Abstract
Published MRI evidence of structural and resting-state functional brain abnormalities in MDD has been inconsistent. To eliminate interference by repeated disease episodes and antidepressant treatment, we conducted the first multimodal voxel-wise meta-analysis of studies of voxel-based morphometry (VBM) and the amplitude of low-frequency fluctuation (ALFF) in first-episode drug-naive MDD patients, using the Seed-based d Mapping method (SDM). Fifteen VBM data sets and 11 ALFF data sets were included. SDM-based multimodal meta-analysis was used to highlight brain regions with both structural and functional abnormalities. This identified conjoint structural and functional abnormalities in left lateral orbitofrontal cortex and right supplementary motor area, and also dissociated abnormalities of structure (decreased grey matter in right dorsolateral prefrontal cortex and right inferior temporal gyrus; increased grey matter in right insula, right putamen, left temporal pole, and bilateral thalamus) and function (increased brain activity in left supplementary motor area, left parahippocampal gyrus, and hippocampus; decreased brain activity in right lateral orbitofrontal cortex). This study reveals a complex pattern of conjoint and dissociated structural and functional abnormalities, supporting the involvement of basal ganglia-thalamocortical circuits, representing emotional, cognitive and psychomotor abnormalities, in the pathophysiology of early-stage MDD. Specifically, this study adds to Psychoradiology, an emerging subspecialty of radiology, which seems primed to play a major clinical role in guiding diagnostic and treatment planning decisions in patients with mental disorder.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is predicted to be the leading cause of disability in high-income countries by the year 20301. It is important to understand the early-stage abnormalities of MDD in the processing and regulation of emotions. Widely-accepted models suggest that MDD is underpinned by structural and functional abnormalities in multiple neuronal circuits, such as the fronto-limbic circuitry2 and the default mode network (DMN)3, and are generally supported by evidence from neuroimaging, notably magnetic resonance imaging (MRI).
There are several MRI analytic approaches to quantifying structural abnormalities, including traditional hand-drawn regions of interest (ROIs) and whole-brain morphometrics. The ROI method has substantial anatomical validity, but has two major limitations: it is time-consuming and vulnerable to ROI selection bias4. There are two whole-brain analytic methods for quantifying structural abnormalities: voxel-based morphometry (VBM) and vertex-based morphometry. Vertex-based morphometry is often applied to cortex thickness. In the present work we wished to focus on GM volume, for which VBM is well-suited. VBM is an automated whole-brain technique which calculates local concentrations of GM in an unbiased way without a priori specification of ROIs5. GM volume reduction has been reported in anterior cingulate cortex (ACC)6, orbitofrontal cortex (OFC)7, 8, and dorsolateral prefrontal cortex (DLPFC)6, 9, which are all prefrontal regions involved in the automatic regulation of emotional behavior10. Structural alterations have also been reported in hippocampus11, 12 and amygdala9, the key regions of the limbic system theoretically identified as important in mood regulation in the pathophysiology of MDD. Additionally, a GM abnormality in the cerebellum13, which is involved in cognitive processing, has been reported in MDD14.
MRI methods for identifying functional abnormalities are broadly of two kinds: resting-state functional MRI and task-based functional MRI. Task-based functional MRI maps specific brain regions recruited during a target-detection task designed to evaluate responses to target stimuli15. However, task-related changes in neural activation represent only a small fraction of the brain’s total activity16, 17, because intrinsic activation is energetically more costly than responses to external stimuli18. Knowing how the brain allocates the majority of its resources is therefore essential for understanding neural mechanisms associated with MDD. In addition, there is a lack of agreement about task paradigms19. Resting-state MRI analytic methods to define the local features of the spontaneous BOLD signal include amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo): ALFF quantifies the intensity of low-frequency oscillations in spontaneous neural activity20, 21 while ReHo reflects the statistical similarity of spontaneous neural activity among spatially adjacent brain tissues22. Because of its physiological correlates23, ALFF is a more direct index of regional spontaneous neuronal activity, and can be used to locate specific impaired brain regions24, 25. ALFF also helps to avoid the potential bias induced by selection of the ‘seed’ voxels or the number of components in resting-state functional connectivity analysis24, 26 such as graph theory, ROI-to-ROI matrix analysis, seed-to-voxel analysis and independent component analysis. Resting-state studies in MDD have reported increased ALFF in the frontal cortex, including ACC, OFC27, 28 and posterior cingulate cortex/precuneus29, as well as the fusiform gyrus29,30,31 and lingual gyrus30, 32, which have been thought to reflect the excessive self-referential processing of MDD. Decreased ALFF has been reported in the cerebellar hemispheres33, 34 and superior temporal gyrus32, 35, and this has been linked to deficits in cognitive control of emotional processing. Reduced ALFF in the OFC has also been reported in MDD31, 36.
To date, volumetric and resting-state functional differences have been inconsistent and are poorly replicated for some brain regions. This is partly explained by considerable variation between studies in sample size (limiting the power to detect subtle brain differences and yielding both false-positive and false-negative findings37), in patients’ demographic and clinical characteristics, and in imaging protocols. We aimed to conduct a whole-brain voxel-wise meta–analysis to explore in a preliminary way the most robust findings across a range of published VBM and ALFF studies. Furthermore, to report on multimodally affected brain regions (the frontal-limbic regions where both structural and functional alterations have been reported), we performed an additional meta-analysis to display abnormalities in both VBM and ALFF in a single map. There are, we suggest, two ways to look at this analysis. On the working assumption that both modalities reflect a common pathophysiology, it is important to know that putatively-affected brain regions show conjoint abnormalities of both GM and brain function in MDD. The most plausible interpretation is that functional abnormalities observed in MDD are mediated by the underlying structural abnormalities38, potentially by complementary mechanisms where the direction of structural and functional changes are opposite. Alternatively, clearly dissociated abnormalities may throw an interesting light on pathophysiology. One such study applied VBM and ALFF together in drug-naive MDD, finding decreased GM in the parietal-temporal regions and decreased ALFF in the temporal regions and cerebellum39; however, no overlap was observed in the same template39. One reason for such failure to detect conjoint GM and brain function abnormalities might be that, hypothetically, structural damage in one region might cause functional abnormality in another. Alternatively, it might be simply an artifact of the relatively small sample size. In either case, a multimodal meta-analysis approach to identifying conjoint abnormalities from VBM studies of GM volume and ALFF studies of brain activity in MDD should be illuminating.
Other factors may contribute to the variability among MRI results in MDD. Antidepressant medication might increase heterogeneity and limit the interpretability and generalizability of the results, especially in the light of evidence that drugs may have important effects, such as upregulating neurotrophin expression40, altering neuronal remodeling41 and protecting against GM loss42, 43, in both animal and human studies44,45,46. In addition, studies on the course of the illness have reported brain structure and function differences between patients with first-episode (FE) and recurrent depression. For instance, compared with patients with recurrent MDD and with healthy controls, patients with FE depression showed increased amygdala volume47. However, depressed subjects with multiple depressive episodes showed hippocampal volume reductions which were not found in FE patients48. In view of this we restricted our analysis to FE and drug-naive MDD patients to eliminate interference by repeated episodes and antidepressant treatment.
In the present meta-analysis, we provide an up-to-date quantitative summary of studies investigating GM and ALFF abnormalities in FE drug-naive MDD patients, using Seed-based d Mapping (formerly “Signed Differential Mapping”) (SDM)49, a new version of effect-size signed differential mapping (ES-SDM)50 which has previously been applied to e.g. studies of dementia with Lewy bodies51, childhood maltreatment52, alcohol dependence53, and migraine54. Furthermore, we used a multimodal meta-analytical method integrated into SDM which enables combination of the results of the separate meta-analyses conducted from studies using different modalities to detect brain regions which display both structural and functional abnormalities55; this has previously been applied to studies of subjects at familial high risk for schizophrenia56, obsessive-compulsive disorder57 and FE psychosis50, but this seems to be its first application in MDD.
In brief, we conducted separate meta-analyses of VBM studies and ALFF studies on FE drug-naive MDD, followed by a multimodal meta-analysis of VBM studies and ALFF studies on FE drug-naive MDD to determine whether individuals exhibit brain regions with both structural and functional abnormalities.
Results
Included studies and sample characteristics
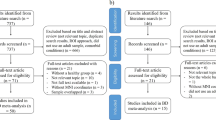
Figure 1 shows a flow diagram of the identification and attrition of studies. The search strategy identified 2124 structural and 786 functional neuroimaging studies. Though we did not apply any language restriction in the search, all the abstracts yielded were in English; any articles in other languages were translated into English or Chinese for assessment. Of the 38 studies which met our inclusion criteria, we excluded 12 which reanalyzed previously published data, 1 in which coordinates were unavailable, and 2 which reported VBM and ALFF results synchronously, leaving 23 peer-reviewed and published original studies10, 11, 19, 30, 33,34,35,36, 39, 58,59,60,61,62,63,64,65,66,67,68,69,70,71. One of the ALFF studies analysed two different subgroups of MDD patients, namely early treatment responsive and nonresponsive patients33, both compared with the same HC group; each subgroup comparison was included as a data set in the present meta-analysis. Specifically, the structural meta-analysis included 15 data sets of 15 VBM studies with 471 FE drug-naive MDD subjects (194/277 male/female; mean age 34.0 years), matched with 521 controls (226/295 male/female; mean age 33.6 years); the resting state functional imaging meta-analysis included 11 data sets of 10 ALFF studies with a total of 261 FE drug-naive MDD subjects (126/135 male/female; mean age 31.9 years), matched with 278 controls (138/140 male/female; mean age 30.7 years). Table 1 and Table 2 summarise clinical and demographic data and technique details from all the included studies. The quality scores ranged from 9 to 12 (mean score 11.1), showing that these studies were of high quality. In no study was there any significant difference in age and sex between the MDD group and the HC.
Meta-analysis of voxel-based morphometry and amplitude of low-frequency fluctuation studies in first-episode drug-naive patients with major depressive disorder. Study selection was done according to “Preferred reporting items for systematic reviews and meta-analysis” (PRISMA) guidelines. Abbreviations: ALFF, amplitude of low-frequency fluctuation; FE, first-episode; HC, healthy control; N, number; MDD, major depressive disorder; VBM, voxel-based morphometry.
Changes in regional grey matter
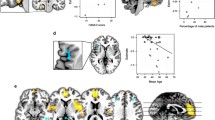
A group comparison of FE drug-naive MDD patients with HC across the 15 data sets in the main meta-analysis of VBM studies revealed decreased GM relative to controls in right DLPFC, right supplementary motor area (SMA), and right inferior temporal gyrus (ITG) extending to fusiform gyrus, and increased GM relative to controls in the right insula extending to putamen and striatum, left lateral OFC, left temporal pole (TP), and bilateral thalamus (Table 3 and Fig. 2).
Areas of increased (red) and decreased (blue) grey matter or resting-state brain activity in first-episode drug-naive with major depressive disorder compared with healthy controls in the meta-analyses of (A) voxel-based morphometry studies and (B) amplitude of low-frequency fluctuation studies. Abbreviations: DLPFC, dorsal lateral prefrontal cortex; ITG, inferior temporal gyrus; L, left; PHG, parahippocampal gyrus; R, right; SMA, supplementary motor area; TP, temporal pole.
In whole-brain jackknife sensitivity analysis the findings of decreased GM in MDD patients in the right DLPFC, right SMA, and right ITG remained significant in all but 1 combination, and increased GM in the right insula, left lateral OFC, and left STG in all but 2 combinations. The right thalamus and left thalamus remained significant in all but 4 and 5 combinations, respectively (Table 3).
In analysis of heterogeneity the right insula and left TP with increased GM showed significant statistical heterogeneity between studies (p < 0.005), while the remaining regions with altered GM did not show significant between-study heterogeneity (p > 0.005) (see Supplementary Table S2).
In analysis of publication bias, the Egger test was significant in the right SMA (P = 0.034) and right ITG (P = 0.025) but not for right DLPFC (P = 0.051), right ITG (P = 0.112), right insula (P = 0.371), left lateral OFC (P = 0.645), left TP (P = 0.641), left thalamus (P = 0.971) or right thalamus (P = 0.936) in the VBM metaanalysis.
Changes in resting state regional brain activity
The main meta-analysis of the ALFF studies on MDD patients showed significantly enhanced brain activities in the bilateral SMA and left PHG extending to hippocampus and attenuated brain activities in the bilateral lateral OFC (Table 3 and Fig. 2).
In whole-brain jackknife sensitivity analysis the findings of attenuated brain activity in the bilateral lateral OFC and SMA were highly replicable, being preserved throughout all but 1 combinations of the data sets. The results in left PGH remained significant in all but 2 combinations (Table 3).
In analysis of heterogeneity, the regions with altered brain activities did not showed significant statistical heterogeneity between studies (p > 0.005).
In analysis of publication bias, the Egger test was significant for left SMA (P = 0.031), right SMA (P = 0.027) and right PGH (P = 0.031), but not for the left lateral OFC (P = 0.870) and right lateral OFC (P = 0.928) with decreased brain activities in the brain activity meta-analysis.
Multimodal analysis of grey matter and brain activity
The results were then summarised by putting structural and functional findings in a single meta-analytic map to demonstrate regions which showed both structural and functional abnormalities. This revealed increased GM with decreased brain activity in the left lateral OFC, and decreased GM with increased brain activity in the right SMA (Table 3 and Fig. 3). The left lateral OFC finding was observed in the two separate structural and functional meta-analyses and preserved in the jackknife sensitive analyses, and was without publication bias or heterogeneity. The right SMA finding was observed in the two separate structural and functional meta-analyses and preserved in the jackknife sensitive analyses, and was without heterogeneity, but failed in both publication bias analyses.
Multimodal meta-analysis reveals (A) increased grey matter with decreased brain activity in the left OFC and (B) Decreased GM with increased brain activity in the right SMA in first-episode drug-naive patients with MDD compared with healthy controls. Abbreviations: L, left; MDD, major depressive disorder; R, right; SMA, supplementary motor area.
Subgroup meta-analyses
Details of the results of subgroup analysis are presented in Supplementary Materials.
Discussion
Aims and strengths of the study
This is to our knowledge the first multimodal neuroimaging meta-analysis which attempts to localise the neural substrates of MDD by combining information from whole-brain VBM studies investigating GM with ALFF studies of spontaneous brain activity.
Methodological strengths are the novel techniques combining features from coordinate meta-analytic approaches and standard meta-analytic methods, and the multimodal approach. The restriction to FE MDD patients helps distinguish the intrinsic brain features of the disease from potential effects of episode times, and the restriction to drug-naive MDD patients minimises the interference from medication effects.
Both anatomical and functional brain abnormalities in MDD were observed, characterised by decreased GM mainly localizing in the right DLPFC, right SMA, and right ITG extending to the fusiform gyrus, and increased GM in the right insula extending to putamen and striatum, left lateral OFC, left TP, and bilateral thalamus, along with increased brain activity in the bilateral SMA and left PHG extending to hippocampus, and decreased brain activity in the bilateral lateral OFC. Because of the publication bias in the right SMA and right ITG findings of the VBM study, as well as in the bilateral SMA and right PHG findings of the ALFF study, these results should be interpreted with some caution. The multimodal meta-analysis identified conjoint structural and functional differences in the left lateral OFC and right SMA in MDD.
These main findings can be thought of in terms of three circuits (DLPFC-striatum-thalamus circuit, lateral OFC-striatum-thalamus circuit, and SMA-striatum-thalamus circuit) broadly representing emotion, cognition and motor dysregulation respectively70.
Grey matter abnormalities in MDD
The most prominent finding was decreased GM in the right DLPFC, part of the central executive network which plays an important role in working memory and attention72, and is related to impaired cognitive function in FE drug-naive patients with MDD. This GM loss may reflect the reductions in glial cell density and neuronal size in the prefrontal cortex reported in postmortem studies73, 74. As the lateral prefrontal lobe is a well-known neural substrate involved in the pathophysiology of MDD, being associated with cognitive dysfunction75, 76, the GM loss in this region may be causally important. Furthermore, repetitive transcranial magnetic stimulation of the DLPFC is an established treatment for depression77. In rat models of depression, a lower expression of synaptic-function-related genes and correspondingly reduced number of synapses in the DLPFC has been reported78, and a similar phenomenon might underlie the decreased DLPFC volume in MDD.
The striatum has been associated with mood, cognitive processes and movement regulation79 and has connections with the DLPFC, OFC, SAM and temporal lobe70. The GM deficit in the putamen may contribute causally to the symptoms of MDD80, 81.
The thalamus is a complex structure, associated with the experience and expression of emotion in mood disorders12. We found symmetrical increased GM in thalamus in FE drug-naive MDD patients, consistent with a postmortem report of elevated neuron number in thalamus82, and also with a previous study in which increased GM in thalamus was related to pre-apoptotic osmotic changes or hypertrophy in FE drug-naive MDD patients68. However, structural studies and a previous meta-analysis have reported decreased GM in the thalamus83, 84. A possible explanation for this discrepancy may be that the latter enrolled data sets with a different course of illness or number of episodes. Consequently, we speculate that the increased volume of bilateral thalamus may be involved in the early stage of MDD, and is not likely to be the result of medication exposure.
MDD patients also showed increased GM in the right insula and left TP. The insula has extensive connections to several areas of the cortex and limbic system implicated in monitoring interceptive awareness85, 86, high-level cognitive control and attentional processes87. Increased insular activation to facial expressions of disgust in MDD may reflect an emotion processing bias88. Contrary to our finding, several studies reported reduced GM in insula25, 89, 90, and so this may be an effect of recurrent episodes91. Furthermore, increased GM in insula could be interpreted as resulting from neuroinflammation92: there is significantly elevated translocator protein density, an important aspect of neuroinflammation, in insula during a major depressive episode92. The TP is a visual and auditory-related brain region implicated in the processing of working memory and facial emotions93. Our findings are in line with previous reports of morphological alteration in the TP, an apparently early sign of MDD unlikely to result from treatment with antidepressants94, 95. Moreover, longitudinal MRI studies have reported progressive GM loss in the temporal lobe in MDD patients95, 96. Accordingly, the increased GM in right insula, bilateral thalamus and left temporal lobe might represent a specific character of early-stage MDD.
Regional brain activity abnormalities in MDD
The meta-analysis of ALFF studies found increased spontaneous brain activity in the left PHG extending to hippocampus. Both structures belong to the limbic system and play a central role in regulation of emotions, motivation, memory, affective dimension of pain6, 21, 23 and cognitive processes in MDD10. Surprisingly, no differences in GM volumes were detected in the hippocampus despite the fact that a variety of studies have reported abnormalities in that region12, 83. Possible reasons for this are differences in illness duration, medication status, age of onset and the number of episode. The volume deficit of hippocampus reportedly correlates with illness duration9, 84, and decreased hippocampus volume was detected in patients with long illness duration compared with short duration60. A newly-published meta-analysis suggests that the lower hippocampus volume is associated with the number of episodes, whilst no difference was detected between FE MDD patients and controls97. In our meta-analysis, all patients were FE and drug-naive, and most of studies were of short duration, which may explain why we found no structural hippocampus abnormality.
Subgroup meta-analyses
In addition to the results in the pooled meta-analysis, we found increased ALFF in the left posterior cingulate gyrus and right precuneus in subgroup meta-analyses of studies with large sample size. These regions belong to the DMN, which has a role in the balance between processing of external stimuli and internal and self-directed processing, which has long been thought to be involved in the pathophysiology of MDD. However, they failed in the pooled ALFF meta-analysis. This suggests that the detection of changes in DMN may be influenced by sample size; larger studies are needed to confirm this finding.
Conjoint abnormalities in grey matter and brain activity in MDD
The multimodal meta-analysis identified conjoint structural and functional differences in the left pars orbitalis (increased GM with decreased brain activity), which is a part of the inferior frontal gyrus (Brodmann area 47), belonging to lateral OFC. The OFC, being important parts of the affective network, are involved in the emotional processing of mental states98, 99. However, there is a difference between the medial part and lateral part, processing negative and positive emotion separately71. This is reconcilable with the conceptualization of MDD as a disorder of emotion regulation100, 101. However, a number of studies have found decreased GM in this region7, 102, corroborated by a previous meta-analysis of volumetric MRI studies79. It should be noted that most previous studies included patients on antidepressant treatment, while we included only studies of drug-naive patients. We hypothesise that increased GM may be related to temporal hypertrophy103, marking areas of early neuronal pathology without the confounding factors of repeat episodes and treatment. One study has reported volume being larger at illness onset, and then declining with multiple episodes or treatment in mood disorder103. Regarding brain activity, the presence of anxiety symptoms of MDD is reportedly associated with decreased OFC activation104. In MDD the severity of depression correlates negatively with activity in the left lateral OFC105. Reduced baseline resting state connectivity within the orbitofrontal component was predictive of clinical response in medication-free MDD patients106, 107. Thus, the imbalance between structure and brain activity may represent a distinctive alteration of FE drug-naive MDD patients.
In addition, conjoint structural and functional differences were found in the right SMA (decreased GM with increased brain activity), which forms part of the SMA-striatum-thalamus circuit. This is traditionally considered the cortical area necessary for voluntary movement as well as implicated in psychomotor retardation81, the key feature of MDD, but it also participates in cognitive activities such as working memory108, implicit learning ability109, and attention and executive function110. As most of these are impaired in MDD, it is tempting to infer a causal link. Our finding was in accord with previous studies relating the reduced regional volumes of the right SMA to psychomotor retardation in early-onset depression patients60, 109. If a primary decrease of GM volume in SMA were accompanied by a compensatory hyperfunctionality of the remaining GM, involving higher regional cerebral metabolism111 and cerebral blood flow112, this would likely increase local ALFF. Conversely, primary hyperfunction might lead to a decrease in GM by glutamate-induced ‘excitotoxicity’96. Of particular importance, a previous review indicated that the reduced GM volume in some structures may produce partial volume effects in functional images19. For instance, MDD subjects relative to controls show metabolic activity that appears reduced in the subgenual prefrontal cortex113. However, when this anatomical deficit is taken into account by correcting the metabolic data for the partial volume averaging effect associated with the corresponding GM reduction, metabolism instead appears increased in the subgenual prefrontal cortex in the unmedicated-depressed patients114. Whatever the pathophysiology, the regions identified by the multimodal meta-analysis could serve as a specific ROIs template for both individual postmortem histopathological and in vivo imaging studies.
Limitations
Firstly, combining numerous potentially underpowered studies with SDM meta-analysis using peak voxels, as we have done, may not reveal subtle widespread changes which are undetected by individual studies. A traditional meta-analysis, or an SDM meta-analysis using raw SPM images, gains much of its advantage by pooling raw data, including nonsignificant results, from all studies to increase power115. Our SDM meta-analysis using only significant co-ordinates as input is actually a tool for spatial integration of already-significant results. It is possible, given the hypothesis of subtle GM or brain activity abnormalities, that some areas of altered GM volume or brain activity do not reach significance in smaller studies but would prove significant if raw SPM maps were combined for SDM analysis.
Secondly, we discussed the findings that were not significant in the multimodal analysis as dissociated abnormalities in grey matter and brain activity in MDD. This dissociated distribution may not represent the real pattern of MDD abnormalities, because it may rather be due to failure to reach statistical significance in the multimodal analysis.
In the Egger test, we found publication bias in right SMA and right ITG in the VBM analysis, and in bilateral SMA and right PGH in the ALFF analysis, so another important limitation is the possibility of selective positive reporting and publication bias.
Finally, most of the primary studies have been so far conducted in China, thus limiting the generalizability of the current findings to other populations.
Summary
The present meta-analysis revealed a complex pattern of neural abnormalities in first-episode drug-naive MDD patients, characterised by conjoint and dissociated structural and functional brain abnormalities in brain regions involved in motor, cognition and emotional processing. These volumetric and functional alterations support the notion that multiple parallel basal ganglia-thalamocortical circuits70, together with other limbic regions (parahippocampus, hippocampus) contribute to the underlying pathophysiology of early-stage MDD. First-episode drug naive MDD patients showed increase in GM as well as decrease in brain activity in the left lateral OFC, and decrease in GM as well as increase in brain activity in right SMA, which could therefore serve as a specific ROI template for future studies. Of note, this study adds to Psychoradiology (https://radiopaedia.org/articles/psychoradiology), an emerging subspecialty of radiology, which seems primed to play a major clinical role in guiding diagnostic and treatment planning decisions in patients with mental disorder116, 117.
Methods
Study selection
Meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA)118. A systematic strategy was used to search for relevant studies published in PubMed, Embase, Web of Science, Science Direct and Google Scholar. Candidate structural imaging studies were sought using the keywords “depression” or “depressive” or “unipolar depression” or “major depression” or “major depressive disorder” or “MDD” plus “voxel-based morphometry” or “VBM” or “voxel*” or “morphometry”. Resting state functional imaging studies were sought using the keywords “depression” or “depressive” or “unipolar depression” or “major depression” or “major depressive disorder” or “MDD” plus “amplitude of low-frequency fluctuation” or “ALFF” or “low-frequency fluctuation” or “LFF”. The search was conducted up to July 2016, with no time-span specified for date of publication. Language of publication was not a specific search criterion. The reference lists of these studies were checked to identify further studies for inclusion.
Structural neuroimaging studies were included according to the following criteria: 1) used VBM to analyze whole-brain GM changes in adult (age range 18 to 60 years) MDD patients, to minimise the effect of neurodevelopment and neurodegeneration as potential confounders; 2) compared MDD patients with healthy control (HC) subjects; 3) investigated first-episode and drug naive MDD patients, who had never received antidepressant medications before MRI scanning. Functional studies were included according to the following criteria: 1) used ALFF to analyze whole-brain resting state brain activity in adult MDD patients; 2) compared MDD patients with HC; 3) investigated first-episode and drug naive MDD patients. We excluded: 1) studies from which peak coordinates could not be retrieved from the published article or after contacting the authors; 2) studies in which different thresholds were used in different regions of the brain; 3) findings based on ROIs. For studies where multiple independent patient samples were compared with HC, the appropriate coordinates were included as separate data sets. For studies using overlapping samples, the study with the most subjects was included.
Three authors (W.N.W., Y.J.Z and X.Y.H.) independently conducted the literature search. The results were compared, any inconsistencies were discussed, and a consensus decision was obtained.
Quality assessment
We assessed the quality of the included studies using a 12-point checklist that focused on both the clinical and demographic aspects of individual study samples and on the imaging methodology. The checklist was based on previous meta-analytic studies91, 119, and included structural measures from MRI, modified to reflect critical variables that are important to assess VBM studies56 and resting state fMRI studies. This assessment included the quality of the diagnostic procedures, the demographic and clinical characterization, the prospective (or otherwise) nature of the patient and control studies, the sample size, the MRI acquisition parameters, the analysis technique and the quality of the reported results (see Supplementary Table S1). Although this checklist was not designed as an assessment tool, it provides an objective index of the rigor of individual studies. The quality scores are presented in Table 1.
Recorded variables
For each included study we recorded: sample size, gender and mean age of subjects; illness duration, depression symptom severity and mean number of episodes; drug status; the statistical threshold of the main findings, and the method employed to correct whole-brain results for multiple comparisons. These data are presented in Tables 1 and 2.
Standard meta-analysis of structural abnormalities
Separate voxel-based meta-analysis of regional GM abnormalities was conducted using the SDM software package57 (www.sdmproject.com), which implements a refinement of methods50, 120 which have been applied to neuroimaging studies of neurological and psychiatric disorders such as Alzheimer’s disease34, Attention-deficit/Hyperactivity Disorder121, late-life depression56 and MDD58. SDM uses the reported peak coordinates and effect sizes to recreate, based on the spatial correlation between neighbouring voxels, brain maps of the effect size of the GM differences between patient and comparison subjects, and accounts for sample size and variance as well as between-study heterogeneity. The SDM methods have been described in detail elsewhere50, 120, so we merely summarise the main features here. First, peak coordinates and effect sizes (derived, for example, from t values) of GM differences between MDD individuals and comparison subjects were extracted from each study. Any peaks not statistically significant at the whole brain level were excluded; thus, while different studies may employ different thresholds, we ensured that in each study the same statistical threshold was used throughout the brain. This avoids bias toward liberally threshold brain regions, which is common for ROIs. Second, a standard Montreal Neurological Institute map of the differences in GM was separately recreated for each study by means of an anisotropic Gaussian kernel, which assigns higher effect sizes to the voxels more correlated with peaks. This has been found to optimise the recreation of the effect size maps, and is robust because it does not depend on a full width at half-maximum49. Third, a map of the effect size variance was derived for each study from its effect size map and its sample size. Fourth, the mean map was obtained by voxel-wise calculation of the random-effects mean of the study maps, weighted by the sample size and variance of each study and the between-study heterogeneity50. Details of the effect size are presented in the online Supplementary Materials.
Considering possible methodological differences between the studies, we then performed subgroup meta-analyses included studies with large sample size (n > 30), studies with small sample size (n < 30), studies that utilized 1.5 T and 3.0 T MRI, studies with a correction for multiple comparisons or not, and patients with short duration (less than 6 months).
The main analysis was complemented with three analyses of robustness to ensure that only the most replicable and robust of the results were retained. First, a jackknife sensitivity analysis was performed, systematically repeating the meta-analyses excluding one study at a time: if a region remains significant in all or most of these combinations of studies, this finding is deemed highly replicable120. Second, a random-effects model with Q statistics was used to detect the statistical (between-studies) heterogeneity of individual clusters. Third, Egger tests were used to assess publication bias.
Standard meta-analysis of resting state functional abnormalities
The separate main meta-analysis and the analyses of robustness of regional resting state brain activity were methodologically identical to those of regional GM.
Multimodal Meta-Analysis
Finally, the meta-analyses of regional GM and resting state functional abnormalities were combined in order to detect those brain regions showing differences in both imaging modalities. We followed the approach described in Radua et al.55, which aims to obtain the overlap between the abnormal regions in the two modalities. In the current meta-analysis, the multimodal meta-analysis is used to detect those brain regions which display both structural and functional abnormalities. However, the exact relationship between these changes cannot be defined further.
References
Wiles, N. et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 381, 375–384 (2013).
Monkul, E. S. et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Molecular Psychiatry 12, 360–366 (2007).
Gong, Q. & He, Y. Depression, neuroimaging and connectomics: a selective overview. Biological Psychiatry 77, 223–235 (2015).
Pearlson, G. D. & Calhoun, V. Structural and functional magnetic resonance imaging in psychiatric disorders. Canadian journal of psychiatry 52, 158–166 (2007).
Ashburner, J. & Friston, K. J. Voxel-based morphometry–the methods. NeuroImage 11, 805–821 (2000).
Salvadore, G. et al. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. NeuroImage 54, 2643–2651 (2011).
Bremner, J. D. et al. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry 51, 273–279 (2002).
Scheuerecker, J. et al. Orbitofrontal volume reductions during emotion recognition in patients with major depression. Journal of Psychiatry & Neuroscience 35, 311–320 (2010).
Frodl, T. S. et al. Depression-related variation in brain morphology over 3 years: effects of stress? Archives of General Psychiatry 65, 1156–1165 (2008).
Price, J. L. & Drevets, W. C. Neurocircuitry of mood disorders. Neuropsychopharmacology 35, 192–216 (2010).
Zou, K. et al. Changes of brain morphometry in first-episode, drug-naive, non-late-life adult patients with major depression: an optimized voxel-based morphometry study. Biological Psychiatry 67, 186–188 (2010).
Frodl, T. et al. Hippocampal changes in patients with a first episode of major depression. The American Journal of Psychiatry 159, 1112–1118 (2002).
Peng, J. et al. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. European Journal of Radiology 80, 395–399 (2011).
Baillieux, H., De Smet, H. J., Paquier, P. F., De Deyn, P. P. & Marien, P. Cerebellar neurocognition: insights into the bottom of the brain. Clinical Neurology and Neurosurgery 110, 763–773 (2008).
Dichter, G. S., Felder, J. N., Bodfish, J. W., Sikich, L. & Belger, A. Mapping social target detection with functional magnetic resonance imaging. Social Cognitive and Affective Neuroscience 4, 59–69 (2009).
Raichle, M. E. Neuroscience. The brain’s dark energy. Science 314, 1249–1250 (2006).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience 8, 700–711 (2007).
Raichle, M. E. & Mintun, M. A. Brain work and brain imaging. Annual Review of Neuroscience 29, 449–476 (2006).
Drevets, W. C., Price, J. L. & Furey, M. L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure & Function 213, 93–118 (2008).
Zang, Y. F. et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & Development 29, 83–91 (2007).
Lu, H. et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proceedings of the National Academy of Sciences of the United States of America 104, 18265–18269 (2007).
Zhu, C. Z. et al. Discriminative analysis of brain function at resting-state for attention-deficit/hyperactivity disorder. Medical Image Computing and Computer-Assisted Intervention 8, 468–475 (2005).
Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine 34, 537–541 (1995).
Lu, D. et al. Altered baseline brain activity in children with bipolar disorder during mania state: a resting-state study. Neuropsychiatric Disease and Treatment 10, 317–323 (2014).
Takeuchi, H. et al. Regional homogeneity, resting-state functional connectivity and amplitude of low frequency fluctuation associated with creativity measured by divergent thinking in a sex-specific manner. NeuroImage 152, 258–269 (2017).
Wang, L., Hermens, D. F., Hickie, I. B. & Lagopoulos, J. A systematic review of resting-state functional-MRI studies in major depression. Journal of Affective Disorders 142, 6–12 (2012).
Liu, J. et al. Alterations in amplitude of low frequency fluctuation in treatment-naive major depressive disorder measured with resting-state fMRI. Human Brain Mapping 35, 4979–4988 (2014).
Du, L. et al. Early life stress affects limited regional brain activity in depression. Scientific Reports 6, 25338 (2016).
Wang, L. et al. Frequency-dependent changes in amplitude of low-frequency oscillations in depression: A resting-state fMRI study. Neuroscience Letters 614, 105–111 (2016).
Wang, L. et al. Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI study. PLoS ONE 7, e48658 (2012).
Zhang, X. et al. Imbalanced spontaneous brain activity in orbitofrontal-insular circuits in individuals with cognitive vulnerability to depression. Journal of Affective Disorders 198, 56–63 (2016).
Guo, W. B. et al. Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Progress in Neuro-psychopharmacology & Biological Psychiatry 40, 153–159 (2013).
Wang, L. J., Kuang, W. H., Xu, J. J., Lei, D. & Yang, Y. C. Resting-state brain activation correlates with short-time antidepressant treatment outcome in drug-naive patients with major depressive disorder. The Journal of International Medical Research 42, 966–975 (2014).
Guo, W. B. et al. Alterations of the amplitude of low-frequency fluctuations in treatment-resistant and treatment-response depression: a resting-state fMRI study. Progress in Neuro-psychopharmacology & Biological Psychiatry 37, 153–160 (2012).
Zhu, Z. et al. Spatial patterns of intrinsic neural activity in depressed patients with vascular risk factors as revealed by the amplitude of low-frequency fluctuation. Brain Research 1483, 82–88 (2012).
Zhang, X. et al. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS ONE 9, e85241 (2014).
Schmidt, A. F. et al. Exploring interaction effects in small samples increases rates of false-positive and false-negative findings: results from a systematic review and simulation study. Journal of Clinical Epidemiology 67, 821–829 (2014).
de Kwaasteniet, B. et al. Relation between structural and functional connectivity in major depressive disorder. Biological Psychiatry 74, 40–47 (2013).
Guo, W. et al. Functional and anatomical brain deficits in drug-naive major depressive disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry 54, 1–6 (2014).
Duman, R. S. & Monteggia, L. M. A neurotrophic model for stress-related mood disorders. Biological Psychiatry 59, 1116–1127 (2006).
Bessa, J. M. et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Molecular Psychiatry 14, 764–773, 739 (2009).
Sheline, Y. I., Gado, M. H. & Kraemer, H. C. Untreated depression and hippocampal volume loss. The American Journal of Psychiatry 160, 1516–1518 (2003).
Lavretsky, H., Roybal, D. J., Ballmaier, M., Toga, A. W. & Kumar, A. Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. The Journal of Clinical Psychiatry 66, 964–967 (2005).
Sheline, Y. I. et al. Treatment course with antidepressant therapy in late-life depression. The American Journal of Psychiatry 169, 1185–1193 (2012).
Cousins, D. A., Aribisala, B., Nicol Ferrier, I. & Blamire, A. M. Lithium, gray matter, and magnetic resonance imaging signal. Biological Psychiatry 73, 652–657 (2013).
Vernon, A. C. et al. Contrasting effects of haloperidol and lithium on rodent brain structure: a magnetic resonance imaging study with postmortem confirmation. Biological Psychiatry 71, 855–863 (2012).
Frodl, T. et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biological Psychiatry 53, 338–344 (2003).
MacQueen, G. M. et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America 100, 1387–1392 (2003).
Radua, J. et al. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Frontiers in Psychiatry 5, 13 (2014).
Radua, J. et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry 27, 605–611 (2012).
Zhong, J., Pan, P., Dai, Z. & Shi, H. Voxelwise meta-analysis of gray matter abnormalities in dementia with Lewy bodies. European Journal of Radiology 83, 1870–1874 (2014).
Lim, L., Radua, J. & Rubia, K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. The American Journal of Psychiatry 171, 854–863 (2014).
Xiao, P. et al. Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug and Alcohol Dependence 153, 22–28 (2015).
Dai, Z. et al. Gray matter correlates of migraine and gender effect: A meta-analysis of voxel-based morphometry studies. Neuroscience 299, 88–96 (2015).
Radua, J., Romeo, M., Mataix-Cols, D. & Fusar-Poli, P. A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Current Medicinal Chemistry 20, 462–466 (2013).
Cooper, D., Barker, V., Radua, J., Fusar-Poli, P. & Lawrie, S. M. Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Research 221, 69–77 (2014).
Radua, J. et al. Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacology 39, 1547–1557 (2014).
Zhao, Y. J. et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychological Medicine 44, 2927–2937 (2014).
Lai, C. H. & Wu, Y. T. The patterns of fractional amplitude of low-frequency fluctuations in depression patients: the dissociation between temporal regions and fronto-parietal regions. Journal of Affective Disorders 175, 441–445 (2015).
Cheng, Y. Q. et al. Brain volume alteration and the correlations with the clinical characteristics in drug-naive first-episode MDD patients: a voxel-based morphometry study. Neuroscience Letters 480, 30–34 (2010).
Tang, Y. et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Research 156, 83–86 (2007).
Watanabe, K. et al. Relationship between the catechol-O-methyl transferase Val108/158Met genotype and brain volume in treatment-naive major depressive disorder: Voxel-based morphometry analysis. Psychiatry Research 233, 481–487 (2015).
Liu, F. et al. Classification of different therapeutic responses of major depressive disorder with multivariate pattern analysis method based on structural MR scans. PLoS ONE 7, e40968 (2012).
Tang, Y., Wu, F., Kong, L. & Xu, K. Gray matter volume changes in first-episode, medication naive patients with major depressive disorder: a voxel-based morphometric 3.0 T MRI study. Chinese Journal of Clinicians (Electronic Edition) 5, 2926–2929 (2011).
Xu, C. & B., Y. fMRI study on the spontaneous activity of the brain in primary depression patients and their immediate family members. Journal of Practical Medical Imaging (2010).
Yan, R., Yao, Z., Wei, M., Tang, H. & Lu, Q. Amplitude of low frequency fluctuation in female depression patients: a resting-state functional magnetic resonance imaging study. Chinese Journal of Psychiatry 47, 195–199 (2014).
Zhang, X., Yao, S., Zhu, X., Wang, X. & Zhong, M. Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel-based morphometry study. Journal of Affective Disorders 136, 443–452 (2012).
Kong, L. et al. Frontal-subcortical volumetric deficits in single episode, medication-naive depressed patients and the effects of 8 weeks fluoxetine treatment: a VBM-DARTEL study. PLoS ONE 9, e79055 (2014).
Ide, S. et al. Relationship between a BDNF gene polymorphism and the brain volume in treatment-naive patients with major depressive disorder: A VBM analysis of brain MRI. Psychiatry Research 233, 120–124 (2015).
Alexander, G. E., DeLong, M. R. & Strick, P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience 9, 357–381 (1986).
Lu, Y. et al. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. NeuroImage 11, 658–666 (2016).
Seeley, W. W. et al. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. The Journal of Neuroscience 27, 2349–2356 (2007).
Cotter, D. et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cerebral Cortex 12, 386–394 (2002).
Rajkowska, G. et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry 45, 1085–1098 (1999).
Matsuo, K. et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Molecular Psychiatry 12, 158–166 (2007).
Phan, K. L. et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry 57, 210–219 (2005).
George, M. S. et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biological Psychiatry 48, 962–970 (2000).
Kang, H. J. et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nature Medicine 18, 1413–1417 (2012).
Koolschijn, P. C., van Haren, N. E., Lensvelt-Mulders, G. J., Hulshoff Pol, H. E. & Kahn, R. S. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping 30, 3719–3735 (2009).
Heller, A. S. et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America 106, 22445–22450 (2009).
Walther, S. et al. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiology of Disease 47, 13–19 (2012).
Young, K. A., Holcomb, L. A., Yazdani, U., Hicks, P. B. & German, D. C. Elevated neuron number in the limbic thalamus in major depression. The American Journal of Psychiatry 161, 1270–1277 (2004).
Bremner, J. D. et al. Hippocampal volume reduction in major depression. The American Journal of Psychiatry 157, 115–118 (2000).
Bell-McGinty, S. et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. The American Journal of Psychiatry 159, 1424–1427 (2002).
Avery, J. A. et al. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biological Psychiatry 76, 258–266 (2014).
Craig, A. D. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience 10, 59–70 (2009).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function 214, 655–667 (2010).
Surguladze, S. A. et al. Depression is associated with increased sensitivity to signals of disgust: a functional magnetic resonance imaging study. Journal of Psychiatric Research 44, 894–902 (2010).
Elliott, R., Dolan, R. J. & Frith, C. D. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex 10, 308–317 (2000).
Nakagawa, S. & Cuthill, I. C. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society 82, 591–605 (2007).
Shepherd, A. M., Matheson, S. L., Laurens, K. R., Carr, V. J. & Green, M. J. Systematic meta-analysis of insula volume in schizophrenia. Biological Psychiatry 72, 775–784 (2012).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275 (2015).
Pavuluri, M. N., Passarotti, A. M., Fitzgerald, J. M., Wegbreit, E. & Sweeney, J. A. Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: a pharmacological functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry 51, 157–170.e155 (2012).
Ramezani, M. et al. Temporal-lobe morphology differs between healthy adolescents and those with early-onset of depression. NeuroImage 6, 145–155 (2014).
van Eijndhoven, P. et al. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. The American Journal of Psychiatry 170, 1477–1486 (2013).
Takahashi, T. et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Archives of General Psychiatry 66, 366–376 (2009).
Schmaal, L. et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Molecular Psychiatry 21, 806–812 (2016).
Price, J. L. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences 1121, 54–71 (2007).
Murray, E. A. & Izquierdo, A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Annals of the New York Academy of Sciences 1121, 273–296 (2007).
Kringelbach, M. L. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience 6, 691–702 (2005).
Schoenbaum, G., Takahashi, Y., Liu, T. L. & McDannald, M. A. Does the orbitofrontal cortex signal value? Annals of the New York Academy of Sciences 1239, 87–99 (2011).
Lacerda, A. L. et al. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biological Psychiatry 55, 353–358 (2004).
Adler, C. M., Levine, A. D., DelBello, M. P. & Strakowski, S. M. Changes in gray matter volume in patients with bipolar disorder. Biological Psychiatry 58, 151–157 (2005).
Townsend, J. D. et al. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Research 183, 209–217 (2010).
Drevets, W. C. Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences 1121, 499–527 (2007).
Fu, C. H. et al. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry 15, 82 (2015).
Frodl, T. et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biological Psychiatry 67, 161–167 (2010).
Wager, T. D. & Smith, E. E. Neuroimaging studies of working memory: a meta-analysis. Cognitive Affective & Behavioral Neuroscience 3, 255–274 (2003).
Exner, C., Lange, C. & Irle, E. Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. Journal of Affective Disorders 119, 156–162 (2009).
Ottowitz, W. E., Dougherty, D. D. & Savage, C. R. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harvard Review of Psychiatry 10, 86–99 (2002).
Frodl, T. et al. Different effects of mirtazapine and venlafaxine on brain activation: an open randomized controlled fMRI study. The Journal of Clinical Psychiatry 72, 448–457 (2011).
Liang, X., Zou, Q., He, Y. & Yang, Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences of the United States of America 110, 1929–1934 (2013).
Drevets, W. C. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827 (1997).
Drevets, W. C. & Price, J. L. Neuroimaging and neuropathological studies of mood disorders. In: Licinio JWM (ed) Biology of depression: from novel insights to therapeutic strategies. WileyVCH Verlag GmbH & Co., Weinheim, (2005).
Nortje, G., Stein, D. J., Radua, J., Mataix-Cols, D. & Horn, N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. Journal of Affective Disorders 150, 192–200 (2013).
Lui, S., Zhou, X. J., Sweeney, J. A., & Gong, Q. Psychoradiology: The Frontier of Neuroimaging in Psychiatry. Radiology 281, 357–372 (2016).
Kressel, H. Y. Setting Sail: 2017. Radiology 282, 4–6 (2017).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery 8, 336–341 (2010).
Strakowski, S. M., DelBello, M. P., Adler, C., Cecil, D. M. & Sax, K. W. Neuroimaging in bipolar disorder. Bipolar Disorders 2, 148–164 (2000).
Radua, J. & Mataix-Cols, D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. The British Journal of Psychiatry 195, 393–402 (2009).
Hart, H., Radua, J., Nakao, T., Mataix-Cols, D. & Rubia, K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 70, 185–198 (2013).
Acknowledgements
This study was supported by the National Natural Science Foundation (Grant Nos. 81621003, 81761128023, 81220108013, 81227002 and 81030027), National Key Technologies R&D Program (Program No. 2012BAI01B03) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT16R52) of China.
Author information
Authors and Affiliations
Contributions
Q.G. conceived the project. W.W., Y.Z. and X.H. designed the protocol and wrote the main manuscript. W.W., Y.Z. and X.H. obtained the data. Y.Z., X.H., X.H., W.K., S.L. and G.K. analysed the results. All authors reviewed the manuscript. G.K. and Q.G. revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, W., Zhao, Y., Hu, X. et al. Conjoint and dissociated structural and functional abnormalities in first-episode drug-naive patients with major depressive disorder: a multimodal meta-analysis. Sci Rep 7, 10401 (2017). https://doi.org/10.1038/s41598-017-08944-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08944-5
- Springer Nature Limited
This article is cited by
-
Functional and structural alterations in different durations of untreated illness in the frontal and parietal lobe in major depressive disorder
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Co-alteration Network Architecture of Major Depressive Disorder: A Multi-modal Neuroimaging Assessment of Large-scale Disease Effects
Neuroinformatics (2023)
-
Translational application of neuroimaging in major depressive disorder: a review of psychoradiological studies
Frontiers of Medicine (2021)
-
Divergent Anatomical Correlates and Functional Network Connectivity Patterns in Temporal Lobe Epilepsy with and Without Depression
Brain Topography (2021)
-
Psychoradiological investigations of gray matter alterations in patients with anorexia nervosa
Translational Psychiatry (2018)