure does not explain why the two mtHsp60-mtHsp10 molecules associate into the (mtHsp60-mtHsp10)2 football conformation. Thus, whether mtHsp60-mtHsp10, or broadly the chaperonin system, may operate via a single-ring mechanism is still not certain. The ability to function as single ring suggests an evolutionary adaptability of the chaperonin family.
To identify a functional single-ring chaperonin system, we set out to convert a nonfunctional single-ring GroEL variant, GroELSR, by modifying its interaction with GroES. GroELSR has four mutations (R451A/E461A/S463A/V464A) to disrupt the inter-ring contact19. Although the GroELSR-GroES cavity allows misfolded substrates to undergo folding to the native conformation20,21,22,23, it traps and does not release the substrates. GroELSR-GroES has t1/2 = 300 min−1 19, considerable longer than the ~15 s lifetime of the GroEL-GroES complex24, 25. Failure to release folding substrates accounts for the inability of GroELSR–GroES to support cell growth26. Mutations in GroELSR allow the single-ring GroELSR-GroES to substitute the double ring GroEL-GroES in supporting cell growth under the normal condition27, 28, and some mutations also support cell growth under the heat stress condition29. However, mechanistic understandings of these single-ring variants are limited as the mutational effects are most likely allosteric. Similarly, genetic analysis of GroES residues (G24/I25/V26/L27) on the GroEL-GroES interface has identified GroES mutants collaborate with GroELSR at lower temperatures (18 °C and 30 °C); however, little biochemical characterization of the mutational effects is available30.
A direct mutation on groES impacts all seven GroES subunits in the GroES heptamer. To avoid this inherent mutational amplification and to incorporate mutations selectively into specific GroES subunits, we generated a concatenated gene groES 7 that links seven groES genes to express a continuous polypeptide GroES7 with seven GroES modules31. We used groES 7 to incorporate mutations in specific GroES modules in GroES7 to modify the GroEL-GroES interface in a controlled manner. We hypothesized that modifying the chaperonin/cochapernonin interaction would activate the single-ring GroELSR-GroES. In our earlier study, we generated GroES7 variants with reduced affinities for GroELSR and identified active GroES7 variants including GroES7I25D1,4 for GroELSR-mediated folding of malate dehydrogenase (MDH). Based on these previous findings, in the current study we designed and generated comprehensive GroES7 variants, to systematically modulate binding of GroES7 to GroELSR. We characterized their interaction with GroELSR, their activity in assisting in protein folding and their in vivo chaperone function. We found that three GroES7 variants functioned with GroELSR in supporting cell growth under both normal and heat shock conditions.
Results
We sought to create functional single-ring GroELSR-GroES chaperonin systems that support cell growth under normal and heat shock conditions. GroELSR-GroES has been shown to perform folding of substrate proteins, but its inability to release the folding substrate arrests the folding cycle, obstructing the chaperone function. To weaken GroELSR-GroES interaction thereby to resume cycling of the folding reaction, here we systematically modified the GroELSR-GroES interaction using a concatenated gene groES 7 we generated previously31.
Mutations of GroES I25A and L27A have the same effect as I25D and L27D in abolishing GroEL-GroES interaction
The GroEL-GroES interaction can be characterized via three assays: the ATPase activity, since binding of GroES inhibits GroEL’s ATPase activity by 50%25, 32, 33, the enzymatic activity of malate dehydrogenase (MDH) since efficient folding of MDH requires not only the formation but also dissociation of the GroEL-GroES folding cavity34, and measurement of dissociation constant (Kd). GroES interacts with GroEL via a tri-peptide I25/V26/L27 region11, and our previous study showed mutations of either I25D or L27D but not V26D in GroES abolish GroES’s interaction with GroEL31. Specifically, we showed that both GroESI25D and GroESL27D mutants did not inhibit GroEL’s ATPase activity, did not participate in GroEL-mediated MDH folding, and no stable GroEL-GroES complex could be isolated. We reasoned that a conserved mutation to Ala would have a less detrimental effect and would not completely abolish the hydrophobic GroEL-GroES interaction. As shown in Fig. 1A, both GroESI25A and GroESL27A did not inhibit GroEL’s ATPase activity, suggesting that both Ala mutations abolished GroEL-GroES interaction. Additionally, no MDH activity was observed in either GroEL-GroESI25A or GroEL-GroESL27A (Fig. 1B), indicating that neither GroESI25A nor GroESL27A collaborated with GroEL in assisting folding of MDH. Finally, we measured GroEL-GroES interaction using microscale thermophoresis (MST). Both I25A and L27A mutations decreased GroES’s binding affinity to GroEL by >1,000 fold from Kd’s values of 3.83 (±0.93) nM to >5 uM (Supplementary Table S1).
Effects of substituting I25 and L27 with Asp or Ala. (A) ATPase activities of GroEL (grey columns) and GroELSR (black columns) in the presence of various GroES variants. GroES inhibited the ATPase activities of GroEL and GroELSR to ~50% and ~10%, respectively. Mutations of I25A, I25D, L27A, and L27D relieved the inhibition on both GroEL and GroELSR. Experiments were carried out at least three times, and error bars are standard deviations of the experiments. (B) Refolding of MDH in the presence of GroEL or GroELSR with various GroES variants. The enzymatic activity of native MDH is set to 100%. GroES participated in GroEL-mediated MDH folding with ~80% yield, and in GroELSR-mediated MDH folding with ~10% yield. The GroES variants are associated with minimal MDH folding yield.
We next evaluated the Ala mutational effect on GroES’s interaction with the single-ring GroELSR. Wild type GroES has a strong binding affinity for GroELSR as shown in the three aspects: it inhibits the ATPase activity of GroELSR by ~90%19, GroELSR-GroES traps the refolding MDH resulting in lack of MDH activity21, and the GroELSR-GroES complex is highly stable with a slow dissociation rate19. We have shown that either I25D or L27D mutations in GroES abolish GroELSR–GroES interaction31. Figure 1A shows that like GroESI25D and GroESL27D, neither GroESI25A nor GroESL27A affected ATP hydrolysis of GroELSR, suggesting that they did not interact with GroELSR. Also similar to their Asp counterparts, neither GroESI25A nor GroESL27A collaborated with in GroELSR in actively refolding MDH (Fig. 1B). Finally, like the Asp variants, the GroES Ala variants did not show binding affinity for GroELSR based on MST (data not shown).
Together, the Ala mutations at I25 and L27 drastically abolished GroES’s binding to both GroEL and GroELSR, the same effect as observed with the Asp mutations. These findings suggest that the hydrophobic residue with extended side chain at positions 25 and 27 are important for productive GroES-GroEL interaction. Consistent with this finding, residues at these two positions in the GroES sequences from bacteria to human are mostly Ile and Leu and sometimes Met1.
One-, two- and three I25A or I25D mutated GroES modules in GroES7 gradually decreased GroEL-GroES7 interaction
The drastic mutational effect on abolishing GroEL-GroES interaction can be explained by the amplification effect that one mutation in groES affects all seven subunits in GroES. To control GroES’s affinity for GroEL in a systematic manner, we created a gene groES 7 in our previous study31. groES 7 links seven copies of groES to express a continuous polypeptide GroES7 with seven GroES modules, allowing us to mutate specific residue(s) at desired GroES module(s) in GroES7 to create combinations of the mutated and wild type GroES modules. We have shown that mutations of either I25D or L27D in one (1st), two (1st and 4th) and three (1st, 4th and 7th) GroES modules in GroES7 gradually decrease GroEL-GroES7 interaction and steadily relieve the strong inhibition on ATPase activity of GroELSR. Since I25D mutation displays greater mutational effect than L27D mutation31, we focused the current study on investigating the I25 mutational effects in GroES7. We generated extensive GroES7 variants with two- or three-I25D or I25A GroES modules. There are three unique ways to place two mutated GroES modules, so we had all six two-mutated variants, GroESI25A1,2, GroESI25A1,3, GroESI25A1,4, GroESI25D1,2, GroESI25D1,3 and GroESI25D1,4. We generated four variants with three mutated GroES modules: GroESI25A1,4,6, GroESI25A1,4,7, GroESI25D1,4,6 and GroESI25D1,4,7.
As the number of either the I25A or I25D modules increased in GroES7, the GroEL-GroES7 interactions decreased. For the I25A series, one mutated module, GroESI25A1, inhibited ATPase of GroEL to a level (53.2%) higher than that of GroES (42.5%). Two-mutated modules, GroESI25A1,2, GroESI25A1,3 and GroESI25A1,4, had markedly reduced inhibitions with the remaining ATPase activities of 58.5–62.8%. Three-mutated modules, GroESI25A1,4,6 and GroESI25A1,4,7, further relived the inhibition with the remaining ATPase activity of 79.0–82.9% (Fig. 2A and Supplementary Table S1). As expected, binding affinity of GroES7 for GroEL decreased as the number of the mutated module increased, with one-mutated module only moderately affecting affinity, two-mutated modules reducing the affinity by two folds, and three-mutated modules by more than 25 folds (Fig. 3A and Supplementary Table S1).
An increased number of the mutated GroES module also decreased both the yield and kinetics of GroEL-mediated MDH folding (Fig. 4A). One mutated module, GroESI25A1, decreased the folding yield from 80% to 72% and the folding kinetics modestly. Two mutated modules, GroESI25A1,2, GroESI25A1,3 and GroESI25A1,4, reduced the folding yield further to 62–65%, and slowed the folding kinetics by ~50% (Fig. 4A). Three mutated modules, GroESI25A1,4,6 and GroESI25A1,4,7, decreased the yield drastically to 30–37% and reduced the folding kinetics by ~85%. Paralleled trends in gradual increase in ATPase activity (Fig. 2B), decrease in binding affinity (Fig. 3A) and decrease in MDH folding activity (Fig. 4B) were found in presence of GroES7 variants with one-, two- and three-mutated GroESI25D modules (Supplementary Table S1).
Effects of substitutions in GroES7 on the GroEL-mediated (A and B) and GroELSR-mediated MDH folding (C and D). The enzymatic activity of native MDH is set to 100%. Experiments were repeated more than three times, and representative data from individual runs were shown. The MDH yields are summarized in Supplementary Tables S1 and S2.
Large mutational effect of GroES7 on GroELSR-GroES7 interaction
The above mutational effects of GroES7 were more pronounced in interactions with the single-ring GroELSR than with the double-ring GroEL. For the I25A mutation series, one mutated module, GroESI25A1, lifted the inhibition on ATPase of GroELSR from ~90% to 80% (Fig. 2C and Supplementary Table S1). Two mutated modules, GroESI25A1,2, GroESI25A1,3 and GroESI25A1,4, drastically relieved the inhibition to ~50%, a level as seen in the canonical GroEL-GroES system where GroES inhibits the ATPase activity of GroEL by 50%19, 24, 25. Three mutated modules, GroESI25A1,4,6 and GroESI25A1,4,7, further relived the inhibition to ~20%. In line with these ATPase studies of GroELSR, large mutational effects of GroES7 on the binding affinity for GroELSR were observed. One mutated module, GroESI25A1, reduced binding affinity for GroELSR by ~50% (Kd of 8.5 ± 3.3 nM from ~3.7 ± 2.2 nM), which is comparable to the effect of the two-mutated modules on binding affinity for the double ring GroEL (Fig. 3 and Supplementary Tables S1 and S2). Two mutated modules, GroESI25A1,2, GroESI25A1,3 and GroESI25A1,4, markedly reduced the binding affinity of GroES7 for GroELSR by > 10 folds (Fig. 3b and Supplementary Table S2), which is comparable to the effect of the three-mutated module of GroESI25A1,4,7 on binding affinity of GroES7 for GroEL. Three mutated modules, GroESI25A1,4,6 and GroESI25A1,4,7, appeared to abolish the binding affinity for GroELSR as no detectable binding was observed.
Mutational effects on GroELSR-mediated MDH folding
One mutated module in GroES7, GroESI25A1 and GroESI25D1, increased the GroELSR-mediated MDH folding yield from <10% to 20–30% (Fig. 4C and D; Supplementary Table S2). Two mutated modules, GroESI25A1,2, GroESI25A1,3 and GroESI25A1,4, GroESI25D1,2, GroESI25D1,3 and GroESI25D1,4, further improved the MDH folding with both the yield and kinetics comparable to the canonical double ring GroEL-GroES (Fig. 4C and D; Supplementary Tables S1 and S2). However, adding a third mutated module in GroES7I25A1,4 and GroES7I25D1,4, to create GroES7I25A1,4,7, GroES7I25A1,4,6, GroES7I25D1,4,7, and GroES7I25D1,4,6, reverted the folding yield to the minimum as seen with GroES (Fig. 4C and D). These findings using different types of mutation, Ala and Asp mutations, and extensive combinations of the mutated module recapitulate our previous results using a representative group of GroES7 variants, GroES7I25D1, GroES7I25D1,4 and GroES7I25D1,4,7 31. Thus, we concluded that GroES7 variants with two-mutated modules, irrespective to the positions of the mutated modules and the types of mutation, were effective and efficient in GroELSR-mediated MDH folding.
Mutational effects on in vivo chaperone function
We reasoned that the MDH-folding active chaperonin systems should have chaperone function, and examined whether the single-ring GroELSR-GroES7 systems were able to substitute the canonical double ring GroEL-GroES in supporting growth via a conditional lethal E. coli strain MGM10035. Interestingly, the ability to refold MDH is not correlated with the in vivo chaperone function. For example, of the six GroES7 variants with two-mutated modules, only GroES7I25A1,3 and GroES7I25D1,4 were able to function with GroELSR at both the optimal temperature of 37 °C and under heat shock temperature of 42 °C (Fig. 5). The three GroES7 variants with two-mutated modules, GroES7I25A1,2, GroES7I25A1,4 and GroES7I25D1,2, might partially function with GroELSR at 37 °C, but they did not function with GroELSR under heat shock. One GroES7 variants with two-mutated modules, GroES7I25D1,3, did not function even at 37 °C. In addition, all four GroES7 with three-mutated modules, despite their little activity in MDH folding, functioned with GroELSR at 37 °C; moreover, one of them, GroES7I25A1,4,7-GroELSR, was functional also at 42 °C. Finally, GroESI25A, with all seven mutated subunits, was functional with GroELSR at 37 °C, despite its inability to interact with GroELSR based on ATPase and MST assays and to refold MDH. The reason for the lack of correlation between MDH folding activity and in vivo chaperone function is not clear, however, it is noted that MDH is not the authentic cellular substrate for GroEL-GroES although MDH folding assay is commonly used in the chaperone field. Nevertheless, we identified three GroES7 variants, GroES7I25A1,3, GroES7I25D1,4 and GroES7I25A1,4,7, to function with the single-ring GroELSR in supporting cell growth under both the optimal and heat shock conditions. These GroES7 variants have mutations on the interface with GroEL that directly weaken the GroELSR-GroES interaction, providing the molecular basis for functional single-ring chaperonin system.
Discussion
The chaperonin system is essential for cellular viability by mediating folding of cellular proteins. The double-ring assembly of bacterial GroEL is required for the chaperone function, because the trans-ring allostery is required to dissociate the stably formed GroEL-GroES complex and to release the enclosed folding substrate protein. The human mitochondrial mtHsp60 may adopt a distinct single-ring mechanism because mtHsp60 exists as a single heptameric ring and has a lower affinity for mtHsp10. A recent model for mtHsp60-mtHsp10 suggests, however, that during the mtHsp60-mtHsp10 reaction cycle two mtHsp60-mtHsp10 complexes associate to form a football shape (mtHsp60-mtHsp10)2, suggesting that mtHsp60-mtHsp10 may not truly function in a single-ring mechanism. We sought to show that the chaperonin system may rely solely on the single-ring mechanism to execute the chaperone function, by activating a single-ring form of GroEL, GroELSR.
GroELSR is not functional with GroES because without the allostery from the absent second ring the tight GroELSR–GroES interaction traps folding protein substrates and arrests the chaperone reaction cycle. To obtain functional single-ring GroELSR-GroES by selectively weakening GroELSR–GroES interaction in a systematic manner, we utilized a novel reagent groES 7, that links seven groES to express GroES7 with seven genetically independent GroES modules. We created extensive GroES7 variants with one, two and three modules of either GroESI25A or GroESI25D mutations. We systematically characterized mutational effect on various activities of GroEL and GroELSR. We found that as the number of the mutated modules increased the inhibition on ATPase activity, the binding affinity and MDH folding activity of GroEL steadily decreased, suggesting that gradual decrease in GroEL-GroES7 interaction. Decreases in inhibiting ATPase activity of and in binding affinity for GroELSR were greater than as seen in GroEL, and suggested that GroES7 variants with mutated modules resumed a recyclable reaction with the single ring GroELSR. Notably in mediating MDH folding, GroES7 variants with two mutated modules were active with GroELSR with both the folding yield and kinetics comparable to the canonical double ring GroEL-GroES. Importantly, we found three GroES7 variants, GroES7I25A1,3, GroES7I25D1,4 and GroES7I25A1,4,7, were functional with GroELSR under both normal and heat shock temperatures.
The chaperonin-cochaperonin interaction is central for chaperonin to function as single ring. Early genetic screens isolated GroELSR variants that are functional with GroES at 37 °C27, and the chaperonin-cochaperonin interaction in these functional GroELSR-GroES systems is much weaker compared to GroEL-GroES27, 29, 36. Since these mutated GroELSR residues are not located in the GroEL-GroES interface, the mutational effects on GroELSR-GroES interaction are presumably allosteric and molecular basis for the allosteric effect remains unclear. Direct mutations on the GroELSR-GroES interface, G24/I25/V26/L27, in the GroES mobile loop, identified GroES variants GroESI25F and GroESI25L that appear functional with GroELSR at 37 °C30. Both variants decreased inhibition on the ATPase activity of GroELSR, suggesting their reduced interaction with GroELSR; however, no further characterizations on the GroELSR-GroES interaction have been reported. Our abilities to directly modulate the chaperonin-cochaperonin interface, shown in this and previous31 studies, confirm that reduced chaperonin-cochaperonin interaction is key to create functional single ring. We found that modifying two or three of the seven individual GroEL-GroES interactive surfaces is effective in rendering single ring GroELSR-GroES functional in vivo. Positions of the modified individual interfaces, 1,2, 1,3 and 1,4 or 1,4,6 and 1,4,7, have different effects on functionality of GroELSR-GroES. These findings support the structural observations that each of the GroEL-GroES interfaces, including conformations of both the GroES mobile loop and the GroEL Helix H and I, is unique37. In terms of interaction strength, we found that the working chaperonin-cochaperonin interaction for a functional single ring GroEL-GroES-based system follows the Goldilocks principle: interaction must not be too loose or too tight. Our studies provide the first step for future mechanistic investigations on the Goldilocks chaperonin-cochaperonin interaction of the single-ring chaperonin system.
Our results that the chaperonin system may rely on the single-ring mechanism are informative to the human mitochondrial chaperonin mtHsp60-mtHsp10. mtHsp60 exists predominately as single heptameric ring12 in equilibrium with the monomeric form16. The lack of the double ring conformation is consistent with its absence of the two conserved salt bridges (K105-D435 and E461-R452; residue naming according to GroEL) that are important to stabilize the inter-ring interaction38. In addition, compared to the stable GroEL-GroES complex (Kd of 0.1–26 nM20, 33, 34, 39, or 3.83 ± 0.93 nM of this study, in the presence of ADP), the reduced mtHsp60-mtHsp10 interaction14 supports the dispensable role of a second ring in the chaperoning reaction cycle. Further support for mtHsp60-mtHsp10 functioning in a single ring mechanism comes from the functional single ring GroELSR/mtHsp60 chimera14, 15. Interestingly, in the presence of both ATP and mtHsp10 two mtHsp60 heptameric rings appear to associate, forming the football (mtHsp60-mtHsp10)2 conformation16. Investigations on whether mtHsp60 undergoes an association to form a double ring conformation in the mtHsp60-mtHsp10 reaction cycle are hindered by the dynamic nature of mtHsp60 quaternary assembly and mtHsp60-mtHsp10 interaction. Genetic screens identified a mutant mtHsp60E321K with high affinity for mtHsp10, forming stable mtHsp60E321K-mtHsp10 and arresting the chaperone cycle40, reminiscent of GroELSR arresting GroEL-GroES cycle. mtHsp60E321K-mtHsp10 crystalized in the football conformation17, 18, that is, two heptameric mtHsp60E321K-mtHsp10 complexes associate via mtHsp60E321K. The two mtHsp60E321K heptameric rings interface via the equatorial domains as seen in GroEL, and as expected no charge-charge interactions in the place of the two conserved inter-ring salt bridges (K105-D435 and E461-R452) are observed. Strikingly, the inter-ring interface in mtHsp60E321K is twice as that in the naturally occurring double-ring GroEL. Such extensive inter-ring interface suggests a stable, GroEL-like double ring conformation, which is in direct contrast to the observed, single-ring conformation. Such extensive inter-ring interface may suggest cross-ring communication and regulation, justifying the assembly of the double ring conformation for biochemical activities. For example, the ATP-induced cross-ring allostery manifests in various aspects in GroEL-GroES. Notably, binding of ATP to one GroEL ring prevents ATP binding to the opposite ring41, and ATP binding in one ring initiates GroES dissociation from the opposite GroEL ring21. For mtHsp60-mtHsp10, the negative ATP binding cooperativity has not been reported, and mtHsp10 dissociates readily from mtHsp60 due to the weak interaction. Besides the lack of biochemical support, structure of (mtHsp60E321K-mtHsp10)2 does not offer structural insights into either cross-ring communication or the double ring assembly of the football conformation important for the mtHsp60-mtHsp10 reaction cycle. Thus, the mechanistic significance for association of two mtHsp60-mtHsp10 to form a football conformation of (mtHsp60-mtHsp10)2 is not clear, and whether the football conformation is the productive intermediate in the chaperone cycle is unknown. However, considering the complex cellular conditions, it is probable that two heptameric mtHsp60-mtHsp10 (mtHsp60) molecules might associate to form the double ring assembly as seen in structure of (mtHsp60E321K-mtHsp10)2. The cellular conditions favorable for molecular association include the abundance of cellular chaperonin (2.6 μM for GorEL42), the high concentration of cellular macromolecules (300–400 mg/ml in E. coli 43) and the macromolecular crowding effect43 that results in increasing the effective concentration of mtHsp60. While investigations on these important mechanistic aspects of mtHsp60-mtHsp10 continue, here we, in conjunction with previous studies14, 15, 27,28,29, show that the chapreonin can rely on the single-ring mechanism to function. Our results demonstrate the mechanistic adaptability of the chaperonin system, and our functional single ring GroELSR-GroES7 variants will provide valuable tools to study the molecular evolution of this ancient protein family from bacterial double-ring to human mitochondrial single-ring conformations.
Methods
Protein expression and purification
groEL and groEL SR (GroEL R452A/E461A/S463A/V464A) were in pTrc vector, groES was in pET3b, and groES 7 and the groES 7 variants were in a modified pET28b31. E. coli BL21(DE3) cells were used to express the proteins. Conditions for cell growth, induction of protein expression, and protein purification are described in ref. 31. To remove the residual proteins bound to GroEL or GroELSR, the chaperonins (1 mg/ml) were dialyzed against 50 mM TrisCl pH 7.5, 1 mM EDTA and 30% methanol, loaded onto a FastQ column (GE Healthcare), and eluted with 0–1 M NaCl gradient. The chaperonin-containing fractions were combined, dialyzed with TEA buffer (50 mM triethanolamine 7.5, 50 mM KCl and 20 mM MgCl2) and 0.1% NaN3 at 4 °C overnight. The purified chaperonins were verified with minimal Trp fluorescence.
ATPase activity assays via Malachite green
Chaperonins and cochaperonins were dialyzed into TEA reaction buffer containing 50 mM KCl and 20 mM MgCl2, to 0.125 μM tetradecameric chaperonins, and 0.3 μM heptameric cochaperonins. ATPase activity was measured via malachite green as described in ref. 31 at room temperature (22 °C) with 2 mM ATP as the starting concentration. Absorption at 660 nm (A660) was measured, and the final A660 values were averaged over three readings. The amount of hydrolyzed free phosphate was derived from a standard curve, and the hydrolysis rate was normalized to GroEL monomer. At least three independent experiments were performed.
MDH refolding assay
Chaperonins and cochaperonins were dialyzed into TEA reaction buffer. Malate dehydrogenase (Roche) was unfolded in TEA buffer including 3 M GdmHCl to a final concentration of 36.7 μM (monomeric MDH) for 60 minutes prior to the experiments. MDH refolding assay via monitoring the enzymatic activity of the refolded MDH at A340, was described in ref. 31. The final protein concentrations were 1 μM of GroEL or 2 μM GroELSR, 4 μM of cochaperonin, and 0.7 μM of monomeric MDH. The enzymatic activity of native MDH was set to 100%, and at least three independent experiments were performed.
Chaperonin-cochaperonin binding via microscale thermophoresis (MST) assay
GroES, GroES7 and GroES7 variants were fluorescently labeled with DyLightTM 650 NHS Ester Amine Reactive Dye (ThermoScientific) according to manufacturer’s protocol. The labeled chaperonin was separated from the free dye using MidiTrap (GE Healthcare) followed by dialysis (to 50 mM TrisCl pH 7.5, 100 mM KCl, 10 mM MgCl2, and 1 mM EDTA), and its concentration was measured using the Bradford assay. For each unlabeled proteins (GroEL or GroELSR), a serial dilution of 15 samples were prepared in the binding buffer (50 mM TrisCl pH 7.5, 100 mM KCl, 10 mM MgCl2, 1 mM EDTA, 2 mM ADP, and 0.5 mg/mL BSA). 10 ul of the unlabeled protein was incubated with 10 ul of the labeled cochaperonin for 30 min, and the solution was loaded into a glass capillary (NanoTemper Technologies) for MST measurements. The thermophoresis measurements were carried out using NanoTemper Monolith NT115 (NanoTemper Technologies) with 80% LED power and 40% IR-Laser power. At least three independent experiments were performed. Initial MST data were processed using Monolith NT115, and dissociation constant (Kd) was determined using KalidaGraph by fitting the following equation:
where m1 is the thermophoresis reading of the labeled cochaperonin in the absence of the unlabeled titrating protein, m2 is the thermophoresis reading when all the labeled cochaperonin was bound with the unlabeled titrating protein, and m3 is the Kd.
In vivo complementation assay
The MGM100 E. coli cell strain (kanamycin resistant, KanR) was obtained from the E. coli Genetic Stock Center at Yale University. pTrc is a lac promoter-based expression vector; the lac-based vector pBbE5c44 was used to express GroES, GroES7 and GroES7 variants. CaCl2 competent MGM100 cells were co-transformed with both plasmids and plated onto LB agar containing 50 μg/mL kanamycin, 100 μg/mL ampicillin, 50 μg/mL chloramphenicol, and 0.2% w/v arabinose. Conditions for cell growth and titration are described in ref. 29.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gupta, R. S. In The Chaperonins (ed R.J., Ellis) 27–64 (Academic Press, Inc., 1996).
Fayet, O., Ziegelhoffer, T. & Georgopoulos, C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol 171, 1379–1385 (1989).
Hartl, F. U. & Hayer-Hartl, M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858 (2002).
Horwich, A. L., Farr, G. W. & Fenton, W. A. GroEL-GroES-mediated protein folding. Chem Rev 106, 1917–1930 (2006).
Thirumalai, D. & Lorimer, G. H. Chaperonin-mediated protein folding. Annu Rev Biophys Biomol Struct 30, 245–269 (2001).
Sigler, P. B. et al. Structure and function in GroEL-mediated protein folding. Annu Rev Biochem 67, 581–608 (1998).
Lin, Z. & Rye, H. S. GroEL-mediated protein folding: making the impossible, possible. Crit Rev Biochem Mol Biol 41, 211–239 (2006).
Braig, K. et al. The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature 371, 578–586 (1994).
Hunt, J. F., Weaver, A. J., Landry, S. J., Gierasch, L. & Deisenhofer, J. The crystal structure of the GroES co-chaperonin at 2.8 A resolution. Nature 379, 37–45, doi:10.1038/379037a0 (1996).
Mande, S. C., Mehra, V., Bloom, B. R. & Hol, W. G. Structure of the heat shock protein chaperonin-10 of Mycobacterium leprae. Science 271, 203–207 (1996).
Xu, Z., Horwich, A. L. & Sigler, P. B. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature 388, 741–750 (1997).
Viitanen, P. V. et al. Purification of mammalian mitochondrial chaperonin 60 through in vitro reconstitution of active oligomers. Methods in enzymology 290, 203–217 (1998).
Viitanen, P. V. et al. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J Biol Chem 267, 695–698 (1992).
Nielsen, K. L. & Cowan, N. J. A single ring is sufficient for productive chaperonin-mediated folding in vivo. Mol Cell 2, 93–99 (1998).
Nielsen, K. L., McLennan, N., Masters, M. & Cowan, N. J. A single-ring mitochondrial chaperonin (Hsp60-Hsp10) can substitute for GroEL-GroES in vivo. J Bacteriol 181, 5871–5875 (1999).
Levy-Rimler, G. et al. The effect of nucleotides and mitochondrial chaperonin 10 on the structure and chaperone activity of mitochondrial chaperonin 60. European journal of biochemistry 268, 3465–3472 (2001).
Nisemblat, S., Parnas, A., Yaniv, O., Azem, A. & Frolow, F. Crystallization and structure determination of a symmetrical ‘football’ complex of the mammalian mitochondrial Hsp60-Hsp10 chaperonins. Acta crystallographica. Section F, Structural biology communications 70, 116–119, doi:10.1107/S2053230X1303389X (2014).
Nisemblat, S., Yaniv, O., Parnas, A., Frolow, F. & Azem, A. Crystal structure of the human mitochondrial chaperonin symmetrical football complex. Proceedings of the National Academy of Sciences of the United States of America 112, 6044–6049, doi:10.1073/pnas.1411718112 (2015).
Weissman, J. S. et al. Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell 83, 577–587, doi:0092-8674(95)90098-5 (1995).
Hayer-Hartl, M. K., Martin, J. & Hartl, F. U. Asymmetrical interaction of GroEL and GroES in the ATPase cycle of assisted protein folding. Science 269, 836–841 (1995).
Rye, H. S. et al. Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL. Nature 388, 792–798, doi:10.1038/42047 (1997).
Weissman, J. S., Rye, H. S., Fenton, W. A., Beechem, J. M. & Horwich, A. L. Characterization of the active intermediate of a GroEL-GroES-mediated protein folding reaction. Cell 84, 481–490, doi:S0092-8674(00)81293-3 (1996).
Ueno, T., Taguchi, H., Tadakuma, H., Yoshida, M. & Funatsu, T. GroEL mediates protein folding with a two successive timer mechanism. Mol Cell 14, 423–434, doi:S1097276504002618 (2004).
Burston, S. G., Ranson, N. A. & Clarke, A. R. The origins and consequences of asymmetry in the chaperonin reaction cycle. J Mol Biol 249, 138–152, doi:S0022-2836(85)70285-9 (1995).
Todd, M. J., Viitanen, P. V. & Lorimer, G. H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science 265, 659–666 (1994).
Weber, F., Keppel, F., Georgopoulos, C., Hayer-Hartl, M. K. & Hartl, F. U. The oligomeric structure of GroEL/GroES is required for biologically significant chaperonin function in protein folding. Nat Struct Biol 5, 977–985, doi:10.1038/2952 (1998).
Sun, Z., Scott, D. J. & Lund, P. A. Isolation and characterisation of mutants of GroEL that are fully functional as single rings. J Mol Biol 332, 715–728, doi:S0022283603008301 (2003).
Chatellier, J., Hill, F., Foster, N. W., Goloubinoff, P. & Fersht, A. R. From minichaperone to GroEL 3: properties of an active single-ring mutant of GroEL. J Mol Biol 304, 897–910, doi:10.1006/jmbi.2000.4278 (2000).
Illingworth, M., Salisbury, J., Li, W., Lin, D. & Chen, L. Effective ATPase activity and moderate chaperonin-cochaperonin interaction are important for the functional single-ring chaperonin system. Biochemical and biophysical research communications 466, 15–20, doi:10.1016/j.bbrc.2015.08.034 (2015).
Liu, H., Kovacs, E. & Lund, P. A. Characterisation of mutations in GroES that allow GroEL to function as a single ring. FEBS letters 583, 2365–2371, doi:10.1016/j.febslet.2009.06.027 (2009).
Illingworth, M., Ramsey, A., Zheng, Z. & Chen, L. Stimulating the Substrate Folding Activity of a Single Ring GroEL Variant by Modulating the Cochaperonin GroES. J Biol Chem 286, 30401–30408, doi:M111.255935 (2011).
Chandrasekhar, G. N., Tilly, K., Woolford, C., Hendrix, R. & Georgopoulos, C. Purification and properties of the groES morphogenetic protein of Escherichia coli. J Biol Chem 261, 12414–12419 (1986).
Jackson, G. S. et al. Binding and hydrolysis of nucleotides in the chaperonin catalytic cycle: implications for the mechanism of assisted protein folding. Biochemistry 32, 2554–2563 (1993).
Todd, M. J., Viitanen, P. V. & Lorimer, G. H. Hydrolysis of adenosine 5′-triphosphate by Escherichia coli GroEL: effects of GroES and potassium ion. Biochemistry 32, 8560–8567 (1993).
McLennan, N. & Masters, M. GroE is vital for cell-wall synthesis. Nature 392, 139, doi:10.1038/32317 (1998).
Kovacs, E. et al. Characterisation of a GroEL single-ring mutant that supports growth of Escherichia coli and has GroES-dependent ATPase activity. J Mol Biol 396, 1271–1283, doi:S0022-2836(09)01468-5 (2010).
Fei, X., Ye, X., LaRonde, N. A. & Lorimer, G. H. Formation and structures of GroEL:GroES2 chaperonin footballs, the protein-folding functional form. Proceedings of the National Academy of Sciences of the United States of America 111, 12775–12780, doi:10.1073/pnas.1412922111 (2014).
Brocchieri, L. & Karlin, S. Conservation among HSP60 sequences in relation to structure, function, and evolution. Protein science: a publication of the Protein Society 9, 476–486, doi:10.1110/ps.9.3.476 (2000).
Kawata, Y. et al. The role of ATP hydrolysis in the function of the chaperonin GroEL: dynamic complex formation with GroES. FEBS letters 369, 283–286 (1995).
Parnas, A. et al. Identification of elements that dictate the specificity of mitochondrial Hsp60 for its co-chaperonin. PloS one 7, e50318, doi:10.1371/journal.pone.0050318 (2012).
Yifrach, O. & Horovitz, A. Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry 34, 5303–5308 (1995).
Lorimer, G. H. A quantitative assessment of the role of the chaperonin proteins in protein folding in vivo. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 10, 5–9 (1996).
Zimmerman, S. B. & Trach, S. O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol 222, 599–620 (1991).
Lee, T. S. et al. BglBrick vectors and datasheets: A synthetic biology platform for gene expression. Journal of biological engineering 5, 12, doi:10.1186/1754-1611-5-12 (2011).
Acknowledgements
This work was partially supported by a research fund from Department of Molecular and cellular Biochemistry, Indiana University, and by Individual Research Award from Institute for Advanced Study, Indiana University.
Author information
Authors and Affiliations
Contributions
M.I., H.E. and L.C. performed experiments. M.I. and L.C. analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Illingworth, M., Ellis, H. & Chen, L. Creating the Functional Single-Ring GroEL-GroES Chaperonin Systems via Modulating GroEL-GroES Interaction. Sci Rep 7, 9710 (2017). https://doi.org/10.1038/s41598-017-10499-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10499-4
- Springer Nature Limited