Abstract
We investigated the characteristics of bladder mechanosensitive single-unit afferent activities (SAAs) in rats with a bladder outlet obstruction (BOO) and their relationship with bladder microcontractions. Male Wistar rats were divided into Sham and BOO groups. Four or 10 days after the surgery, rats were anesthetized with urethane. The SAAs of Aδ- or C-fibers from the L6 dorsal roots were recorded during bladder filling. The BOO group showed a higher number of microcontractions and lower SAAs of Aδ-fibers compared with those of the Sham group. These findings were significant at day 10 post-operatively. In contrast, SAAs of C-fibers were not significantly different between the groups at either day 4 or 10. In the BOO group at day 10, the SAAs of both Aδ- and C-fibers at the “ascending” phase of microcontractions were significantly higher than those at the other phases (descending or stationary), and a similar tendency was also observed at day 4. Taken together, during bladder filling, the bladder mechanosensitive SAAs of Aδ-fibers were attenuated, but SAAs of both Aδ- and C-fibers were intermittently enhanced by propagation of microcontractions.
Similar content being viewed by others
Introduction
Detrusor overactivity (DO) has been defined as “a urodynamic observation characterized by involuntary detrusor contractions during the filling phase which can be spontaneous or provoked”1, and is commonly associated with the overactive bladder syndrome (OAB)2. Although the similarities are questionable3, a microcontraction during the storage phase in rodents has been widely used as a surrogate parameter for DO, and these microcontractions are frequently observed in pathophysiological conditions, especially in animal models of bladder outlet obstruction (BOO)3, 4. In isolated whole bladder preparations of a rat model of BOO, coordination of micromotions (similar to the microcontractions) was enhanced by stretch, leading to increased pressure fluctuations5. In addition, in women with OAB, micromotions were enhanced and concomitantly observed with urinary urgency during filling cystometry (CMG)6. These previous studies suggest that the microcontractions or micromotions may partly contribute to the development of urgency in humans with OAB. This has also been discussed in a recent review7.
Bladder afferent nerves are composed by Aδ- and C-fibers. A previous study in cats revealed that more than 90% of C-fibers do not respond to normal bladder distension, being so called “silent” fibers8. However, at least in rats, C-fibers can respond to normal bladder distension like Aδ-fibers, although they may also fulfill a potentially different role in the bladder sensory function in response to abnormal stimuli9,10,11. We previously demonstrated that bladder microcontractions may be related to the bladder mechanosensitive Aδ-fiber activities even in normal rats12. However, so far, there has been no study directly investigating the possible relationship between the microcontractions and sensory afferent transduction in pathophysiological conditions such as BOO. Thus, in the present study, we investigated the characteristics of bladder mechanosensitive single-unit afferent activities (SAAs) in male rats with BOO, and their relationship with bladder microcontractions.
Results
CMG measurements (at day 4)
In the present study, we further investigated CMG parameters at day 4, and found that the number and amplitude of NVCs in the BOO rats were significantly higher than in the Sham rats, whereas there were no significant differences between the groups in the other parameters (Fig. 1 upper traces, Table 1). In a previous study, we analyzed CMG parameters at day 10 postoperatively in our BOO model and found that bladder capacity (BC), residual volume (RV), and the mean amplitude and number of non-voiding contractions (NVCs) in the BOO rats were significantly higher than in the Sham rats13. However, in this study, CMG parameters were analyzed using only the third measurement in the three-times repeated CMG measurements. When the parameters were reanalyzed by the same methods used in the present study (Supplemental table) we found similar changes in the number and amplitude of NVCs as at day 4.
Histological findings (at day 4) and bladder weights (at day 4 and 10)
At day 4, the bladders in the BOO rats showed remarkable thickening of the detrusor muscle layer compared with the Sham rats (Fig. 1 lower traces), which was similar to our previous findings observed at day 1013. At day 4 and 10, the mean bladder weight in the BOO rats was significantly higher than in the Sham rats (day 4: 160.9 ± 18.1 mg vs. 94.5 ± 5.0 mg, P < 0.01; day 10: 190.9 ± 14.5 mg vs. 82.7 ± 2.3 mg, P < 0.001).
SAA measurements (at day 4 and 10)
During the SAAs measurements, bladder compliance in the BOO rats at day 10 was significantly higher than in the Sham rats, whereas no such differences were found at day 4 (Table 2). The number of microcontractions in the BOO rats was significantly higher than in the Sham rats at day 10, whereas there was no significant difference in the number of microcontractions between the Sham and BOO rats at day 4, although a similar tendency was observed (Table 2).
One hundred and twenty-seven single afferent fibers (more than 14 fibers in each group) were isolated, and there were no significant differences in conduction velocities (CVs) in either Aδ-fibers or C-fibers between the Sham and BOO rats (Table 2). On both study time-points (day 4 and 10), the BOO rats showed lower SAAs of Aδ-fibers than the Sham rats. These differences were more remarkable and significant at day 10, in which lower SAAs were observed throughout the entire filling phase, whereas those at day 4 only appeared during the last half of the filling phase (left panels in Fig. 2). In contrast, SAAs of C-fibers were not remarkably and significantly different between the Sham and BOO rats although slight differences were observed at day 4 (Right panels in Fig. 2). During the measurements, intermittently enhanced SAAs responses synchronized with bladder microcontractions, especially at the ascending phase, were frequently observed in the BOO rats, but not in the Sham rats (Fig. 3). The SAAs of both Aδ- and C-fibers during the ascending phase of microcontractions were significantly higher than those during the other phases (descending and stationary) at day 4 and 10 except those of Aδ-fibers between the ascending and descending phases at day 4 (Figs 3 and 4). Such higher afferent activities during the ascending phase of microcontractions were found in 86% (6/7 rats) and 100% (6/6 rats) for Aδ- and C-fibers, respectively, at day 4, and in 100% (8/8 rats) and 86% (6/7 rats) for Aδ- and C-fibers, respectively, at day 10.
Influence of BOO-induction on mechanosensitive SAAs of the Aδ- and C-fibers. The horizontal and vertical axes indicate the intravesical pressure and firing rate of SAAs, respectively. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01: significant differences from the Sham rats (unpaired Student’s t-test). On both study time-points (day 4 and 10), the BOO rats showed lower SAAs of Aδ-fibers than the Sham rats. These differences were more remarkable and significant at day 10 (Left panels). In contrast, SAAs of C-fibers were not remarkably and significantly different between the Sham and BOO rats (Right panels).
Representative traces of intravesical pressure and the firing rate of the Aδ- and C-fiber in the Sham (upper traces) and BOO rats (lower traces) at day 10. Each square in left panel is corresponding to the right panel. IP: intravesical pressure, FR: firing rate, NA: nerve activity. A: ascending phase, D: descending phase, S: stationary phase. During the measurements, intermittently enhanced SAAs of both Aδ- and C-fiber responses synchronized with bladder microcontractions were frequently observed, especially at the ascending phases, in the BOO rats, but not in the Sham rats.
SAAs responses in the BOO rats during three phases of the microcontractions on filling CMG. The vertical axis indicates firing rate of SAAs. Values are expressed as mean ± SEM. # P < 0.05, ## P < 0.01: significant differences between each phase (repeated measures ANOVA followed by Tukey’s multiple comparison test).
Discussion
In the SAAs measurements of the present study, no reflex arc through the L6 dorsal roots was preserved, thus the rats could not void. Therefore, we used the term NVCs in CMG and microcontractions in SAAs measurements. It is conceivable that both contractions are similar, and they have been demonstrated to be of myogenic origin4. The nature and importance of myogenic microcontractions during the bladder filling phase have been discussed by many investigators. Microcontractions were originally proposed as a motor component of a motor/sensory system14,15,16,17, and their importance for urinary storage and voiding dysfunction such as overactive bladder and detrusor underactivity was recently discussed7. Microcontractions have been found to be enhanced in pathological situations such as BOO18.
The CMG measurements in the present study demonstrated that the BOO rats showed increases in the number and amplitude of NVCs at day 4 post-operatively. Our previous13 and re-analyzed data (Supplemental table) by using the same CMG measurements showed that the number and amplitude of NVCs in the BOO rats were increased also at day 10. In addition, in the present SAAs measurements, the BOO rats showed an increase in the number of microcontractions, and these contractions were conspicuous at day 10 rather than day 4. These findings may partly support a previous study, in which increased coordination of micromotion waves with stretch and the consequent enhancement of intravesical pressure fluctuations following BOO may be involved in the pathophysiology of DO5. Moreover, the BOO rats showed higher bladder weights compared with the sham rats accompanied with thickening of the detrusor muscle layer at day 4, and such changes were more pronounced at day 1013.
In the pathophysiological condition of lower urinary tract dysfunction, bladder efferent function can be deteriorated, e.g. due to peripheral denervation19,20,21,22,23. In this condition, loss of efferent function may enable unregulated microcontraction activity, and afferent stimulation, predisposing to urinary urgency. During the storage phase, the bladder is not totally quiescent. In humans, there are low amplitude microcontractions in the bladder, which have barely-detectable effect on intravesical pressure6. In addition, Parsons et al. demonstrated that microcontractions (termed micromotions in the article) occurred over the bladder surface, with variable correlation with intravesical pressure fluctuations (microcontractions) in the pig bladder24. Exaggerated microcontractions are seen in animal models of lower urinary tract pathologies. For example, Drake et al. demonstrated that an increased coordination of microcontractions in isolated bladder strips with stretch and the consequent enhancement of bladder microcontractions in the isolated rat bladder after creating BOO5. These previous reports suggest that microcontraction propagation and general bladder tone at any given moment are key factors determining whether autonomous bladder activity results in proportionate intravesical pressure fluctuations.
The present BOO rats showed lower SAAs of Aδ-fibers and this finding was more conspicuous at day 10 than day 4, whereas C-fibers in the BOO rats did not show such lower activities compared with the Sham rats at either day 4 or day 10. These results suggest that BOO causes time-dependent denervation in myelinated Aδ-fibers, but not in unmyelinated C-fibers, of the bladder mechanosensitive afferent nerves at least within 10 days after mechanically creating BOO. Saito et al. demonstrated a linear correlation between the severity of obstruction and the degree of reduction in blood flow to the bladder25, 26, these changes leading to hypoxia and/or ischemia. In ischemic rabbit bladder tissue with atherosclerosis, Azadzoi et al. showed degenerating and collapsed axons and Schwann cells surrounded by dense connective tissue and splitting of the myelin sheaths27. In addition, Dahlin et al. reported that the thinner myelinated fibers were more susceptible to deprivation of oxygen under ischemic condition than the thicker ones, whereas unmyelinated fibers were resistant to ischemic induction28. These studies support our findings that mechanosensitive myelinated Aδ-fiber activity was attenuated following BOO condition.
We further investigated the characteristics of the SAAs related with microcontractions, and showed the SAAs of both Aδ- and C-fibers under the BOO condition were enhanced during the ascending phases. Even in un-treated (normal) rats, microcontractions and their synchronized SAAs have been observed in Aδ-fibers, but not in C-fibers. However, these microcontractions were rarely observed and relatively small12. In the present study with the BOO rats, more sustained and exaggerated SAAs of both Aδ- and C-fibers synchronized with microcontractions were demonstrated, suggesting that the myogenic bladder microcontractions promote the mechanosensitive bladder afferent activities at least under the BOO condition. Aδ-fibers are mainly located in the smooth muscle layer, whereas C-fibers distribute throughout the bladder wall from the smooth muscle layer to the urothelium29. It has been demonstrated that microcontractions provoked by BOO were of myogenic origin4, which may be strongly related with the function of the smooth muscle layer. Thus, it is reasonably assumed that propagation of microcontractions under the mechanical BOO condition causes intermittently enhanced SAAs of Aδ-fibers. Interestingly, similar SAAs enhancement by propagation of microcontractions was observed also in C-fibers in the present study. C-fibers in the bladder appear to be mechano-insensitive and “silent” (i.e., responding only to noxious and inflammatory stimuli), and may not participate in normal micturition in cats8, 30. In contrast, previous reports indicated that in rats, some C-fibers may be volume receptors and may not respond to bladder contractions31. Although we need further investigations, C-fibers may potentially fulfill a key role in the bladder abnormal sensation in response to microcontractions under pathophysiological conditions, i.e. it may be speculated that such intermittently enhanced afferent activities synchronized with microcontractions can link to the abnormal bladder sensation such as urgency.
In the present study, there is a limitation of the way of fiber classification, which was solely based on the CV value, i.e. Aδ- and C-fibers. Although this simple classification is helpful for our understanding of the afferent outflow from the bladder, there are several histologically different fiber types, regional variations in innervation and alterations of fiber properties following pathological changes. Thus, the present findings need to be considered in the light of all the new ideas that are emerging in relation to the structural and functional complexities of the bladder.
It has been suggested that myogenic microcontractions during the storage phase contribute to the development of urgency6, 32. To our knowledge, the present study is the first direct demonstration of a relationship between the mechanosensitive afferent fiber activities and bladder microcontractions under pathophysiological conditions. Although there are limitations regarding the experimental setup such as under urethane-anesthesia3, and acute severe BOO compared to chronic slowly progressive BOO from benign prostatic enlargement in human, if valid in humans, this might be supports the view that microcontractions contribute to the development of urgency and/or DO associated with BOO6.
In conclusion, there is a relation between the mechanosensitive afferent fiber activities and bladder microcontractions in a pathophysiological condition such as BOO. During bladder filling, the bladder mechanosensitive SAAs of Aδ-fibers were attenuated, but SAAs of both Aδ- and C-fibers were intermittently enhanced by propagation of microcontractions.
Methods
Ethical approval and animals
The protocol was approved by the Institutional Animal Care and Use Committees of the University of Tokyo and was in line with the NIH guidelines for the care and use of experimental animals. Fifty-seven adult male Wistar rats were used (11 weeks old, 254–295 g, Japan SLC, Shizuoka, Japan). The rats were maintained under standard laboratory conditions with a 12:12 h light: dark cycle, and free access to food and water. Maintenance and killing of the animals followed principles of good laboratory practice in compliance with national laws and regulations. The animals were killed humanely by overdose of anesthesia.
Surgical procedures for creating BOO and experimental schedule
Surgery to create partial BOO was done as described in previous reports13, 33, 34. In brief, the proximal urethra was tightly ligated with a steel rod (1.2 mm in diameter), then the rod was removed. This ligation was kept to the end of the experiments. Sham rats received similar surgery without the urethral ligation. Each animal was housed in a separate cage. CMG measurements and histological examinations were performed at day 4 post-operatively (Sham: N = 7, BOO: N = 6), when the BOO rats showed an increase in voiding frequency and decreases in mean uroflow rate (Qave) and voided volume per micturition (VV) based on our previous findings with voiding behavior measurements in a metabolic cage13. In separate animals, SAAs measurements were performed at day 4 (N = 10, in each group) and 10 (N = 12, in each group).
CMG measurements and histological examinations
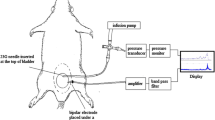
The methods for CMG measurements were slightly modified from our previous study13. In brief, at the same day of the surgery for creation of Sham or BOO, a PE-50 catheter (Clay Adams, Parsippany, NJ) with a cuff was implanted into the bladder. Four days after the catheter-implantation (on day 4), CMG measurements in a conscious restrained condition were performed. Saline was instilled into the bladder at a rate of 6 mL/h until micturition occurred, and voided urine was collected in a cup placed just under the penis and weight of voided urine was measured. After each micturition, the bladder catheter was disconnected and the post-void residual was collected by natural dropping through the catheter for 10 min and measured. CMG recordings were repeated 3 times and the following parameters were averaged and analyzed: basal pressure (BP: minimum intravesical pressure), threshold pressure (TP: intravesical pressure at the onset of micturition), maximum pressure (MP: maximum intravesical pressure during micturition), VV, RV (the volume of urine collected after micturition), BC (VV + RV), Qave (VV/voiding time), and mean amplitude and the number of NVCs. NVCs were defined as bladder contractions without micturition, the amplitudes of which were more than 2 cmH2O, observed for 3 min before micturition.
In some animals (four Sham and five BOO rats) of the CMG measurements, the whole bladder was isolated at the end of the experiments, subsequently fixed in 4% paraformaldehyde-PBS, then embedded in paraffin and cut into 3 µm sections. Finally, the bladder specimens were evaluated with HE staining.
SAAs measurements
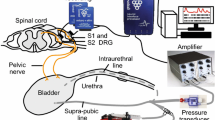
SAAs measurements were performed as described previously under urethane anesthesia (1.2 g/kg, intraperitoneally)35. In brief, after a pair of silver stimulation electrodes was placed around the left pelvic nerve, both L6 dorsal roots were cut near their entrance into the spinal cord. Unitary action potentials of mechanosensitive bladder afferent nerve fibers isolated from left L6 dorsal roots were identified as SAAs by electrical stimulation of the pelvic nerve and by bladder distension with saline via an inserted bladder catheter (PE-50). The SAAs were grouped on the basis of their CV, i.e. those with a CV of less than 2.5 m/s were considered to correspond to unmyelinated C-fibers, whereas those with a CV of 2.5 m/s or greater were considered to correspond to myelinated Aδ-fibers. SAAs and intravesical pressure were recorded during constant saline-instillation into the bladder. Based on the values of BC obtained from the CMG measurements in our previous13 and the present studies, saline-instillation rate was applied at 6 ml/h in the Sham (at day 4 and 10) and BOO rats (at day 4), and at 10 ml/h in the BOO rats (at day 10). Such different rates of saline-instillation may affect the development of microcontractions or SAAs, but our preliminary investigation showed that there were no remarkable differences in the microcontractions or SAAs between the different rates (data not shown). The filling continued until an intravesical pressure of 30 cmH2O was achieved, and the bladder compliance was simply calculated between the start and end of the saline-instillation into the bladder during measurements. The SAAs were expressed as the firing rates (Hz) and evaluated in relation to intravesical pressure at each 5 cmH2O-interval.
A microcontraction was defined as a contraction with an amplitude of more than 2 cmH2O, and where intravesical pressure was ascending at 0.25 cmH2O/s or more and then descending at 0.15 cmH2O/s or more. The microcontraction was divided into two phases: “ascending” and “descending”. The portion between the two microcontractions was termed as the “stationary” phase (Fig. 5).
Definition of the microcontractions (ascending and descending phases) and stationary phase in the present study. Upper trace is intravesical pressure, and lower trace is firing rate of SAAs. The microcontractions were defined as the contractions having more than 2 cmH2O of amplitude, and pressure was ascending at 0.25 cmH2O/s or more, and then descending at 0.15 cmH2O/s or more. And the portions between these phases were defined as the stationary phases.
Statistical Analyses
All data are expressed as mean ± SEM. The results between the Sham and BOO rats were analyzed using an unpaired Student’s t-test. To evaluate the SAAs related with microcontractions, repeated measures ANOVA followed by Tukey’s multiple comparison test was used. P values < 0.05 were considered statistically significant.
References
Abrams, P. et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21, 167–178, doi:10.1002/nau.10052 (2002).
Gratzke, C. et al. EAU Guidelines on the Assessment of Non-neurogenic Male Lower Urinary Tract Symptoms including Benign Prostatic Obstruction. Eur Urol 67, 1099–1109, doi:10.1016/j.eururo.2014.12.038 (2015).
Andersson, K. E., Soler, R. & Fullhase, C. Rodent models for urodynamic investigation. Neurourol Urodyn 30, 636–646, doi:10.1002/nau.21108 (2011).
Igawa, Y., Mattiasson, A. & Andersson, K. E. Micturition and premicturition contractions in unanesthetized rats with bladder outlet obstruction. J Urol 151, 244–249 (1994).
Drake, M. J. et al. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol 170, 276–279, doi:10.1097/01.ju.0000069722.35137.e0 (2003).
Drake, M. J., Harvey, I. J., Gillespie, J. I. & Van Duyl, W. A. Localized contractions in the normal human bladder and in urinary urgency. BJU Int 95, 1002–1005, doi:10.1111/j.1464-410X.2005.05455.x (2005).
Drake, M. J. et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int 119, 22–29, doi:10.1111/bju.13598 (2017).
Habler, H. J., Janig, W. & Koltzenburg, M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425, 545–562 (1990).
Aizawa, N., Igawa, Y., Nishizawa, O. & Wyndaele, J. J. Effects of CL316,243, a beta 3-adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol Urodyn 29, 771–776, doi:10.1002/nau.20826 (2010).
Aizawa, N., Igawa, Y., Nishizawa, O. & Wyndaele, J. J. Effects of nitric oxide on the primary bladder afferent activities of the rat with and without intravesical acrolein treatment. Eur Urol 59, 264–271, doi:10.1016/j.eururo.2010.10.035 (2011).
Shea, V. K., Cai, R., Crepps, B., Mason, J. L. & Perl, E. R. Sensory fibers of the pelvic nerve innervating the Rat’s urinary bladder. J Neurophysiol 84, 1924–1933 (2000).
Aizawa, N., Homma, Y. & Igawa, Y. Effects of mirabegron, a novel beta3-adrenoceptor agonist, on primary bladder afferent activity and bladder microcontractions in rats compared with the effects of oxybutynin. Eur Urol 62, 1165–1173, doi:10.1016/j.eururo.2012.08.056 (2012).
Sugiyama, R. et al. Synergic suppressive effect of silodosin and imidafenacin on non-voiding bladder contractions in male rats with subacute bladder outlet obstruction. LUTS: Lower Urinary Tract Symptoms in press (2015).
Iggo, A. Tension receptors in the stomach and the urinary bladder. J Physiol 128, 593–607 (1955).
Sherrington, C. S. Notes on the Arrangement of some Motor Fibres in the Lumbo-Sacral Plexus. J Physiol 13, 621–772 617 (1892).
Starling, E. H. Elements of human physiology (Churchill, 1892).
Vaughan, C. W. & Satchell, P. M. Urine storage mechanisms. Progress in neurobiology 46, 215–237 (1995).
Lluel, P., Duquenne, C. & Martin, D. Experimental bladder instability following bladder outlet obstruction in the female rat. J Urol 160, 2253–2257 (1998).
Brading, A. F. & Turner, W. H. The unstable bladder: towards a common mechanism. Br J Urol 73, 3–8 (1994).
Mills, I. W. et al. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J Urol 163, 646–651 (2000).
Sibley, G. N. The physiological response of the detrusor muscle to experimental bladder outflow obstruction in the pig. Br J Urol 60, 332–336 (1987).
Drake, M. J. et al. Structural and functional denervation of human detrusor after spinal cord injury. Lab Invest 80, 1491–1499 (2000).
Drake, M. J., Gardner, B. P. & Brading, A. F. Innervation of the detrusor muscle bundle in neurogenic detrusor overactivity. BJU Int 91, 702–710 (2003).
Parsons, B. A., Drake, M. J., Gammie, A., Fry, C. H. & Vahabi, B. The validation of a functional, isolated pig bladder model for physiological experimentation. Front Pharmacol 3, 52, doi:10.3389/fphar.2012.00052 (2012).
Saito, M., Yokoi, K., Ohmura, M. & Kondo, A. Effects of partial outflow obstruction on bladder contractility and blood flow to the detrusor: comparison between mild and severe obstruction. Urol Int 59, 226–230 (1997).
Saito, M., Yokoi, K., Ohmura, M. & Kondo, A. Effect of ischemia and partial outflow obstruction on rat bladder function. Urol Res 25, 207–211 (1997).
Azadzoi, K. M., Chen, B. G., Radisavljevic, Z. M. & Siroky, M. B. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol 186, 2115–2122, doi:10.1016/j.juro.2011.06.047 (2011).
Dahlin, L. B., Shyu, B. C., Danielsen, N. & Andersson, S. A. Effects of nerve compression or ischaemia on conduction properties of myelinated and non-myelinated nerve fibres. An experimental study in the rabbit common peroneal nerve. Acta Physiol Scand 136, 97–105, doi:10.1111/j.1748-1716.1989.tb08634.x (1989).
Gabella, G. The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol 24, 159–187 (1995).
Habler, H. J., Janig, W. & Koltzenburg, M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol 463, 449–460 (1993).
Morrison, J. & Wen, J. & Kibble, A. Activation of pelvic afferent nerves from the rat bladder during filling. Scand J Urol Nephrol Suppl 201, 73–75 (1999).
Brading, A. F. A myogenic basis for the overactive bladder. Urology 50, 57–67; discussion 68–73 (1997).
Saito, M., Longhurst, P. A., Tammela, T. L., Wein, A. J. & Levin, R. M. Effects of partial outlet obstruction of the rat urinary bladder on micturition characteristics, DNA synthesis and the contractile response to field stimulation and pharmacological agents. J Urol 150, 1045–1051 (1993).
Hashimoto, T., Nagabukuro, H. & Doi, T. Effects of the selective acetylcholinesterase inhibitor TAK-802 on the voiding behavior and bladder mass increase in rats with partial bladder outlet obstruction. J Urol 174, 1137–1141, doi:10.1097/01.ju.0000168616.71956.a4 (2005).
Aizawa, N. et al. Effects of intravesical instillation of ATP on rat bladder primary afferent activity and its relationship with capsaicin-sensitivity. Neurourol Urodyn 30, 163–168, doi:10.1002/nau.20940 (2011).
Acknowledgements
We would like to thank Rino Sugiyama for contributing some of the data acquisition. The present study has been supported by a Grant-in-Aid for Scientific Research (Y.I. Grant no. 40159588, N.A. Grant no. 80595257), from the Ministry of Education, Culture, Sport, Science and Technology of the Japanese Government.
Author information
Authors and Affiliations
Contributions
All experiments were performed in Professor Igawa’s laboratory at the University of Tokyo. N.A. and Y.I. were responsible for the conception and design of experiments. N.A. was responsible for the collection, analysis, interpretation of data and drafting of the manuscript. K.I., H.F., T.F., K.-E.A. and Y.H. were involved in revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript and all persons designated as authors qualify for authorship.
Corresponding author
Ethics declarations
Competing Interests
Y.H. is a consultant for Astellas and Pfizer; and has received grants from Asahi Kasei, Daiichi-Sankyo, GlaxoSmithKline, Kissei, Ono, Kyorin, Taiho, Pfizer and Nippon Shinyaku; and speaker’s honoraria from Astellas, Pfizer, Kyorin, Kissei, Ono, Asahi Kasei, Daiichi-Sankyo, GlaxoSmithKline, Taiho and Integral. Y.I. is a consultant for Astellas, Pfizer and Eli Lilly; and has received grants from Astellas, Asahi Kasei, Kissei, Ono, Kyorin, Taiho, RaQualia, Daiichi-Sankyo, Nippon Shinyaku, Lilium Otsuka, Integral, Medicon and Tsukada Medical Research; and speaker’s fees from Astellas, Asahi Kasei, Kissei, Kyorin, Taiho, Nippon Shinyaku and Pfizer.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aizawa, N., Ichihara, K., Fukuhara, H. et al. Characteristics of the mechanosensitive bladder afferent activities in relation with microcontractions in male rats with bladder outlet obstruction. Sci Rep 7, 7646 (2017). https://doi.org/10.1038/s41598-017-07898-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07898-y
- Springer Nature Limited
This article is cited by
-
Potentilla chinensis aqueous extract attenuates cyclophosphamide-induced hemorrhagic cystitis in rat model
Scientific Reports (2022)
-
Role of PTHrP in attenuating transient pressure rises and associated afferent nerve activity of the rat bladder
Pflügers Archiv - European Journal of Physiology (2022)
-
Pathophysiological changes of the lower urinary tract behind voiding dysfunction in streptozotocin-induced long-term diabetic rats
Scientific Reports (2020)