Abstract
Nitrogen (N) is essential for the growth of algae, and its concentration varies greatly in the ocean, which has been regarded as a limitation for phytoplankton growth. Despite its great importance, most of the existing studies on the mechanisms underlying the effects of N on diatoms have focused on physiology, biochemistry and a few target genes and have rarely involved whole genomic analyses. Therefore, in this study, we integrated physiological data with RNA and protein profiling data to reveal the response strategy of Thalassiosira pseudonana under N-depleted conditions. Physiological measurements indicated that the cell growth capacity and chlorophyll content of the cells decreased, as did the expression of photosynthesis- and chlorophyll biosynthesis-related genes or proteins. The RNA-Seq profile results showed that T. pseudonana responded to N deprivation through increases in glycolysis, the TCA cycle and N metabolism as well as down-regulation in the Calvin cycle, gluconeogenesis, pentose phosphate, oxidative phosphorylation and lipid synthesis. These results provide a basic understanding for further research addressing how N affects phytoplankton in terms of genomics.
Similar content being viewed by others
Introduction
As an essential element in living creatures, nitrogen (N) plays a vital role in the composition of macromolecules and in determining the intracellular proportions of key metabolites of N and carbon metabolism1. The N content of the coastal zones has increased for decades because of the human nitrogen economy in agriculture2. However, compared with terrestrial plants, nitrogen is still a major limiting nutrient for primary production in the ocean3. Therefore, N is often used as a stressor treatment to study physiological changes in microalgae3,4,5,6.
Due to the great contribution of diatoms to global primary production7, Thalassiosira pseudonana is one marine diatom that is widely distributed throughout the world’s oceans and is often used as a model for diatom physiology or lipid-accumulation studies8, 9. Because of its wide distribution and small genome size, T. pseudonana was also the first eukaryotic marine phytoplankton whose whole genome sequence was decoded10. In the past few decades, most research has focused on physiological and biochemical phenomena, such as responses to nitrate, ammonium, silicon, phosphorus, light, temperature and CO2 in algae11,12,13,14,15, whereas only a few studies have attempted to examine whole transcripts or changes at the protein level5, 16,17,18. In fact, the algal genome era has become a hot topic in recent years, and an increasing number of algae genomes have been completed, including those of Cyanidioschyzon merolae 19, Ostreococcus tauri 20, Phaeodactylum tricornutum 21 and Porphyridium purpureum 22.
There are have been many studies on relevant stressors based on microarray analyses, 2-dimensional electrophoresis and transcriptome and proteome methods5, 9, 23. A recent study revealed whole-cell alterations stimulated by phosphorus deficiency by analyzing both the global transcriptome and proteome24. Additionally, there have been studies addressing the effects of iron, silicon and other macronutrient stressors on P. tricornutum 17, 25,26,27. In the diatom T. pseudonana, several previous research studies have focused on gene expression responses related to different environment stress conditions using the available technology. According to transcript analysis performed through RT-PCR, the effects of phosphine provide an alternative mechanism to oxidation to release phosphate for uptake9; 16 copper-induced genes were identified under copper stress27; and the expression levels of multiple selective genes were studied under nitrogen, phosphate and iron depletion stress conditions28. Using microarray methods, the dose-response effects of the polycyclic aromatic hydrocarbon benzo[a]pyrene on diatoms were detected29, and a wealth of new genes were identified as potentially being involved in silica formation in response to silicon stress8. Compared with the methods used in these studies, high-throughput sequencing or proteome methods, or de novo transcript expression can generate much more information. A total of 318 transcripts or 136 proteins in multiple biochemical pathways were found to be differentially abundant under diverse forms of phosphorus stress24, and large-scale proteome and transcriptome rearrangement was shown to be induced by cobalamin scarcity18.
Under very severe N-stress, the extent for the N-stress altering cell metabolism and growth depends on how severe the stress is. The traditional research has shown T. pseudonana undergoes little or no additional cell division, and its uptake capacity declines under deeply N-depleted conditions11. There is also a reduction of the intracellular chlorophyll a (Chl a) content, which is responsible for the decreasing light absorption30, and an increase in pigment and dimethyl sulfoniopropionate contents12, 15. Numerous studies have shown that N depletion may lead to lipid accumulation (mainly of triacylglycerol, or TAG) in numerous microalgal species, such as Chlamydomonas reinhardtii, Nannochloris sp., Scenedesmus obliquus, Parietochloris incise, and Haematococcus pluvialis 31,32,33,34,35,36,37. As a model species with one of the earliest available reference genomes for eukaryote algae, the N-stress response of C. reinhardtii is well understood36, 38, 39. In C. reinhardtii, transcript analysis suggested that N depletion can directly lead to the funneling of acetate into fatty acid biosynthesis, causing a significant increase in lipid droplets, whereas protein biosynthesis is down-regulated, and a subset of control genes involved in gametogenesis are activated38. Moreover, rapidly developing post-genomics biological approaches have become practical for obtaining an in-depth understanding of how microbes respond and adapt to variations in the external environment, especially among algae without genome sequences. There are also a few studies based on RNA-seq methods that have focused on the molecular changes in non-sequenced microalgae when N is limited in culture conditions, especially in the biosynthesis pathway responsible for lipid formation and accumulation34, 40,41,42,43,44,45.
To investigate the response and alterations of T. pseudonana metabolic pathways induced by N depletion, we combined physiological data, RNA-Seq analysis and proteomic profiling to analyze diverse responses through mapping using the reference genome in the Joint Genome Institute (JGI) database. The results of this analysis can help us to further understand how this microalga fundamentally responds to physiological N stress. Both RNA-seq and proteome data were used in comparisons and to identify positional polymorphisms as well as differential expression levels. Through integrated analysis of RNA-seq and proteome profiles, we obtained differentially expressed transcripts and proteins as well as correlated items, which were further analyzed through functional annotation and pathway enrichment analyses. In this study, we used Nile Red dye to stain cells to semi-quantify the lipid content in cells for monitoring of intracellular secondary metabolic conditions. When systematic calculation was performed, 782 genes and 362 proteins showing differential expression between the treatments emerged. Our goal was to gain thorough insight into the molecular changes that promote or accompany lipid accumulation in T. pseudonana cells, especially for genes associated with photosynthesis, carbon fixation and N uptake, and we aimed to gain a whole-cell view of adaption and survival to further explain some of the physiological phenomena that are observed in N-depleted environments.
Materials and Methods
Culture conditions
A Chinese T. pseudonana strain was cultivated at 20 ± 1 °C under 220 μmol of photons m−2 s−1 and a 12 h photoperiod. Algal cells were initially activated twice in complete f/2 medium, and sodium nitrate was the only N supplement. The cells were transferred when they reached the exponential stage, at a cell density of approximately 5.5 × 104 cells/ml, and were then cultured under N-replete (NR) (f/2 medium, with an initial 882 μM nitrate concentration) or N-depleted (ND) (f/2 medium minus nitrate, approximately 182 μM initial nitrate concentration) conditions; the day of the transfer was set as day 1. Cell growth conditions were monitored daily, including measurements of cell density, chlorophyll content, Nile Red fluorescence and free N (nitrate and nitrite) remaining in the medium. To estimate chlorophyll contents, 20 ml of cultivated cells were harvested using GF/C filters (Whatman, UK), after which the filters were immersed in 6 ml of 90% acetone. Following incubation of the cells at 4 °C for at least 16 h in darkness, the filters and cells were removed via centrifugation, and the supernatant fluid was subjected to OD measurements using an Infinite M200 Pro microplate reader (Tecan, Switzerland), with 90% acetone as the blank (Mitchell, BG, 1984). The chlorophyll content was estimated using the following formulas46:
To quantify lipid levels, 1 ml samples of the cultures were mixed with Nile Red solution (0.1 mg/ml in acetone) (100/1, v/v) and were then analyzed after 7 min on an Infinite M200Pro microplate reader (Tecan, Switzerland). The fluorescence emission spectra were measured at 580 nm, with 544 nm as the excitation wavelength47. The N concentration remaining in the medium was determined from measurements of nitrate and nitrite concentrations48. All conditions were assessed in triplicate. The cells were harvested in mid-stationary phase using sterile 0.45 μm filters, and all samples were snap-frozen in liquid nitrogen and stored in a −80 °C freezer.
RNA extraction and RNA-Seq data analysis
Total RNA was obtained from the snap-frozen N-replete and N-deprived (day 4) cultures described above using the TRIzol reagent (Invitrogen, USA) according the manufacturer’s protocol. First, the cells were treated with DNase I to remove DNA contamination, and the total RNA was then enriched using oligo(dT) magnetic beads. The procedures for library construction and sequencing were consistent with previous reports49. Briefly, the purified mRNA was fragmented into ~200 bp fragments that were suitable for sequencing. After the first strand of cDNA was successfully synthesized using random hexamer primers, the second strand was synthesized. The double-stranded cDNA was again purified with magnetic beads. After end reparation and ligation of the sequence adaptors, the modified fragments were amplified via PCR. During the QC step, an Agilent 2100 Bioanalyzer (USA) and an ABI StepOnePlus Real-Time PCR System (USA) were used to qualify and quantify the sample libraries. Finally, the library products were sequenced with an Illumina HiSeqTM 2000 system (USA).
For base calling, the raw image data were converted to sequence data with a length of 49 bp, which were defined as the raw data. The adaptor sequences were first removed, and the raw reads were processed by filtering out low-quality data (more than 10% unknown bases; more than 50% of bases showing Q 5 within a read). The retained high-quality reads, which were considered clean reads, were then mapped to reference sequences and reference genes (http://genome.jgi-psf.org/Thaps3/Thaps3.home.html) using SOAP250, allowing 3 and 2 mismatches, respectively, in the alignment. The methods for identifying differentially expressed genes (DEGs) were the same as the methods used in a previous study51 based on the Poisson distribution model. An “FDR (false discovery rate) ≤0.001 and an absolute value of the log2Ratio ≥1 (fold-change ≥2)” were used as the threshold to judge the significance of differences gene expression52. Cluster 3.053 software was subsequently used to perform cluster analysis of gene expression patterns.
Isolation and preparation of total protein
Both samples were ground to a powder in liquid nitrogen. Total protein was then extracted according the manufacturer’s recommendations in the next step. Briefly, the samples were mixed with lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 40 mM Tris-HCl pH 8.5, 1 M PMSF and 2 mM EDTA). After adding 10 mM DTT, the suspension was sonicated and centrifuged. Then, the supernatant was mixed with a 5-fold volume of chilled acetone, followed by incubation at −20 °C for 2 hr. The resultant protein pellets were suspended in lysis buffer, then sonicated and centrifuged again. To reduce disulfide bonds, the supernatant was incubated at 56 °C with 10 mM DTT for 1 hr. Subsequently, the samples were incubated in darkness for 45 min with 55 mM IAM added to block the cysteines. The samples were then pelleted using chilled acetone and reconstituted in 0.5 M TEAB (Applied Biosystems). Finally, the supernatant was transferred to a new tube and quantified using a Bradford Protein Assay Kit. Next, 100 μg of total protein was taken from each sample solution and digested with Trypsin Gold (Promega, USA) into peptides. According the manufacturer’s protocol for the 8-plex iTRAQ reagent (Applied Biosystems, USA), the peptides were labeled with the isobaric tag and dried via vacuum centrifugation. SCX fractionation was performed with an LC-20AB HPLC Pump system (Shimadzu, Japan), and the peptides were pooled into 20 fractions, which were subsequently loaded into an LC-20AD nanoHPLC (Shimadzu, Japan). Data acquisition was performed with a TripleTOF 5600 System (AB SCIEX, USA) fitted with a Nanospray III source (AB SCIEX, USA) and a pulled quartz tip as the emitter (New Objectives, USA).
Proteome analysis
The proteome analysis included peptides identification, protein identification and protein quantification. The mass tolerance of 0.05 Da (ppm) was permitted for intact peptide masses, allowing for one missed cleavage in the trypsin digests. To reduce the probability of false peptide identification, only peptides showing significance scores ≥20 in the 99% confidence interval in a Mascot probability analysis as falling above the “identify” threshold was counted as identified peptides. Then, using Mascot 2.3.02 (Matrix Science, London, United Kingdom)54, mass spectrum data was converted to MGF (Mascot generic format) format and the final protein identification results were obtained against a database containing T. pseudonana sequences. In this process, every confidently identified protein involved at least two unique peptides and FDR for the peptide spectrum level was set as < = 0.01. The detailed procedures are described in http://www.matrixscience.com/help/quant_statistics_help.html. With the Mascot 2.3.02 software, the student’s T-test was performed in this analysis. Briefly, a protein ratio is reported in bold face if it is significantly different from unity. The comparison test is:
There is no significant difference at the stated confidence level when this inequality is true. (N is the number of peptide ratios, s represents the standard deviation and x are the mean of the peptide ratios, both numbers calculated in log space. The true value of the ratio, µ, is 0 in log space. t is student’s t for N-1 degrees of freedom and a two-sided confidence level of 95%). Finally, the protein quantification and statistical significance of the protein quantitative ratios was performed by the software of Iquant55. The ratio between the NR and the ND was obtained directly using the protein abundance for any given protein. We used p-value < 0.05 and only those with fold changes >1.5 were considered as differentially expressed proteins. In IQuant analysis, the mascot percolator algorithm methods was combined56 and protein-level FDR < = 0.01 was used to decrease the false positive rate57. The statistical analysis refers to permutation tests which have been widely used in the fields of microarray and RNA-Seq data analysis58, 59.
Functional annotation
For both the RNA-Seq and proteome data, functional annotations were conducted using the BLAST2GO (Gene Ontology) program, along with the non-redundant protein database (Blastnr; NCBI). The cluster of Orthologous Groups (COG) database (http://www.ncbi.nlm.nih.gov/COG/) was employed to classify and group the putative and definitively identified proteins. The KEGG database (http://www.genome.jp/kegg/) was used to obtain maps of regulated metabolic pathways in molecular interaction and reaction networks.
Results and Discussion
Physiological performance during N deprivation
To monitor their growth, the T. pseudonana cultures were sampled at the same time (12:00) every day and were used to measure cell counts, chlorophyll contents, Nile Red fluorescence and the free N remaining in the medium. Through the examined period, the N deprivation treatment showed a similar growth curve to N repletion and the cell counts obtained under both conditions increased from an initial count of 5.57 × 104 cells/mL to a plateau of 2.57 × 106 cells/mL on day 7. Then, the count under N deprivation slightly declined to 2.40 × 106 cells/mL (Fig. 1A). Different N concentrations also led to other substantial physiological changes. As observed under a microscope, algae cell size was smaller under the N deprivation condition (data not shown). The contents of Chl a and Chl c 1 + c 2 showed a continuous increase overall in f/2 medium, with Chl a increased from 0.15 to 0.27 pg/cell at the early stage (before 96 h). On day 4, a decrease in the chlorophyll a content was observed (Fig. 1B). Similar results were observed during nitrogen depletion in Phaeodactylum tricornutum and Neochloris oleoabundans 60, 61. The fluorescence intensity value obtained under N deprivation was 3.4-fold of that under N repletion, indicating that the neutral lipid content was significantly higher on day 8 (Fig. 1C). Regarding the N content in the culture environment, the trend in changing nitrate levels was like that for Chl a. The nitrate concentration showed a continuous drop and even became exhausted under the N deprivation condition (Figure S1A,B). Results showed that the nitrate and nitrite were indeed consumed in the ND treatment, but there was always remained nitrate in the NR treatment. The observed trend again confirmed the N utilization mechanism. Ammonium is the top priority N source in diatom cells. Once ammonium is used up, the cells will assimilate extracellular nitrate and deoxidize it into nitrite through nitrate reductase, then pump the nitrite out of the cells62. In this process, most of the reduced nitrite is retained in the cell for further incorporation into biomass though some can leak out. After nitrate was exhausted, extracellular nitrite was assimilated into cells to maintain regular physiological metabolism on day 5. N deprivation has been demonstrated to have an impact on chlorophyll synthesis and even photosynthesis in many eukaryotic plants63. Likewise, when diatom cells were cultured in an N-deprived environment in the present study, the chlorophyll content decreased dramatically. The intracellular neutral lipid content increased when N deprivation occurred, which has been shown to occur in many other algae31,32,33,34,35,36,37.
Physiological parameters of T. pseudonana under different N levels. The entire cultivation period was 9 days. (A) Growth of algal cells under N-replete (NR) and N deprivation (ND) conditions. (B) T. pseudonana cellular chlorophyll content in NR and ND treatments. (C) Nile Red fluorescence intensity per 104 cells. The data are displayed as the means ± SD (n = 3).
Processed RNA-Seq and proteome data
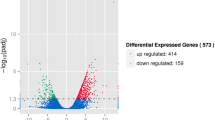
To identify algal N-stress response-related genes in the diatoms, comparative transcript profiling of T. pseudonana cells during the mid-stationary phase (on the eighth day of culture) was performed under different N concentrations using RNA-Seq. A total of 6,264,454 and 6,249,215 high-quality reads (with the adaptors removed and low-quality data filtered out) were obtained, and approximately 64% and 42% of reads could be mapped to the genome or genes of the T. pseudonana genome in the ND and the NR conditions, respectively (Table S1). The expression level for each gene was determined from the numbers of reads that were uniquely mapped to the specific gene and the total number of uniquely mapped reads in the samples, which were calculated using the RPKM method (reads per kilobase per million mapped reads)64. The unique reads were mapped to 11,296 and 11,338 transcripts for ND and NR, respectively, which were clustered to a final consensus transcriptome of 11,385 transcripts (slightly larger than the reference gene model) using SOAP2.21. Based on the RNA-seq analysis, 11,241 transcripts were clustered to T. pseudonana CCMP 1335, and the remaining 145 transcripts were clustered to Thalassiosira oceanica (51), T. pseudonana (48, not the CCMP 1335 strain), Phaeodactylum tricornutum CCAP 1055/1(13), other species (13) and unidentified species (19) using the JGI database for user annotation references and sources for common gene names. A total of 782 differentially expressed genes (DEGs) were identified in this dataset. Two hundred and seven genes were up-regulated, and 575 genes were down-regulated in the ND treatment compared with the NR treatment (Fig. 2). The Blast2GO program was used to provide Gene Ontology (GO) annotation information. WEGO software65 was employed for GO functional classification for DEGs and to understand the overall distribution of gene functions in the species (Figure S2). The greatest numbers of DEGs among the three categories were the 250 terms obtained for metabolic processes (i.e., biological processes), the 174 terms for cell parts (i.e., cellular components) and the 211 terms for catalytic activity (i.e., molecular functions). Through KEGG pathway classification, 320 DEGs were annotated and assigned to at least one of the 86 metabolic pathways. The top 10 categories of pathways were as follows: metabolic pathways (135), biosynthesis of secondary metabolites (71), purine metabolism (24), glycolysis/gluconeogenesis (19), photosynthesis-antenna proteins (18), RNA transporter proteins (15), photosynthesis (13), carbon fixation in photosynthetic organisms (12) and amino sugar as well as nucleotide sugar metabolism (12) (Figure S3). The proteomics profile analysis was based on iTRAQ labels and protein extraction, in parallel with transcriptome profiling. From a total of 8,206 peptides, 8,093 unique peptides and 2,564 proteins were identified in the proteome. The mass of the identified proteins was mainly distributed between 20~50 kDa, and the highest percentage was ~15% in the 30~40 kDa range. The quantitative protein ratios were weighted and normalized based on the median ratio from Mascot2.3.02.The protein quantification and statistical significance of the protein quantitative ratios was performed by the software of Iquant, the candidate differentially expressed proteins ratios was identified with p-value < 0.05 and only fold changes >1.5, then the differentially expressed proteins (DEPs) were identified, among which 152 proteins were more abundant in the ND condition, while 210 proteins were more abundant in the NR condition. Among the total 362 DEPs, 196, 143 and 200 terms were classified as biological processes, cellular components and molecular functions, respectively. In the KEGG annotation analysis, 5 pathways showed at least 10 terms, including metabolic pathways (87), biosynthesis of secondary metabolites (47), photosynthesis (11), oxidative phosphorylation (11) and protein processing in the endoplasmic reticulum (10).
Differentially expressed genes under N repletion (NR) and N deprivation (ND). Distribution of gene expression (log10-transformed RPKM values (reads per kilobase per million mapped reads)) between NR and ND, with the differentially expressed genes highlighted. The colors represent different probability values. The darker regions represent a higher gene density.
Integrated analysis of the transcriptome and proteome led to a total of 1,665 correlations being identified between quantitative transcripts and proteins as well as 80 correlations identified in both DEGs and DEPs. A further analysis showed a rather weak correlation between the protein and gene expression levels, with r = 0.1617. This phenomenon also appears in bacteria, and transcript abundance and protein abundances are not necessarily correlated66. On the other hand, the different datasets (i.e., transcript and protein) may provide key aspects of the physiological state in the cells. Previous studies on P. tricornutum based on several gene RT-PCR and related protein analyses showed similar abundance trends67. Based on whole-genome transcript and protein analyses conducted in this study and in P. tricornutum, some genes may show similar transcript and protein trends, but these trends are not correlated well with each other60. However, the different transcript and protein data demonstrated the same expression trends in 64 proteins, including 13 down-regulated and 51 up-regulated proteins, and a similar trend was found for the correlation coefficient was well (r = 0.5053). These results may further show that this approach may be more comprehensive for assessing genome-wide changes and the complex relationships between the transcripts and proteins.
Transcripts of photosynthesis and carbon-fixation-related genes decreased
N deprivation may influence photosynthetic efficiency in T. pseudonana, as the shown in Fig. 3 ref. 68 (detailed number as Table 1 and Dataset 1). The abundance of transcripts encoding photosynthesis-related proteins was substantially reduced, including those involved in chlorophyll biosynthesis and the efficiency of PSI and PSII. A similar phenomenon was discovered in P. tricornutum during N deprivation69. Chlorophyll is a nitrogenous macromolecule, and reduction of chlorophyll suppresses the nitrogen demand, in addition to affecting the light-harvesting capacity and production of reactive oxygen species3. In this study, the transcript encoding GSA markedly decreased by more than 10-fold. Chlorophyll b reductase, which is an enzyme that catalyzes Chl b to 7-hydroxy-chlorophyll a, also showed a reduction in both the transcriptomic and proteomic datasets. The abundance of transcripts encoding chlorophyll biosynthesis proteins was reduced for both the precursor and key enzymes, and this situation could decrease the chlorophyll content directly, which was consistent with the chlorophyll content indicated above. Most of the genes encoding antenna proteins in PS I and PSII were sharply down-regulated, especially in PS I, and the synthesis of chlorophylls and antenna proteins was inhibited (Dataset 1 and Fig. 3)68. This inhibition may have resulted in excess light energy. To protect itself from light injury, light-harvesting complex II could alter its function from light-harvesting to light-dissipating to reduce excess light energy69. Among all transcripts encoding antenna proteins, PsbA in the D1 protein was the only gene that was up-regulated 2-fold, which suggests that turn-over of the D1 protein became faster to prevent photo-inhibition(Fig. 3)68, 70. In diatoms, the xanthophyll cycle can induce non-photochemical quenching (NPQ) to protect PS II from excess light energy, and a decrease of NPQ therefore has a positive impact and harms PS II71,72,73. In the photosynthetic electron transport pathway, the reaction in the cytochrome b6 subunit of the b 6 f complex is a rate-limiting step, and plastoquinone is the direct electron donor to cytochrome b6f 74, 75. In chloroplasts, dynamic ATP biosynthesis is hypothesized to occur through an ion gradient generated via electron transport74. For the PsbO of photosynthesis proteins and the b 6 subunit of the cytochrome b 6 f complex, both the transcripts and proteins were down-regulated (Fig. 3)68.
The KEGG photosynthesis pathway map for T. pseudonana during N depletion compared to N replete conditions (ND vs. NR). The KEGG photosynthesis pathway map can be found online at http://www.kegg.jp/pathway/map00195. The light green boxes indicate the genes were down-regulated and up-expressed (red boxes). The highlighted in red indicate the protein represent up-expressed and down-expressed (green word). The detail fold-change and annotation of these genes were summarized in Table 1 and Dataset 1.
As one part of the electron transport, NADPH was produced in the last step of the electron chain of the light reactions of photosynthesis. The NADPH-catalyzing enzyme ferredoxin-NADP+ reductase (the fold-change of Thaps25892 and Thaps4914 respectively is 6.0 and 2.8) showed a marked decrease in transcript abundance, but an increase in protein abundance. NADPH, pumped-protons and ATP were used to help turn the carbon dioxide into glucose. When the protons travel through the CF1 structure in F-type ATPase, the energy that is released causes the γ subunit to revolve to induce a conformational change and produce ATP74, 76. Therefore, the oxygen, NADPH and ATP generated through photosynthesis would all decrease, which finally regulated the carbon fixation.
Following N deprivation, the mRNA abundance of most enzymes in the Calvin cycle decreased, leading to a decline of the carbon fixation in T. pseudonana (Fig. 4). Among these genes, two ATP-dependent enzymes, phosphoribulokinase and phosphoglycerate kinase, showed dramatic decreases of 7.1 and 49.7-fold, which demonstrated that ATP biosynthesis had decreased in the photophosphorylation pathway. Due to PEPCKase and PEPCase genes of the T. pseudonana genome10, there is still no agreement whether T. pseudonana contains the C4 pathway77, 78. In this study, the abundance of PEPCase increased by 50% of the protein level observed in cells in f/2 medium, although we did not perform experiments assessing CO2 concentrations. Two key enzymes involved in gluconeogenesis, pyruvate carboxylase and fructose-1,6-bisphosphatase I decreased by 3.2 and 5.8-fold in terms of transcript abundance (Fig. 4). This regulation extended to the expression of the phosphoglucomutase gene, which catalyzes the transformation of glucose-6P to glucose-1P and finally suppresses starch synthesis. Thus, carbohydrate biosynthesis had been suppressed obviously in cells in an N-limited environment.
The KEGG calvin cycle and starch synthesis pathway for T. pseudonana during N depletion compared to N replete conditions (ND vs. NR). The serial number represent the related enzyme and the negative number indicate the down-expressed fold change of the genes (The opposite is up-expressed). The Inside the parentheses is the protein fold change.
Carbohydrate decomposition and utilization
As the key and rate-limiting enzyme in glycolysis, 6-phosphofructokinase transcript abundance was increased 2.9-fold (Fig. 5), which could be activated by AMP and suppressed by ATP. In addition, another key enzyme, pyruvate kinase, displayed a slight decrease in transcript abundance but showed the opposite trend in protein abundance (Fig. 5). The activity of this enzyme has an important influence on fatty acid biosynthesis79. These two enzymes are specific to glycolysis, indicating that this was the direction of carbon flow for producing more ATP and pyruvate. Pyruvate can be converted to acetyl-CoA as a carbon input for the TCA cycle.
The glycolysis, pentose phosphate, TCA cycle and oxidative phosphorylation pathway in T. pseudonana during N depletion compared to N replete conditions (ND vs. NR). The serial number represent the related enzyme and the negative number indicate the down-expressed fold change of the genes (The opposite is up-expressed). The Inside the parentheses is the protein fold change.
The pentose phosphate pathway is another main pathway for degrading glucose and producing NADPH in cells; this pathway consists of an oxidative phase and a non-oxidative phase. However, the abundance of transcripts encoding ribulose-phosphate 3-epimerase and transketolase, which catalyzes the transformation of ribulose-5P to xylulose-5P and sedoheptulose-7P, and then to fructose-6P and glyceraldehyde-3P, both of which were decreased more than 5-fold. Ribose 5-phosphate isomerase, which catalyzes the transformation of ribulose-5P to ribose-5P (precursors of nucleotides), showed a 4.6-fold decrease (Table 1). To maintain regular metabolism during N deprivation, glucose preferentially enters glycolysis to produce ATP, rather than the pentose phosphate pathway for producing NADPH. Thus, as the main pathway for producing NADPH, the decrease in the phosphate pathway could affect fatty acid biosynthesis because fatty acid biosynthesis requires plentiful NADPH.
Pyruvate, as a carbon input into the TCA cycle, is converted to acetyl-CoA by the pyruvate dehydrogenase complex, which consists of pyruvate dehydrogenase (E1), dihydrolipoamide acetyltransferase (E2) and dihydrolipoamide dehydrogenase (E3). Among these three enzymes, both transcripts encoding E1 were increased to 2-fold of the level in the NR treatment. The abundance of the transcript encoding E2 was sharply down-regulated by 36.8-fold, while the level of the E2 protein only decreased slightly (Fig. 5). For E3, there was one down-regulated transcript, and one up-regulated protein was detected. On the other hand, pyruvate can be catalyzed to oxaloacetate by pyruvate carboxylase, and one transcript was decreased to almost one-third of the amount in the NR treatment (Fig. 5). Thus, how is the acetyl-CoA content altered in cells? The acetyl-CoA content shows an important relationship with fatty acid biosynthesis. Does the increased level of acetyl-CoA suppress the activity of E2 via negative feedback? From fatty acid biosynthesis and the degradation of the Val, Leu and Ile pathways, there was no signal for increasing acetyl-CoA. Additionally, acetyl-CoA is a strong activator for pyruvate carboxylase, which was found to be up-regulated in this study, indicating that the content of acetyl-CoA in cells was decreased (Fig. 5). Although the synthesis of acetyl-CoA was suppressed, the TCA cycle was enhanced due to two key enzymes, citrate synthase and isocitrate dehydrogenase, which were increased in both transcript and protein abundance. Additionally, the abundance of dihydrolipoamide succinyltransferase and the succinate dehydrogenase enzyme increased almost 2-fold. Enhancement of the TCA cycle typically indicates that an organism requires more ATP or intermediates as precursors for other pathways.
The oxaloacetate generated from the TCA cycle could be converted to phosphoenolpyruvate (PEP) and gluconeogenesis to produce glucose or serve as a carbon input into glyoxylate metabolism to form acetyl-CoA, which would in turn form oxaloacetate, which again participates in the TCA cycle. The biological function of the TCA cycle is to degrade triacylglycerol to acetyl-CoA and form glucose for cells. Glyoxylate metabolism showed not change, which was consistent with the decrease in gluconeogenesis.
Oxidative phosphorylation re-oxidizes NADH and FADH2, which are mainly generated from glycolysis and the TCA cycle through the electron transport pathway. However, both the transcriptome and proteome datasets showed decreases in NADH dehydrogenase.
N utilization and metabolism
In the ND treatment, inorganic N was exhausted on day 4. Both the transcript and protein abundance of nitrate reductase and ferredoxin nitrite reductase, which catalyze nitrate to nitrite and nitrite to NH3, respectively, declined. After day 4, the nitrogen was not enough, the cellular chlorophyll content significantly declined and the activity of the nitrate reductase and ferredoxin nitrite reductase were affected. Therefore, the cells may produce stress reaction and showed an increased affinity for nitrogen, resulting in the increase of the reduced flavodoxin abundance.
There were changes in two glutamine synthetases responsible for catalyzing the transformation glutamate and NH3 to glutamine. One was decreased in mRNA and protein abundance, while the other one only showed changes in protein abundance, with an almost 2-fold increase being observed.
In amino acid metabolism pathways, both the transcriptome and proteome datasets showed that catabolism was faster than anabolism. Glutamate synthase catalyzes the transformation of glutamine to glutamate, which is then dehydrogenized to form 2-oxoglutarate; phenylalanine is catalyzed to form tyrosine and then fumarate; aspartate is catalyzed to form transaminase; lysine, leucine, valine, isoleucine, cysteine, alanine, glycine and serine are catalyzed to form acetyl-CoA; and these products are finally converted in the TCA cycle.
Following N depletion, citrate synthase and isocitrate dehydrogenase were up-regulated by 2.4 (transcription level), 1.9 (protein level) and 2.7 (transcription level), 1.6-fold (protein level), respectively (Fig. 5). The cells may decompose less important proteins more quickly, and the free amino acids may be used to synthesize new proteins or enter the TCA cycle to produce intermediates for other pathways in terms of maintaining regular catabolism.
Lipid biosynthesis and metabolism
Fatty acid de novo biosynthesis was found to be suppressed in both the transcriptome and proteome datasets, as the key enzyme acetyl-CoA carboxylase showed a decrease in transcript abundance. Acetyl-CoA carboxylase (ACCase), which catalyzes the transformation of acetyl-CoA into malonyl-CoA (an important precursor in the synthesis of fatty acids), decreased dramatically, as did other enzymes in this pathway (Fig. 6). Although ACCase is regarded as a key enzyme in fatty acid biosynthesis, overexpression of ACCase in diatoms does not improve the intracellular lipid content80. As a decrease in fatty acid biosynthesis occurs, palmitic acid elongation is also suppressed, including elongation in mitochondria and on the endoplasmic reticulum (Fig. 6). The restriction of fatty acid biosynthesis could be due to the decrease in acetyl-CoA contents mentioned above as well as the decrease in NADPH, which is treated as the reducing power in fatty acid biosynthesis and is mainly generated through the pentose phosphate pathway. This reduction could also be the reason that ACCase overexpression occurred without any increase in lipid content (Fig. 6).
The lipid synthesis and metabolism pathway in T. pseudonana during N depletion compared to N replete conditions (ND vs. NR). The serial number represent the related enzyme and the negative number indicate the down-expressed fold change of the genes (The opposite is up-expressed). The Inside the parentheses is the protein fold change. There are serval genes in one enzyme.
In this study, there was no significant evidence that the fatty acid β-oxidation pathway became more active. Only long-chain acyl-CoA synthetase, which catalyzes long-chain fatty acids to acyl-CoA, showed a marked increase in both mRNA and protein abundance. Following that increase, acyl-CoA could have been transported into mitochondria through the synergistic effects of carnitine acyltransferase I and carnitine/acylcarnitine translocase and subsequently entered the β-oxidation pathway. In addition, acyl-CoA was is of the sources for TAG synthesis.
According to the Nile Red data, the intracellular lipid content (mainly consisting of TAG) was increased under the ND treatment. However, neither the transcriptome nor proteome dataset showed any enhancement of enzymes related to TAG biosynthesis, including cytosolic glycerol-3-phosphate acyltransferase (GPAT), lysophosphatidic acid acyltransferase (LPAAT), phosphatidic acid phosphatase (PAP) and diacylglycerol acyltransferase (DGAT). This finding is similar with previous studies. Hockin et al. did not detect any obvious changes in the enzymes involved in the fatty acid and lipid biosynthesis pathways via protein analysis81. The abundance of another source of TAG, sn-glycerol-3-phosphate, which is transformed from glycerone phosphate, also showed no change.
Hence, what was the source of TAG? In Chlamydomonas reinhardtii, TAG is assumed to be remodeled from membrane phospholipids38. Phospholipid:diacylglycerol acyltransferase (PDAT) can catalyze the transformation of phospholipids to diacylglycerol (DAG) as a precursor to TAG synthesis82, 83. In the present study, there was no signal of any changes in PDAT in the transcriptome or the proteome. Interestingly, phospholipase D can carry out the catalysis of phosphatidylcholine and phosphatidyl-ethanolamine (both of which are major components in membrane phospholipids) to 1,2-diacyl-sn-glycerol-3-phosphate, and the transcript abundance of this enzyme showed a decrease in the present study. All of these findings indicated that phospholipid degradation was suppressed in T. pseudonana, which was quite different from what occurs in green algae38, 45. However, our results do not allow us to deduce the original source of TAG. Thus, additional experiments will be required to explore this unanswered question.
Conclusion
In this study, T. pseudonana displayed a significant decrease in photosynthesis, including decreases in chlorophyll and antenna protein biosynthesis, electron transport and photophosphorylation, in both RNA-seq and proteome data, which further resulted in decreased carbon fixation and gluconeogenesis abilities under N depletion. The enhancement of glycolysis and the TCA cycle and the maintenance of the pentose phosphate pathway and the glyoxylate cycle indicated that cells preferred to preserve the functions of the main pathways to generate energy and intermediates. Furthermore, oxidative phosphorylation was decreased in the cells to slow their metabolism, with only intermediates for the most required substances being catalyzed, thereby resisting negative external impacts. As N became exhausted in the media, cells began to degrade unnecessary proteins to produce free amino acids to synthesize more important proteins or to enter the TCA cycle to maintain their metabolism. Although the intensity of Nile Red fluorescence showed a marked increase under the ND treatment compared with that under the NR treatment, we could not find any evidence of enhancement of transcripts or proteins involved in TAG biosynthesis pathways. There may be some undetected pathways or mechanisms that produce TAG, and we will need to conduct further experiments to evaluate this possibility. A combination of RNA and protein profiling was shown to be able to reveal most of the genes in pathways associated with physiological activity under nitrogen-depleted conditions in Thalassiosira pseudonana, which could be valuable for future physiological research in phytoplankton.
References
Flynn, K. Algal carbon–nitrogen metabolism: a biochemical basis for modelling the interactions between nitrate and ammonium uptake. Journal of Plankton Research 13, 373–387 (1991).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196, doi:10.1126/science.1186120 (2010).
Hockin, N. L., Mock, T., Mulholland, F., Kopriva, S. & Malin, G. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant physiology 158, 299–312, doi:10.1104/pp.111.184333 (2012).
Pichrtova, M., Kulichova, J. & Holzinger, A. Nitrogen limitation and slow drying induce desiccation tolerance in conjugating green algae (Zygnematophyceae, Streptophyta) from polar habitats. PloS one 9, e113137, doi:10.1371/journal.pone.0113137 (2014).
Alipanah, L., Rohloff, J., Winge, P., Bones, A. M. & Brembu, T. Whole-cell response to nitrogen deprivation in the diatom Phaeodactylum tricornutum. Journal of experimental botany, doi:10.1093/jxb/erv340 (2015).
Takagi, M., Watanabe, K., Yamaberi, K. & Yoshida, T. Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Applied microbiology and biotechnology 54, 112–117 (2000).
Nelson, D. M., Treguer, P., Brzezinski, M. A., Leynaert, A. & Queguiner, B. Production and Dissolution of Biogenic Silica in the Ocean - Revised Global Estimates, Comparison with Regional Data and Relationship to Biogenic Sedimentation. Global Biogeochemical Cycles 9, 359–372, doi:10.1029/95gb01070 (1995).
Shrestha, R. P. et al. Whole transcriptome analysis of the silicon response of the diatom Thalassiosira pseudonana. BMC genomics 13, 499, doi:10.1186/1471-2164-13-499 (2012).
Fu, M., Song, X., Yu, Z. & Liu, Y. Responses of phosphate transporter gene and alkaline phosphatase in Thalassiosira pseudonana to phosphine. PloS one 8, e59770, doi:10.1371/journal.pone.0059770 (2013).
Armbrust, E. V. et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 306, 79–86, doi:10.1126/science.1101156 (2004).
Parslow, J., Harrison, P. & Thompson, P. Saturated uptake kinetics: transient response of the marine diatom Thalassiosira pseudonana to ammonium, nitrate, silicate or phosphate starvation. Marine Biology 83, 51–59 (1984).
Reynolds, R. A., Stramski, D. & Kiefer, D. A. The effect of nitrogen limitation on the absorption and scattering properties of the marine diatom Thalassiosira pseudonana (DTIC Document, 1996).
Berges, J. A., Varela, D. E. & Harrison, P. J. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae). Marine Ecology Progress Series 225, 139–146 (2002).
Stramski, D., Sciandra, A. & Claustre, H. Effects of temperature, nitrogen, and light limitation on the optical properties of the marine diatom Thalassiosira pseudonana. Limnology and Oceanography 47, 392–403 (2002).
Bucciarelli, E. & Sunda, W. G. Influence of CO2, nitrate, phosphate, and silicate limitation on intracellular dimethylsulfoniopropionate in batch cultures of the coastal diatom Thalassiosira pseudonana. Limnology and Oceanography 48, 2256–2265 (2003).
Holzinger, A. et al. Transcriptomics of desiccation tolerance in the streptophyte green alga Klebsormidium reveal a land plant-like defense reaction. PloS one 9, e110630, doi:10.1371/journal.pone.0110630 (2014).
Maheswari, U. et al. Digital expression profiling of novel diatom transcripts provides insight into their biological functions. Genome biology 11, R85, doi:10.1186/gb-2010-11-8-r85 (2010).
Bertrand, E. M. et al. Influence of cobalamin scarcity on diatom molecular physiology and identification of a cobalamin acquisition protein. Proceedings of the National Academy of Sciences of the United States of America 109, E1762–E1771, doi:10.1073/pnas.1201731109 (2012).
Matsuzaki, M. et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428, 653–657, doi:10.1038/nature02398 (2004).
Derelle, E. et al. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proceedings of the National Academy of Sciences of the United States of America 103, 11647–11652, doi:10.1073/pnas.0604795103 (2006).
Bowler, C. et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456, 239–244, doi:10.1038/nature07410 (2008).
Bhattacharya, D. et al. Genome of the red alga Porphyridium purpureum. Nature communications 4, 1941, doi:10.1038/ncomms2931 (2013).
Ai, X. Y. et al. A global view of gene activity at the flowering transition phase in precocious trifoliate orange and its wild-type [Poncirus trifoliata (L.) Raf.] by transcriptome and proteome analysis. Gene 510, 47–58, doi:10.1016/j.gene.2012.07.090 (2012).
Dyhrman, S. T. et al. The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PloS one 7, e33768, doi:10.1371/journal.pone.0033768 (2012).
Sapriel, G. et al. Genome-wide transcriptome analyses of silicon metabolism in Phaeodactylum tricornutum reveal the multilevel regulation of silicic acid transporters. PloS one 4, e7458, doi:10.1371/journal.pone.0007458 (2009).
Osborn, H. L. & Hook, S. E. Using transcriptomic profiles in the diatom Phaeodactylum tricornutum to identify and prioritize stressors. Aquatic toxicology 138–139, 12–25, doi:10.1016/j.aquatox.2013.04.002 (2013).
Davis, A. K., Hildebrand, M. & Palenik, B. Gene expression induced by copper stress in the diatom Thalassiosira pseudonana. Eukaryotic cell 5, 1157–1168, doi:10.1128/EC.00042-06 (2006).
Shi, X., Gao, W. M., Chao, S. H., Zhang, W. W. & Meldrum, D. R. Monitoring the Single-Cell Stress Response of the Diatom Thalassiosira pseudonana by Quantitative Real-Time Reverse Transcription-PCR. Applied and environmental microbiology 79, 1850–1858, doi:10.1128/Aem.03399-12 (2013).
Carvalho, R. N., Bopp, S. K. & Lettieri, T. Transcriptomics responses in marine diatom Thalassiosira pseudonana exposed to the polycyclic aromatic hydrocarbon benzo[a]pyrene. PloS one 6, e26985, doi:10.1371/journal.pone.0026985 (2011).
Sciandra, A. et al. Response of coccolithophorid Emiliania huxleyi to elevated partial pressure of CO2 under nitrogen limitation. Marine Ecology Progress Series 261, 111–122, doi:10.3354/meps261111 (2003).
Yamaberi, K., Takagi, M. & Yoshida, T. Nitrogen depletion for intracellular triglyceride accumulation to enhance liquefaction yield of marine microalgal cells into a fuel oil. Journal of Marine Biotechnology 6, 44–48 (1998).
Mandal, S. & Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Applied Microbiology and Biotechnology 84, 281–291, doi:10.1007/s00253-009-1935-6 (2009).
Dean, A. P., Sigee, D. C., Estrada, B. & Pittman, J. K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresource Technology 101, 4499–4507, doi:10.1016/j.biortech.2010.01.065 (2010).
Rismani-Yazdi, H., Haznedaroglu, B. Z., Hsin, C. & Peccia, J. Transcriptomic analysis of the oleaginous microalga Neochloris oleoabundans reveals metabolic insights into triacylglyceride accumulation. Biotechnology for Biofuels 5, doi:10.1186/1754-6834-5-74 (2012).
Recht, L., Zarka, A. & Boussiba, S. Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Applied Microbiology and Biotechnology 94, 1495–1503, doi:10.1007/s00253-012-3940-4 (2012).
Msanne, J. et al. Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydornonas reinhardtii and Coccomyxa sp C-169. Phytochemistry 75, 50–59, doi:10.1016/j.phytochem.2011.12.007 (2012).
Merzlyak, M. N. et al. Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). Journal of Phycology 43, 833–843, doi:10.1111/j.1529-8817.2007.00375.x (2007).
Miller, R. et al. Changes in Transcript Abundance in Chlamydomonas reinhardtii following Nitrogen Deprivation Predict Diversion of Metabolism. Plant Physiology 154, 1737–1752, doi:10.1104/pp.110.165159 (2010).
Grossman, A., Manodori, A. & Snyder, D. Light-Harvesting Proteins of Diatoms - Their Relationship to the Chlorophyll A/B Binding-Proteins of Higher-Plants and their Mode of Transport into Plastids. Molecular & General Genetics 224, 91–100 (1990).
Liang, C. W. et al. De Novo Sequencing and Global Transcriptome Analysis of Nannochloropsis sp (Eustigmatophyceae) Following Nitrogen Starvation. Bioenergy Research 6, 494–505, doi:10.1007/s12155-012-9269-0 (2013).
Ouyang, L. L., Chen, S. H., Li, Y. & Zhou, Z. G. Transcriptome analysis reveals unique C4-like photosynthesis and oil body formation in an arachidonic acid-rich microalga Myrmecia incisa Reisigl H4301. Bmc Genomics 14, doi:10.1186/1471-2164-14-396 (2013).
Lv, H. X. et al. Transcriptome analysis of Chlamydomonas reinhardtii during the process of lipid accumulation. Genomics 101, 229–237, doi:10.1016/j.ygeno.2013.01.004 (2013).
Lane, T. W. et al. Digital Transcriptomic Analysis of Silicate Starvation Induced Triacylglycerol Formation in the Marine Diatom Thalassiosira Pseudonana. Phycologia 48, 71–71 (2009).
Carrier, G. et al. Comparative Transcriptome of Wild Type and Selected Strains of the Microalgae Tisochrysis lutea Provides Insights into the Genetic Basis, Lipid Metabolism and the Life Cycle. PLoS ONE 9, doi:10.1371/journal.pone.0086889 (2014).
Sun, D. Y. et al. De novo transcriptome profiling uncovers a drastic downregulation of photosynthesis upon nitrogen deprivation in the nonmodel green alga Botryosphaerella sudeticus. Bmc Genomics 14, doi:10.1186/1471-2164-14-715 (2013).
Jeffrey, S. W. & Humphrey, G. F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiologie der Pflanzen 167, 191–194, doi:10.1016/s0015-3796(17)30778-3 (1975).
Liu, Z. Y., Wang, G. C. & Zhou, B. C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresource Technology 99, 4717–4722, doi:10.1016/j.biortech.2007.09.073 (2008).
Strickland, J. & Parsons, T. A practical handbook of seawater analysis Fisheries Research Board of Canada, Ottawa 167, 311 (1972).
Yang, S. Y. et al. RNA-Seq analysis of differentially expressed genes in rice under varied nitrogen supplies. Gene 555, 305–317, doi:10.1016/j.gene.2014.11.021 (2015).
Li, R. et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25, 1966–1967 (2009).
Audic, S. & Claverie, J. M. The significance of digital gene expression profiles. Genome Res 7, 986–995 (1997).
Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Annals of statistics 1165–1188 (2001).
de Hoon, M. J., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004).
Qiao, J. et al. Quantitative iTRAQ LC-MS/MS proteomics reveals metabolic responses to biofuel ethanol in cyanobacterial Synechocystis sp. PCC 6803. Journal of proteome research 11, 5286–5300, doi:10.1021/pr300504w (2012).
Wen, B. et al. IQuant: an automated pipeline for quantitative proteomics based upon isobaric tags. Proteomics 14, 2280–2285, doi:10.1002/pmic.201300361 (2014).
Brosch, M., Yu, L., Hubbard, T. & Choudhary, J. Accurate and sensitive peptide identification with Mascot Percolator. Journal of proteome research 8, 3176–3181, doi:10.1021/pr800982s (2009).
Savitski, M. M., Wilhelm, M., Hahne, H., Kuster, B. & Bantscheff, M. A Scalable Approach for Protein False Discovery Rate Estimation in Large Proteomic Data Sets. Molecular & cellular proteomics: MCP 14, 2394–2404, doi:10.1074/mcp.M114.046995 (2015).
Langmead, B., Hansen, K. D. & Leek, J. T. Cloud-scale RNA-sequencing differential expression analysis with Myrna. Genome biology 11, R83, doi:10.1186/gb-2010-11-8-r83 (2010).
Volinia, S. et al. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proceedings of the National Academy of Sciences of the United States of America 109, 3024–3029, doi:10.1073/pnas.1200010109 (2012).
Valenzuela, J. et al. Potential role of multiple carbon fixation pathways during lipid accumulation in Phaeodactylum tricornutum. Biotechnology for biofuels 5, 40, doi:10.1186/1754-6834-5-40 (2012).
Li, Y., Horsman, M., Wang, B., Wu, N. & Lan, C. Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Applied microbiology and biotechnology 81, 629–636, doi:10.1007/s00253-008-1681-1 (2008).
Young, E. B., Dring, M. J., Savidge, G., Birkett, D. A. & Berges, J. A. Seasonal variations in nitrate reductase activity and internal N pools in intertidal brown algae are correlated with ambient nitrate concentrations. Plant Cell Environ 30, 764–774, doi:10.1111/j.1365-3040.2007.01666.x (2007).
Baker, N. R. In Annual Review of Plant Biology Vol. 59 Annual Review of Plant Biology 89–113 (2008).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5, 621–628, doi:10.1038/nmeth.1226 (2008).
Ye, J. et al. WEGO: a web tool for plotting GO annotations. Nucleic acids research 34, W293–W297 (2006).
Clark, M. E. et al. Transcriptomic and proteomic analyses of Desulfovibrio vulgaris biofilms: carbon and energy flow contribute to the distinct biofilm growth state. BMC genomics 13, 138, doi:10.1186/1471-2164-13-138 (2012).
Roberts, K., Granum, E., Leegood, R. C. & Raven, J. A. C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant physiology 145, 230–235, doi:10.1104/pp.107.102616 (2007).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic acids research 44, D457–462, doi:10.1093/nar/gkv1070 (2016).
Yang, Z. K. et al. Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation. Biotechnology for biofuels 6, 67, doi:10.1186/1754-6834-6-67 (2013).
Adir, N., Zer, H., Shochat, S. & Ohad, I. Photoinhibition–a historical perspective. Photosynthesis Research 76, 343–370 (2003).
Lavaud, J., Strzepek, R. F. & Kroth, P. G. Photoprotection capacity differs among diatoms: Possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnology and Oceanography 52, 1188–1194 (2007).
Heber, U., Bilger, W. & Shuvalov, V. A. Thermal energy dissipation in reaction centres and in the antenna of photosystem II protects desiccated poikilohydric mosses against photo-oxidation. Journal of experimental botany 57, 2993–3006 (2006).
Niyogi, K. K., Li, X.-P., Rosenberg, V. & Jung, H.-S. Is PsbS the site of non-photochemical quenching in photosynthesis? Journal of Experimental Botany 56, 375–382 (2005).
Malkin, R. & Niyogi, K. Biochemistry and Molecular Biology of Plants, eds Buchanan, B. B., Gruissen, W. & Jones, R. L. (Am. Soc. Plant Physiol., Rockville, MD), 568–628 (2000).
Cramer, W. et al. Some new structural aspects and old controversies concerning the cytochrome b 6 f complex of oxygenic photosynthesis. Annual Review of Plant Biology 47, 477–508 (1996).
Wollman, F.-A., Minai, L. & Nechushtai, R. The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1411, 21–85 (1999).
Granum, E., Roberts, K., Raven, J. A. & Leegood, R. C. Primary Carbon and Nitrogen Metabolic Gene Expression in the Diatom Thalassiosira Pseudonana (Bacillariophyceae): Diel Periodicity and Effects of Inorganic Carbon and Nitrogen1. Journal of phycology 45, 1083–1092 (2009).
Roberts, K., Granum, E., Leegood, R. C. & Raven, J. A. C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant physiology 145, 230–235 (2007).
Andre, C., Froehlich, J. E., Moll, M. R. & Benning, C. A heteromeric plastidic pyruvate kinase complex involved in seed oil biosynthesis in Arabidopsis. The Plant Cell Online 19, 2006–2022 (2007).
Dunahay, T. G., Jarvis, E. E. & Roessler, P. G. Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. Journal of Phycology 31, 1004–1012, doi:10.1111/j.0022-3646.1995.01004.x (1995).
Hockin, N. L., Mock, T., Mulholland, F., Kopriva, S. & Malin, G. The Response of Diatom Central Carbon Metabolism to Nitrogen Starvation Is Different from That of Green Algae and Higher Plants. Plant Physiology 158, 299–312, doi:10.1104/pp.111.184333 (2012).
Dahlqvist, A. et al. Phospholipid: diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proceedings of the National Academy of Sciences 97, 6487–6492 (2000).
Yoon, K., Han, D., Li, Y., Sommerfeld, M. & Hu, Q. Phospholipid: diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. The Plant Cell Online 24, 3708–3724 (2012).
Acknowledgements
This project is supported by projects of Guangdong Department of Science and Technology (No. 2011B050300026 and S2011030005257). Special thanks go to LetPub for English editing.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: W.H.L., P.L. and D.Z.Z. Performed the experiments: D.Z.Z., W.W., H.M.L. and P.L. Analyzed the data: J.B.J. and D.Z.Z. Wrote the paper: J.B.J., D.Z.Z. and W.H.L.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jian, J., Zeng, D., Wei, W. et al. The Combination of RNA and Protein Profiling Reveals the Response to Nitrogen Depletion in Thalassiosira pseudonana . Sci Rep 7, 8989 (2017). https://doi.org/10.1038/s41598-017-09546-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09546-x
- Springer Nature Limited
This article is cited by
-
Paclobutrazol induces the concurrent accumulation of chrysolaminarin and lipids in the diatom Phaeodactylum tricornutum
Journal of Oceanology and Limnology (2023)
-
The impact of nitrogen deficiency and subsequent recovery on the photosynthetic performance of the red macroalga Gracilariopsis lemaneiformis
Journal of Applied Phycology (2019)