Abstract
A new series of etherification chalcone derivatives were designed and synthesized through Willimison etherification and Claisen-Schmidt condensation. Among them, compound 2-c which was given chemical name of S17, has been successfully screened out as the most potent one on gastric cancer cell line(MGC803) through the investigation for their effects against the growth of five cancer cell lines (EC109, HepG2, MCF7, MGC803, SKNSH). S17 exhibited strong anti-proliferative activity on other two gastric cancer cells (HGC27 and SGC7901), but less cytotoxicity to non-malignant gastric epithelial cells GES1. S17 potently killed gastric cancer cells with causing modulation of Bcl-2 family proteins and activation of caspase 9/3 cascade. S17 also up-regulated DR5 expression and DR5 knockdown partially reversed S17-induced apoptosis, caspase activation and MMP decrease. S17 robustly induced generation of ROS with Keap/Nrf2 pathway activated and the application of ROS scavenger N-acetyl cysteine (NAC) completely blocked these effects by S17 in MGC803 cells. Intraperitoneal administration of S17 significantly inhibited the growth of MGC803 cells in vivo in a xenograft mouse model without observed toxicity. These results indicated that S17 is a leadbrominated chalcone derivate and deserves further investigation for prevention and treatment of gastric cancer.
Similar content being viewed by others
Introduction
Gastric cancer is a kind of occurring commonly cancer in gastrointestinal tract cancer1. In recent years, attention has been focused on the anti-cancer properties of natural products, which play an important role in the prevention of cancers2. As an important candidates of the subclasses of the flavonoid family, chalcone derivatives are the precursors of the flavones in the biosynthesis of flavonoids and a large amount of which have been applied as antiplatelet, anti-inflammatory, anti-allergic, antimicrobial, antioxidant or anti-tumor agent3, 4. The most classical and general synthetic route of chalcone derivatives was the Claisen-Schmidt condensation among the reported ones5. Chalcone and its derivatives display a wide range of important pharmacological activities and have a huge importance in medicinal chemistry6. As reported, chalcone, coumarins and flavanones from the exudate of Angelica keiskei have chemopreventive effects7. Isobavachalcone exhibits anti-proliferative effects towards several human cancer cells through blocking of Akt signaling8. A chalcone panduratin A isolated from Kaempferia pandurata induce apoptosis and cell cycle arrest in androgen-independent human prostate cancer cells PC3 and DU1459. These observations suggested that naturally-occurring chalcone can be further optimized through synthesis of their derivatives as new anti-cancer agents to effectively treat certain cancers.
Cell apoptosis, or programmed cell death acted as one of the most important manner in regulation of carcinogenesis10. In the initial of apoptotic process, it triggers an activation of apoptotic signaling program leading to cell death rather than kills cells directly. Reactive oxygen species (ROS), a cellular metabolite which regulates multiple cancer-related signalling pathways appears to be an important regulatory signal of cell apoptosis11. Nowadays, it is significantly recognized that ROS are involved in the function of antitumor, because high levels of ROS cause cell damage by oxidation and nitration of macromolecules including RNA, DNA, lipids, and proteins, as well as cause DNA damage and apoptosis12, 13. SL4, a chalcone-based compound, induces apoptosis by activation of the ROS/MAPK signaling pathway in human cancer cells in vitro 14 and in another study, a new synthetic 2′-hydroxy-2,4,6-trimethoxy-5′,6′-naphthochalcone induces cell apoptosis and cycle arrest of human colon cancer cells15. In this study, a series of etherified chalcone derivatives were rationally designed and their antitumor activities evaluated.
Among them S17 which was designed and synthesized for the first time exhibited strong cytotoxic effect against gastric cancer cells. We discussed the mechanism of S17 on gastric cancer cell MGC803 with reactive oxygen species (ROS) causing apoptosis via mitochondria apoptotic pathway and through upregulation of DR5. DR5 knockdown indeed partially reversed the mitochondrial membrane potential decrease and apoptosis. At the same time the increasing ROS activated the Nrf2/HO-1 axis in a short time. We also evaluated antitumor activity of S17 in a MGC803 tumor bearing xenograft mice model in vivo. The significant antitumor effects of S17 have been confirmed both in the vitro and vivo experiments.
Results
S17 showed significant inhibition of proliferation of human gastric cancer cells (MCG803, HGC27 and SGC7901) with minimal toxicity to non-malignant human gastric epithelial cells GES-1
Etherification on ring A and B occurs relatively infrequently (Fig. 1A). Chalcone derivates with multiple methoxy substituted on ring A and B have never been reported. Their anticancer activities haven’t been elaborated. Therefore, a new series of etherification chalcone derivatives were designed and synthesized through Willimison etherification and Claisen-Schmidt condensation (Fig. 1B,C). Based on the screening results of the synthesized compounds for inhibiting the growth of five cancer cell lines, S17 was prioritized to perform further experiment for evaluating its anti-cancer potential in gastric cancer (Fig. 1D). Furthermore, the IC50 value of S17 for MGC803 is 6.754 ± 0.830 μM, SGC7901 is 9.285 ± 0.968 μM and HGC27 is 12.292 ± 1.090 μM, exhibiting better cytotoxicity than other cell lines. Therefore, we chose S17 and gastric cancer cells for the further experiment.
To evaluate the effects of S17 on human gastric cancer cells, three gastric cancer cell lines (MGC803, HGC27 and SGC7901) and human gastric epithelial cells (GES1) were incubated with S17(10 μM) for indicated time points, and then the effects of S17 on reducing cell viabilities were measured by an MTT assay. As shown in Fig. 1E, following treatment with S17, the viability of the gastric cancer cells decreased in a time-dependent manner. MGC803 cells showed the most sensitivity to S17 treatment, causing 60.77% viability reduction at 48 h in related to control treatment. However, it is almost no toxicity to human gastric epithelial cells (GES1) (Fig. 1E). Taken together, these results suggested that S17 has selective cytotoxicity against gastric cancer cells versus normal human gastric epithelial cells.
S17 induced caspase-dependent apoptosis of MGC803 cells
Further experiments were conducted to determine whether the inhibition of S17 on the viability of gastric cancer cells was the result of apoptotic cell death. In Fig. 2A, we evaluated the apoptotic induction of S17 on MGC803 with Annexin V and PI staining and found that the numbers of Annexin V positive cells showed gradually increase in a time-dependent manner from 12.0% to 58.7% in MGC803, whereas control treatment only resulted in 5.0% Annexin V positive staining cells. Morphological changes of MGC803 cells were then determined using DAPI staining, as shown in Fig. 2B, a significant number of cells with chromatin condensation were observed in cells treated with S17, whereas these features were not observed in control cells. Caspases are known as key executioners of apoptosis through the cleavage of various cellular substrates16. To further investigate whether S17-induced apoptosis is associated with the caspase cascade leading to proteolytic cleavage of poly(ADP-ribose) polymerases-1 (PARP-1) in MGC803 cells17, we assessed cleavage of caspases (3, 8 and 9) and PARP-1. As shown in Fig. 2C, Western blot analyses revealed that treatment of MGC803 cells with S17 (10 μM) down-regulated the levels of pro-caspase3, pro-caspase9 and pro-PARP-1 proteins accompanied by an increase in levels of cleaved caspase3, 8, 9 and PARP-1 proteins compared to the control treatment in MGC803 cells.
S17 induced apoptosis in MGC803 cells. (A) S17 induced cell apoptosis of MGC803 cells in a time-dependent manner by Flow Cytometer analysis. (B) MGC803 cells were stained with DAPI solution. Stained nuclei were then observed under a fluorescence microscope using a blue filter (magnification, 400X). The arrow was indicating chromatin condensation. (C) Western blotting assay showed the effects of S17(10 μM) on the expression CF-PARP, CF- caspase3, CF- caspase8 and CF-caspase9. (D) Flow Cytometric analysis demonstrated the effect of the inhibitor of caspases (Z-VAD-fmk, 100 μM) on S17(10 μM)-induced cell apoptosis of MGC803 cells for 48 h.
To further evaluate the significance of caspase activation in S17-induced apoptosis, MGC803 cells were pretreated with z-VAD-fmk (100 μM) for 1 h, followed by treatment with 10 μM S17 for 48h18. As shown in Fig. 2D, Pretreatment with z-VAD-fmk efficiently attenuated S17-induced apoptosis from 54.5% to 23.3% according to flow cytometry analysis. Taken together, these results suggest that S17-induced apoptosis is dependent of caspase activation in MGC803 cells.
S17-induced apoptosis involves the modulation of Bcl-2 and IAP family proteins, attenuation of Mitochondrial membrane potential (MMP/ΔΨ) in MGC803 Cells
Mitochondria play an essential role in cell apoptosis pathway19, 20 and the mitochondria-dependent apoptotic pathway is regulated by the Bcl-2 family of pro- and anti-apoptotic proteins21, 22. To determine the role of the mitochondria in S17-induced apoptosis of gastric cancer cells, the change of MMP (ΔΨ) was measured. As shown in Fig. 3A, S17 significantly reduced the MMP level in a time-dependent manner in MGC803 cells. And pretreatment with z-VAD-fmk (the pan-Caspase inhibitor) which can block the process of apoptosis significantly reversed S17-induced decrease of the MMP level as shown in Fig. 3B. The role of the Bcl-2 and the IAP family proteins was determined by western blotting to explore the possible mechanisms involved in S17-induced apoptosis in MGC-803 cells. As shown in Fig. 3C, in comparison with the control cells, S17 treatment of MGC803 cells led to a significant increase in the protein levels of BimEL, Bax, t-Bid and a reduction in the protein levels of XIAP in a time-dependent manner, whereas the levels of anti-apoptotic Bcl-2, Bcl-xL and death receptor family protein DR4 were not changed. These results revealed that the mitochondrial pathway plays an important role in S17 induced apoptosis in gastric cancer cells.
S17-induced apoptosis involves Mitochondrial pathway in MGC803 Cells. (A) S17 decreased the membrane potential (ΔΨ) of mitochondria. Membrane potential was measured by JC-1 dye retention using Flow Cytometry. (B) Flow Cytometric analysis demonstrated the effect of the inhibitor of caspases (Z-VAD-fmk, 100 μM) on S17 (10 μM)-induced decrease of the mitochondria membrane potential (ΔΨ) of MGC803 cells for 48 h. (C) The protein levels of Bax, BimEL, Bcl-2, t-Bid, Bcl-XL, Bad, XIAP and Survivin were determined by Western blotting assay.
S17-induced apoptosis is related to Death Receptor 5
Apoptosis usually happens following the death ligand binding to corresponding receptors such as DR4 and DR523. And we found that the death receptor DR5 expression level was obviously increased after treatment of 10 μM S17 for 24 hours while DR4 wasn’t influenced as shown in Fig. 4A. And this was confirmed by analysis of expression of DR5 in MGC803 cells by flow cytometry as shown in Fig. 4B. Then we knocked down the protein DR5 in MGC803 cells. The cell death induced by S17 were attenuated significantly as shown in Fig. 4C,D. We also detected that the cleavage of Caspase3 and PARP-1 decreased to the modest degree as shown in Fig. 4E. Interestingly we also found that S17-induced MMP decrease was partially reversed in DR5−/− MGC803 cells as shown in Fig. 4F. These results together revealed that DR5 is associated with S17-induced apoptosis and mitochondria pathway.
S17 induced cell apoptosis of MGC803 cells involved with extrinsic pathway. Both wild type and DR5−/− MGC803 cells were treated of 10 μM S17 for indicated time. (A) S17 increases DRs protein expression in MGC803 cells at a time-dependent manner. (B) DR5 protein expressions in MGC803 cells were determined by flow cytometer analysis after S17 incubation for 24 hours. (C) Cell viabilities were measured by MTT assay after 30 hours S17 incubation. **p < 0.01 vs. untreated group. (D) The ratios of apoptotic cells were determined by Flow Cytometric analysis after S17 incubation for 48 hours. (E) The expression levels of apoptosis-related proteins were tested by western blotting assay after S17 incubation for 24 hours. (F) Flow Cytometric analysis demonstrates MMP(ΔΨ) decrease after 48 hours treatment of S17.
S17 induces generation of ROS in MGC803 cells and activation of keap1/Nrf2 pathway
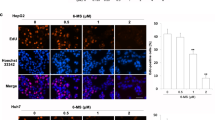
The generation of intracellular ROS has been shown to be related to the induction of apoptosis in various cell types24. We therefore measured ROS production in MGC803 cells by flow cytometry analysis of cellular DCFH-DA fluorescence intensity. As shown in Fig. 5A, treatment of MGC803 cells with 10 μM of S17 resulted in a time-dependent increase of ROS generation. Since elevated ROS activates Nrf2 and its subsequent nuclear-translocation and increases the downstream proteins expression25, as shown in Fig. 5B,C, we confirmed the increasing expression of proteins like p-Nrf2, HO-1 and NQO1 and the immunofluorescence assay also showed accumulation of Nrf2 around the nuclear and on the chromatin. However, we also noted that Nrf2 expression dramatically decreased if not vanished and NQO1 expression also reached its peak at 3 h after S17 treatment indicating Nrf2 didn’t happen accompanied with S17 all the time. These results together suggest S17 indeed induced the generation of ROS and the activation of keap1/Nrf2 pathway was meant to protect cells from the injury by ROS, although it didn’t reverse S17-induced cell death.
S17 induced ROS elevating in gastric cancer cells. (A) Measurement of ROS. MGC803 cells were treated with S17 (10 μM) for 0,1,2,4,6,8 hours. The level of ROS was measured by DCFH-DA with Flow Cytometry. (B) Western blotting demonstrated S17 (10 μM)-induced protein expression changes at indicated time points. (C) MGC803 cells were treated with S17 (10 μM) for 8 hours. The treated and untreated samples are stained with Nrf2 antibody (Green) and DAPI (Blue) (magnification, 400X). The arrow was indicating Nrf2 nuclear-translocation.
S17-induced Apoptosis is associated with the generation of ROS
Next, to determine whether S17-induced ROS production was attributable to the cell inhibition and apoptosis induction, the cells were pretreatment with NAC for 1 h and co-incubated with 10 μM of S17 for a further 48 h. Figure 6A,B revealed that pretreatment with NAC almost completely attenuated S17-induced cell inhibition in three gastric cancer cells and completely attenuated apoptosis in MGC803 cells. Blocking the generation of ROS by pretreating MGC803 cells with NAC prevented S17-induced modulation of Bcl-2 and IAP family proteins, activation of caspases, cleavage of PARP-1, up-regulation of DR5 and also abrogated the decrease of MMP level. And in SGC7901 cells, NAC also decreased the expression levels of Bax, DR5 and CF-PARP proteins induced by S17, which were consistent with the effect of NAC on MGC803 (Fig. 6C,D). In order to demonstrate how S17 induces lethal ROS generation in gastric cancer cells, MGC803 cells were pretreated 1 h with the different functional ROS inhibitors include Catalase (CAT), ButyIhydroxyanisole (BHA), GKT137831 (GKT), Rotenone (ROT), Neohesperidin (NEO), and Apocynin (APO). Figure 6E exhibited that the cytotoxicity of S17 was significantly attenuated by BHA and CAT compared with the rest of ROS inhibitors. These data suggested that the generation of ROS is required for the S17-induced apoptosis in gastric cancer cells.
S17 induced apoptosis is related with elevating ROS level in gastric cancer cells. (A) MTT assay demonstrated the effect of NAC (5 mM) on S17-induced cell death of MGC803, HGC27 and SGC7901cells at 48 h. **p < 0.01 vs untreated group. (B) Flow Cytometric analysis showed the effects of NAC (5 mM) on S17(10 μM)-induced cell apoptosis of MGC803 cells. (C) Western blotting demonstrated the effect of NAC (5 mM) on S17(10 μM)-induced protein expression changes at 48 h. (D) Flow Cytometric analysis showed the effect of NAC (5 mM) on S17(10 μM)-induced loss of membrane potential (ΔΨ) in MGC803 cell mitochondria at 48 h. (E) MTT assay demonstrated the effect of different ROS inhibitors (CAT (250 μM), BHA (15 μM), GKT (200 μM), ROE (125 nM), NEO (200 μM) and APO (10 μM)) on S17 (10 μM)-induced cell death of MGC803 at 48 h. **p < 0.01 vs S17 treatment group.
S17 inhibits in vivo tumor growth in the xenograft nude mouse model of MGC803 cells
There is no significant difference in mean body weights over time between control and S17 treated groups (Fig. 7A) (Student’s t test; P > 0.05). The growth rate of MGC803 xenograft tumors from mice which were treated with S17 was lower than those from the vehicle control-treated mice. (Fig. 7B). The mean of wet tumor weights in the S17 treated mice was about 60% less than that of the control treated mice (Student’s t test; P < 0.01) (Fig. 7C). In order to determine whether xenograft tumor cells treated with S17 underwent ROS-mediated apoptosis, immunohistochemical assay was performed to determine changes of cleaved-caspase3, cleaved-caspase8 proteins expression and the levels of ROS in xenograft tumors treated with S17. As shown in Fig. 7D, S17 treatment led to an increase in expression of cleaved caspase 3, cleaved caspase 8 and ROS. These results demonstrated the anti-tumor activity and the apoptotic effect of S17 against gastric cancer cells in vivo.
S17 inhibited tumor growth in MGC803 xenograft model. Mice wererespectively treated or not treated with S17. (A) The body weight-time bar charts.Statistical analyses demonstrated that the average volume (B) and weight (C) of MGC803 xenografts from S17-treated mice were significantly reduced. **P < 0.01 vs Control group. Treatment was initiated when the average size of the tumor reached 100 mm3. The test group were treated with indicated dosage in 20 μl of DMSO of S17 every day and the control group received injection of DMSO alone. The mice (n = 5 per group) were treated for 21days. (D) Immunohistochemical assays were performed in xenograft tumors to demonstrate the expression levels of cleaved caspase3, cleaved caspase8 and ROS induced by 40 mg/kg S17.
Discussion
Carcinogenesis is a complex process which consists of a series proliferative signaling, including evasion of growth suppression, damaged extracellular matrix (ECM) components, resistance to cell death, uncontrolled proliferation as well as increasing invasion and metastasis of cancer cells26, 27. Thus polypharmacological approaches for developing anti-tumour drugs attarct much interests of investigator. In recent years, naturally occurring botanicals and there derivatives are attracting considerable attention as cancer chemopreventive agents28, 29. Chalcones which belong to a specific class of flavonoids frequently occur in fruits, vegetables and beverages (tea, coffee, beer, wine and fruit drinks)30, and a new brominated chalcone derivative H72 in our previous work has been proved to effectively induce apoptosis in gastric cancer cells31.
In this study, we have demonstrated that S17, a novel chalcone derivate, selectively inhibits the growth of gastric cancer cells MGC803 by inducing cell apoptosis through ROS-mitochondria apoptotic pathway. S17 induced robust ROS obviously detected by flow cytometer analysis in MGC803 cells. It leaded to the apoptosis through DR5 and mitochondrial apoptotic pathway.
Since mitochondrial respiration are the main source of ROS, mitochondria are considered to be the major target of ROS damaging and increase in ROS level can damage mitochondrial membrane and result in apoptosis by oxidizing mitochondrial pores, thereby disrupting the mitochondrial membrane potential(MMP)32, 33. In this study, we demonstrated the ability of S17 to induce mitochondrial dysfunction, by detecting the MMP.
In addition, ROS inhibitors with different function were utilized to investigate how S17 induces lethal ROS generation in MGC803 cells. ButyIhydroxyanisole (an antioxidant acts as a radical-scavenger) and catalase (H2O2 enzyme) decreased the death of MGC803, due to oxygen free radicals and H2O2 are mainly derived from mitochondrial aerobic respiration and the metabolic process, and is the byproduct source of mitochondrial electron transport chain (ETC, includes complex members I-IV)34. We utilized Rotenone (an irreversible inhibitor of complex I) and Apocynin (a selective NADPH-oxidase inhibitor) to detect whether the generation of ROS is associated with ETC, but Rotenone and Apocynin failed to reverse the cytotoxicity of S17, ETC may be unrelated with the generation of ROS induced by S17. However, ROS from mitochondria are not all derived from mitochondrial electron transport chain, mitochondrial H2O2 also available from monoamine oxidase, and generated from oxidation of cytochrome c directly by the oxidordeuctase p66shc35, which may be as the target for S17 to induce apoptosis and deserve to be further investigated. Furthermore, the associated mechanisms of Neohesperidin (an antioxidant activity in scavenging the DPPH radical) and GKT137831 (dual Nox1/Nox4 inhibitor) could be excluded, however, how S17 induces lethal ROS generation in gastric cancer cells MGC803 needs to be further investigated.
The mitochondrial associated apoptosis pathway involves mitochondrial outer membrane permeabilization followed by release of cytochrome c into the cytosol, which induces a caspase cascade culminating in cell death36, 37. On the other hand, in the cytosol, cytochrome c triggers activation of initiator caspase 9 could then activate the effector caspase 3 to contribute to activation the mitochondrial apoptosis pathway. Executioner caspase-3 is responsible for cleaving its substrates, such as poly ADP-ribose polymerase 1 (PARP-1), subsequently inducing apoptosis38, 39. Western blot analysis showed that caspase 9, caspase 8, caspase 3 and PARP-1 were all activated in a time-dependent manner by treatment with S17 in MGC803 cells (Fig. 3A), and the activated caspase 8 can make Bid cleaved into t-Bid triggering the mitochondria apoptotic pathway, elucidating the interplay between extrinsic and intrinsic apoptosis pathway induced by S17 is essential. Mechanistically, S17 activated caspases and modulated XIAP and Bcl-2 member proteins involved in mitochondrial apoptotic pathway in vitro. Inhibitors of apoptosis (IAP) proteins are endogenous inhibitors of apoptosis40, and the best characterized of these are XIAP and survivin. They respectively inhibit the activation of caspase-9 and caspase-3, thereby negatively regulating apoptosis.Our results showed that the decreased expression of XIAP, which might contribute to the reason of caspase-9 activation induced by S17. However, there was no change in survivin after treatment with S17, indicating that activation of caspase-3 by S17 was mediated by upstream signaling. Bcl-2 family proteins are important regulators of mitochondrial apoptosis approach, which govern MMP and can be either pro-apoptotic, such as Bax, or anti-apoptotic such as and Bcl-2, and Bcl-xL. Even if caspase proteins are generally required in apoptosis process, Since z-VAD-fmk didn’t completely reversed S17-induced apoptosis, we supposed there was another caspase-independent way involved in it. Thus, drugs which use ROS-mitochondrial apoptosis approaches in cancer cells may prove to be valuable anti-cancer therapeutics.
We detected that DR5 increased greatly after S17 treatment. To investigate the role of DR5 in the regulation of S17-induced cell apoptosis. According to our results, MMP decrease was significantly reversed in DR5−/− cells comparing to that in wild type MGC803 cells after 10 μM S17 treatment for 48 hours (Fig. 4F), indicating that DR5-related apoptotic pathway activated mitochondria pathway as was elaborated that caspase 8 makes Bid cleaved into t-Bid triggering the mitochondria apoptotic pathway41.
In addition, we found that the elevating level of ROS reflectively activated Nrf2 and promoted its translocation into nuclear and increased antioxidant enzymes expression such as HO-1, NQO1. Similarly as reported 15d-PGJ2, a stable PGD2 degradation product, causing intracellular redox imbalance in p53-deficient MG-63 OS cells induced p38 activation and Akt phosphorylation within 4 h followed by Nrf2 translocation into nuclear which acted as a protective pathway42. In addition, compared with H72 in our previous work31, Nrf2 activation is a special mechanism and the apoptotic effects of S17 on gastric cancer cell lines may be better than H72 if the Nrf2 pathway be blocked. Nrf2/HO-1 signaling indeed play protection effect on the cells in spite of it didn’t reverse apoptosis, Furthermore, our results showed Nrf2 decreased and NQO1 reached its peak at 3 h after S17 treatment but HO-1 increased all the time. The underlying reason was until unknown and need further investigation.
This study showed that a novel chalcone derivate, S17, inhibited tumor growth in vivo. Moreover, we found that S17 reduced tumor burdens in MGC803 xenografted mice without gross toxicity. In the MGC803 xenograft model, S17 showed good antitumor activity and less toxicity for gastric cancer cells. Furthermore, immunohistochemical analysis showed S17 treatment led to the increased levels of cleaved caspase 3 and cleaved caspase 8 in xenografted tumors. More importantly, the levels of ROS also were increased induced by S17 compared with control, indicating the anti-tumor activity of S17 against gastric cancer cells in xenograft tumors via ROS-mediated apoptosis manners.
Overall, S17 may be a novel lead compound as a cancer drug candidate with polypharmacological properties. Therefore for our research work, we made a summary that S17-induced apoptosis through ROS dependent DR5 up-regulation and Nrf2-mediated relief in gastric cancer cells (Fig. 8), and we found S17 of new synthetized chalcone is a promising candidate in drug discovery.
Methods
Reagents and chemicals
Fetal bovine serum (FBS), RPMI-1640, and penicillin–streptomycin were purchased from HyClone (Victoria, Australia). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra- zolium bromide (MTT), N-acetyl-L-cysteine (NAC) and JC-1 fluorescent dye (Sigma-Aldrich, St Louis, MO). ButyIhydroxyanisole, Rotenone and Apocynin were purchased from MCE. GKT137831, Neohesperidin and z-VAD-fmk were purchased from Selleck. FITC/Annexin V Apoptosis Detection Kit (BestBio, Shanghai, China), Catalase and 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) was purchased from Beyotime Biotechnology (Shanghai, China). Antibodies specific for β-actin (sc-1615, goat, 1:1000), Bim (sc-11425, rabbit, 1:1000), Bax (sc-493, rabbit, 1:1000), Bad (sc-8044, mouse, 1:1000), caspase-3 (sc-7272, rabbit, 1:1000), caspase-9 (sc-7885, rabbit, 1:800), poly (ADP-ribose) polymerase-1 (PARP-1) (sc-7150, rabbit, 1:1000), DR4 (sc-7863, rabbit, 1:1000), DR5 (sc-65314, mouse, 1:1000) were obtained from Santa Cruz Biotechnology(Santa Cruz, CA). Antibodies specific for Bid (#2002, rabbit, 1:1000), Bcl-xL (#2764, rabbit, 1:1000), XIAP (#14334, rabbit, 1:1000) and cleaved caspase-8 (#9496, rabbit, 1:1000) were purchased from Cell Signaling Technology (Danvers, MA). The antibody specific for survivin (ab76424, rabbit, 1:2000) was purchased from Abcam (Cambridge, MA). Anti-DR5 (ab1675, goat, 1: 500) antibody for flow cytometer were purchased from Abcam(Cambridge, MA). Peroxidase-labeled anti-goat (1:5000), anti-rabbit (1:5000) and anti-mouse (1:5000) polyclonal immunoglobulins were purchased from Bioss (Shanghai, China). The enhanced chemiluminescence (ECL) kit was purchased from Thermo Fisher (Waltham, MA).
Chemosynthesis
The synthetic method of the etherification chalcone derivatives was shown in Fig. 1B,C. Substituted benzaldehydes, which were synthsized by willimison etherification, reacted with 2-hydroxyl-4, 6-dimethoxyl acetophenone (Compound 1 Fig. 1B) or 2,4,6-trimethoxyl acetophenone (Compound 2 Fig. 1B) by Claisen-Schmidt condensation reaction to afford a novel series of flavokawain A derivates. The chemical structure of S17 was identified using using 1H-NMR and 13C-NMR.
S17 was dissolved in DMSO to 10 mM stock solution and stored at room temperature. For each experiment, the stock solution were diluted in the culture medium to obtain the desired concentration. The final DMSO content in cell culture was ≤ 0.5% (v/v), which was found to be nontoxic to cells.
Cell lines and cultures
EC109 (human esophagus cancer), HepG2(human hepatoma) cells, MCF7 (human breast cancer) cells, SKNSH (human neuroblastoma), MGC803(human gastric cancer), HGC27(human gastric cancer), SGC7901(human gastric cancer) and GES1(human gastric epithelial cell) were purchased from the American Type Culture Collection (Manassas, VA), and cultured at 37 °C in an atmosphere containing 5% CO2, with RPMI1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 0.1 mg/ml streptomycin. MGC803 of DR5−/− cell line were cultured by our group through a lentiviral system and the primer sequence is 5′-GCAAGUCUUUACUGUGGAA-3′.
Cell proliferation
The cells were seeded into a 96-well plate at a density of 4,000 (100 μl) cells per well for 24 h, followed by compounds added (200 μl) to the respective wells in the indicated final concentrations at different times. Next, 20 μl of 5 mg/ml MTT per well was added to the medium, and the cells were incubated for 4 h at 37 °C and 5% CO2. After removing the culture medium, 150 μl of DMSO was added to dissolve the formazan crystals. The absorbance was read by enzyme labeling instrument with 570 nm wavelength measurement. The viability of treated cells for 0 hour was set as 100%, and the viability in the other groups was calculated by comparing the optical density reading with the control. The IC50 values were calculated using nonlinear regression analysis.
Nuclear staining with DAPI
Morphological changes of nuclei were visualized following DAPI staining. Following incubation with 10 μM S17 for 24 h, cell were fixed with 4% paraformaldehyde for 10 min at room temperature. Fixed cells were washed with PBS, and stained with 2 μg/ml DAPI solution (dissolved with PBS containing 0.1% TritonX-100) for 40 min at room temperature. Cells were washed two more times with PBS and analyzed via a fluorescence microscope.
Apoptosis analysis
MGC-803 cells were seeded at 1.5 × 105 cells/well in 6-well plates and cultured for 24 h. Next, the cells were exposed of S17 (10 μM) to different times. After that, cells were collected and washed with PBS twice, and incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin V and PI using FITC Annexin-V/PI apoptosis kit following the step-by-step protocol provided by the manufacturer. The cell apoptosis was detected by flow cytometer. Annexin V+/PI− cells were considered as early apoptotic while Annexin V+/PI+ cells as late apoptotic/necrotic.
Analysis of DR5 expression by flow cytometer
5 × 105 MGC803 cells were harvested, fixed by 1% PA (paraformaldehyde) and washed three times with PBS. Washed cells were blocked in 1X PBS/10% normal goat serum for 30 min at 4 °C to eliminate the non-specific protein-protein interactions followed by the antibody (at 1:200 dilution) for 45 min. Goat anti-mouse Ig G was used at 1:500 dilution for 30 min at 4 °C. Then cells were washed three times by PBST and detected by flow cytometer.
Measurement of loss of mitochondrial membrane potential (MMP, ΔΨ)
JC-1 probe was used as a measure of loss of MMP. Briefly, Cells were seeded at 1.5 × 105/well in 10% FBS RPMI-1640 into 6-well plates, and treated with S17 (10 μM) for indicated times. Then JC-1 (2.5 μg/ml), were added to incubate with an equal volume of cell suspension at 37 °C for 10 min and rinsed twice with PBS. The concentration of retained JC-1 dye was determined by a flow cytometer.
Measurement of ROS
The levels of intracellular reactive oxygen species (ROS) were determined using 2, 7-dichlorodihydro fluorescent diacetate (DCFH-DA), as described previously. MGC-803 cells were plated into a 6 well plate for 24 h prior to the experiment. On the following day, cells were subjected to S17 (10 μM) for a certain range of time. Following treatment, cells were incubated with 20 mM DCFH-DA dissolved in cell-free medium at 37 °C for 30 min in dark. After incubating, the cells were washed by PBS, trypsinized, and collected by centrifugation, and then washed three times with PBS. ROS generation was assessed in fluorescence intensity (FL-1, 530 nm) using a flow cytometer. Control cells were subjected to the same manipulation, except for treatment with S17.
Western blotting analysis
MGC-803 cells (1 × 106) were cultured in each 100 mm plates. After treatment with S17 (10 μM) for indicated times, cells were collected and lysed with ice-cold lysis buffer (Beyotime, Shanghai, China). After centrifugation at 12,000 rpm/min for 30 min, protein concentrations of the lysates were determined by the micro-BCA protein assay kit. The total cellular protein extracts were boiled with loading buffer and separated by SDS-PAGE and transferred to nitrocellulose membrane. After blocking with 5% skimmed milk in PBS with 0.1% Tween-20 for 2 h, the membranes were incubated with appropriate antibodies overnight at 4 °C, followed by HRP conjugated anti-mouse, anti-goat or anti-rabbit secondary antibodies. The detection of specific proteins was carried out with an ECL Western blotting kit according to the recommended procedure.
Immunofluorescence analysis of Nrf2
MGC803 cells were treated with 10 μM of S17 for 8 h. Cells were fixed with 4% paraformaldehyde in PBS for 30 min, permeabilized with 0.1% Triton X-100, and blocked with 10% normal goat serum for 30 min. Incubation with primary antibodies against Nrf2 was done overnight at 4 °C. After washing, cells were exposed to FITC-conjugated antibody (goat-anti-rabbit Ig (H + L)-FITC). After washing, the nuclei were visualized with 2 μg/ml DAPI solution (dissolved with PBS) and added 10 min before imaging. The confocal microscopy (Nikon) was used for co-localization analysis.
Immunohistochemistry (IHC)
Paraffin-embedded tissue sections were dewaxed and rehydrated, washed by PBS, following the antigen retrieval, endogenous peroxidase were blocked by 3% H2O2 for 20 min, and normal goat serum was used to block non-specific binding sites for 20 min, the sections were then incubated with cleaved caspase3 and cleaved caspase 8 antibody diluted 1:50 in PBS at 4 °C overnight. Peroxidase-conjugated anti-rabbit antibodies were used for secondary detection, the reaction was revealed with diaminobenzidine (DAB). And ROS was detected according to ROS Immunohistochemistry kit direction (Y-J biological company, Shanghai, China). Sections were counterstained with hematoxylin. Images were acquired on a microscope (Nikon).
Tumor xenograft growth assay in vivo
Animals were treated according to protocols established by the ethics committee of Zhengzhou University and the experiments in vivo were conducted according to the approved guidelines and approved by the ethics committee of Zhengzhou University. Thirty male nude mice (5 weeks-old) were purchased from the Chinese Academy of Sciences (Beijing, China). Cells were digested and resuspended with PBS at a density of 2.5 × 107 cells/ml. Cell suspension (200 μl) was subcutaneously injected into the nude mice on the backside. Tumor growth was monitored by tumor volume which was measured with calipers and calculated according to the formula, V = 0.5 × (length × width2). Finally, tumors were harvested after 21 days (21 injections), body weight, tumor volume and tumor weight were measured.
Statistical Analysis
The data are expressed as means ± SD. Significant differences between the groups were determined using the unpaired Student’s t-test. * and ** respectively represent p < 0.05 and p < 0.01.
References
Overby, A., Zhao, C. M., Bones, A. M. & Chen, D. Naturally occurring phenethyl isothiocyanate-induced inhibition of gastric cancer cell growth by disruption of microtubules. J Gastroen Hepatol 29, 99–106, doi:10.1111/jgh.12732 (2014).
Li, J. et al. Natural therapeutic agents for neurodegenerative diseases from a traditional herbal medicine Pongamia pinnata (L.) Pierre. Bioorg Med Chem Lett 25, 53–58, doi:10.1016/j.bmcl.2014.11.015 (2015).
Radhakrishnan, S. K., Shimmon, R. G., Conn, C. & Baker, A. T. Evaluation of Novel Chalcone Oximes as Inhibitors of Tyrosinase and Melanin Formation in B16 Cells. Archiv der Pharmazie (2015).
Wani, Z. A. et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem Toxicol 87, 1–11, doi:10.1016/j.fct.2015.11.016 (2015).
Matos, M. J., Vazquez-Rodriguez, S., Uriarte, E. & Santana, L. Potential pharmacological uses of chalcones: a patent review (from June 2011–2014). Expert Opin Ther Pat 25, 351–366, doi:10.1517/13543776.2014.995627 (2015).
Mahapatra, D. K., Bharti, S. K. & Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur J Med Chem 98, 69–114, doi:10.1016/j.ejmech.2015.05.004 (2015).
Akihisa, T. et al. Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Letters 201, 133–137, doi:10.1016/S0304-3835(03)00466-X (2003).
Jing, H. et al. Abrogation of Akt signaling by Isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett 294, 167–177, doi:10.1016/j.canlet.2010.01.035 (2010).
Yun, J. M., Kweon, M. H., Kwon, H., Hwang, J. K. & Mukhtar, H. Induction of apoptosis and cell cycle arrest by a chalcone panduratin A isolated from Kaempferia pandurata in androgen-independent human prostate cancer cells PC3 and DU145. Carcinogenesis 27, 1454–1464, doi:10.1093/carcin/bgi348 (2006).
Hikita, H. et al. Bak deficiency inhibits liver carcinogenesis: A causal link between apoptosis and carcinogenesis. J Hepatol 57, 92–100, doi:10.1016/j.jhep.2012.01.027 (2012).
Ralph, S. J., Rodriguez-Enriquez, S., Neuzil, J. & Moreno-Sanchez, R. Bioenergetic pathways in tumor mitochondria as targets for cancer therapy and the importance of the ROS-induced apoptotic trigger. Mol Aspects Med 31, 29–59, doi:10.1016/j.mam.2009.12.006 (2010).
O’Donovan, P. et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 309, 1871–1874, doi:10.1126/science.1114233 (2005).
Waszczak, C. et al. Sulfenome mining in Arabidopsis thaliana. P Natl Acad Sci USA 111, 11545–11550, doi:10.1073/pnas.1411607111 (2014).
Wang, L. H. et al. SL4, a chalcone-based compound, induces apoptosis in human cancer cells by activation of the ROS/MAPK signalling pathway. Cell Prolif 48, 718–728, doi:10.1111/cpr.12226 (2015).
Lee, J. M. et al. A new synthetic 2′-hydroxy-2,4,6-trimethoxy-5′,6′-naphthochalcone induces G2/M cell cycle arrest and apoptosis by disrupting the microtubular network of human colon cancer cells. Cancer Lett 354, 348–354, doi:10.1016/j.canlet.2014.08.041 (2014).
Venero, J. L., Burguillos, M. A., Brundin, P. & Joseph, B. The executioners sing a new song: killer caspases activate microglia. Cell Death Differ 18, 1679–1691, doi:10.1038/cdd.2011.107 (2011).
Corbiere, C., Liagre, B., Terro, F. & Beneytout, J. L. Induction of antiproliferative effect by diosgenin through activation of p53, release of apoptosis-inducing factor (AIF) and modulation of caspase-3 activity in different human cancer cells. Cell Research 14, 188–196, doi:10.1038/sj.cr.7290219 (2004).
Chen, S. Y. et al. zVAD-induced autophagic cell death requires c-Src-dependent ERK and JNK activation and reactive oxygen species generation. Autophagy 7, 217–228 (2011).
Guerra, M. T. et al. Mitochondrial Calcium Regulates Rat Liver Regeneration Through the Modulation of Apoptosis. Hepatology 54, 296–306, doi:10.1002/hep.24367 (2011).
Wang, X. et al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev 27, 1351–1364, doi:10.1101/gad.215855.113 (2013).
Zi, X. & Simoneau, A. R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res 65, 3479–3486, doi:10.1158/0008-5472.CAN-04-3803 (2005).
George, N. M., Evans, J. J. D. & Luo, X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Gene Dev 21, 1937–1948, doi:10.1101/gad.1553607 (2007).
Mellier, G., Huang, S., Shenoy, K. & Pervaiz, S. TRAILing death in cancer. Molecular aspects of medicine 31, 93–112, doi:10.1016/j.mam.2009.12.002 (2010).
Yee, C., Yang, W. & Hekimi, S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157, 897–909, doi:10.1016/j.cell.2014.02.055 (2014).
Levonen, A. L. Activation of stress signaling pathways by oxidized and nitrated lipids. Free radical biology & medicine 75 Suppl 1, S8, 10.1016/j.freeradbiomed.2014.10.846 (2014).
Lin, S. H. & Shih, Y. W. Antitumor effects of the flavone chalcone: inhibition of invasion and migration through the FAK/JNK signaling pathway in human gastric adenocarcinoma AGS cells. Mol Cell Biochem 391, 47–58, doi:10.1007/s11010-014-1986-6 (2014).
Wang, R. A. et al. Apoptosis drives cancer cells proliferate and metastasize. J Cell Mol Med 17, 205–211, doi:10.1111/j.1582-4934.2012.01663.x (2013).
Dong, Y. Z., Morris-Natschke, S. L. & Lee, K. H. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat Prod Rep 28, 529–542, doi:10.1039/c0np00035c (2011).
Walsh, C. T. The chemical versatility of natural-product assembly lines. Accounts Chem Res 41, 4–10, doi:10.1021/ar7000414 (2008).
Lee, Y. H. et al. A new synthetic chalcone derivative, 2-hydroxy-3′,5,5′-trimethoxychalcone (DK-139), suppresses the Toll-like receptor 4-mediated inflammatory response through inhibition of the Akt/NF-kappa B pathway in BV2 microglial cells. Exp Mol Med 44, 369–377, doi:10.3858/emm.2012.44.6.042 (2012).
Zhang, S. et al. A new brominated chalcone derivative suppresses the growth of gastric cancer cells in vitro and in vivo involving ROS mediated up-regulation of DR5 and 4 expression and apoptosis. Toxicology and applied pharmacology 309, 77–86, doi:10.1016/j.taap.2016.08.023 (2016).
Perier, C. et al. Apoptosis-Inducing Factor Deficiency Sensitizes Dopaminergic Neurons to Parkinsonian Neurotoxins. Ann Neurol 68, 184–192, doi:10.1002/ana.22034 (2010).
Kalghatgi, S. et al. Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells. Sci Transl Med 5, doi:10.1126/scitranslmed.3006055 (2013).
Szczepanek, K., Chen, Q., Larner, A. C. & Lesnefsky, E. J. Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion 12, 180–189, doi:10.1016/j.mito.2011.08.011 (2012).
Giorgio, M., Trinei, M., Migliaccio, E. & Pelicci, P. G. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nature reviews. Molecular cell biology 8, 722–728, doi:10.1038/nrm2240 (2007).
Herrera, B. et al. Activation of caspases occurs downstream from radical oxygen species production, Bcl-x(L) down-regulation, and early cytochrome C release in apoptosis induced by transforming growth factor beta in rat fetal hepatocytes. Hepatology 34, 548–556, doi:10.1053/jhep.2001.27447 (2001).
Marsden, V. S. et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419, 634–637, doi:10.1038/nature01101 (2002).
Moorjani, N. et al. Activation of apoptotic caspase cascade during the transition to pressure overload-induced heart failure. J Am Coll Cardiol 48, 1451–1458, doi:10.1016/j.jacc.2006.05.065 (2006).
Gray, D. C., Mahrus, S. & Wells, J. A. Activation of Specific Apoptotic Caspases with an Engineered Small-Molecule-Activated Protease. Cell 142, 637–646, doi:10.1016/j.cell.2010.07.014 (2010).
Wang, X. D. The expanding role of mitochondria in apoptosis. Gene Dev 15, 2922–2933 (2001).
Jin, C. Y. et al. Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anti-cancer drugs 20, 713–722, doi:10.1097/CAD.0b013e32832e8998 (2009).
Koyani, C. N. et al. Activation of the MAPK/Akt/Nrf2-Egr1/HO-1-GCLc axis protects MG-63 osteosarcoma cells against 15d-PGJ2-mediated cell death. Biochemical pharmacology 104, 29–41, doi:10.1016/j.bcp.2016.01.011 (2016).
Acknowledgements
This work was supported by National Natural Science Foundation of China (Project no. 81273393 for Yanbing Zhang and Project no. U1404821 for Cheng-Yun Jin); The Scientific Innovation Talent Award from Department of Education of Henan Province (no. 15HASTIT036 for Cheng-Yun Jin).
Author information
Authors and Affiliations
Contributions
C.Y.J. designed the study and revised the article, S.Y.Z., D.J.F., Y.B.Z. and H.M.L. were responsible for the chalcone derivatives synthesis. T.Y.L. contributed most of the experiments in vitro and X.Y.W. was in charge of instruments assay. H.Q.D. screened and cultured the DR5−/− MGC803 cell line. H.D.X., Y.C.L., L.L.L. made the research work in vivo. L.Z. cooperated to write the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, S., Li, T., Zhang, L. et al. A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up-regulation in gastric cancer cells. Sci Rep 7, 9873 (2017). https://doi.org/10.1038/s41598-017-10400-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10400-3
- Springer Nature Limited
This article is cited by
-
Design and development of innovative microparticulate/nanoparticulate inhalable dry powders of a novel synthetic trifluorinated chalcone derivative and Nrf2 agonist
Scientific Reports (2020)
-
SP600125 enhances C-2-induced cell death by the switch from autophagy to apoptosis in bladder cancer cells
Journal of Experimental & Clinical Cancer Research (2019)
-
RETRACTED ARTICLE: 7-H-Pyrrolo[2,3-d]pyrimidine derivative acts as promising agent for gastric cancer treatment by inducing cell death
3 Biotech (2019)