Abstract
Emerging evidence supports that stem cells are regulated by both intrinsic and extrinsic mechanisms. However, factors that determine the fate of stem cells remain incompletely understood. The Drosophila testis provides an exclusive powerful model in searching for potential important regulatory factors and their underlying mechanisms for controlling the fate of germline stem cells (GSCs). In this study, we have found that Drosophila gilgamesh (gish), which encodes a homologue of human CK1-γ (casein kinase 1-gamma), is required intrinsically for GSC maintenance. Our genetic analyses indicate gish is not required for Dpp/Gbb signaling silencing of bam and is dispensable for Dpp/Gbb signaling-dependent Dad expression. Finally, we show that overexpression of gish fail to dramatically increase the number of GSCs. These findings demonstrate that gish controls the fate of GSCs in Drosophila testis by a novel Dpp/Gbb signaling-independent pathway.
Similar content being viewed by others
Introduction
Adult stem cells (ASCs) are essential for tissue homeostasis by constantly providing new cells to replenish many tissues, including blood, skin, germ-line, and the intestinal epithelium. ASCs are characterized by self-renewal and potentiality to differentiation during all life time, and a balance between self-renewal and differentiation is crucial for tissue homeostasis1,2,3. Previous studies have shown that the intrinsic factors from stem cells are necessary to achieve this balance, extrinsic signaling molecules from microenvironment (also called “niche”) surrounding ASCs also control this balance4,5,6.
Little is known for the mechanisms of stem cell regulation which maintains this balance between self-renewal and differentiation. The Drosophila testis provides an exemplary model for the study of stem cell biology7, 8. In adult Drosophila males, two stem cell populations are located at the apical tip of the testis: germline stem cells (GSCs) and somatic stem cells (SSCs) (Fig. 1a). Both GSCs and SSCs contact with a cluster of non-dividing somatic cells known as the hub. A GSC divides asymmetrically to generate one daughter cell, which maintains adjacent to the hub and retains stem cell identity, and the other one, which is pushed away from the hub and initiates differentiation as a gonialblast (GB). The GB mitotically divides four times to produce a cyst of 16 interconnected spermatogonia, which go on to enter meiosis and differentiate into spermatid, eventually maturing into sperms. During the process of GSCs dividing, the hub functions as a major component of GSCs niche. SSCs serve both as another component of GSCs niche and as stem cells to generate cyst cells (CCs) which encapsulates the differentiating GBs9.
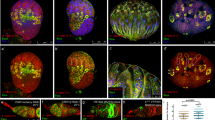
Identification of a gish mutant with defects in male GSCs maintenance. (a) A diagram of a testis tip with different cell types labeled with different colors: Germline stem cells (GSCs) (dark pink), Hub cell (green), Somatic stem cell (SSC) (dark blue), Gonialblasts (GBs) (pink), cyst cells (blue), and fusomes (red). (b–f,h) Testes stained with anti-Fas III antibody to label the hubs (red, indicated by asterisks), anti-Hts antibody to label the fusomes (red), and anti-Vasa antibody to label germ cells (green). GSCs were highlighted by white dots. (b) In wild-type testis, seven GSCs (white dot) directly contact the hub (asterisk). (c) gish 04895 mutant testis from 1-day-old fly showing that six GSCs contact the hub. (d) gish 04895/gish MI08417 testis from 10-day-old fly showing that five GSCs remains adjacent to the hub. (e) gish KG03891/gish MI08417testis from 20-day-old fly showing three remaining GSCs. (f) gish 04895/gish MI08417 testis from 20-day-old fly. Only two GSCs remains. (g) Quantitative PCR analyses of gish mRNA levels in testes between gish mutant and wild-type. (h) The transgene P{gishP-gishF} rescued the gish 04895/gish MI08417 male testis to normal, with eight GSCs close to the hub. Scale bars: 10 μm.
The casein kinase 1 (CK1) family is evolutionarily conserved from yeast to human, and regulates multiple physiological processes, such as membrane transport, circadian rhythm, apoptosis, vesicle transport, cell division and differentiation10. Drosophila gilgamesh (gish), which encodes a homologue of human CK1-γ (casein kinase 1-γ), has been shown to be involved in glial cell migration, olfactory learning, spermatogenesis and the Wg/Wnt pathway11,12,13,14,15,16. Here, we identified a new role for gish in maintaining the fate of germline stem cells in male Drosophila.
Results
Identification of gish mutants with defects in GSC maintenance
To identify novel genes that regulate the self-renewal or differentiation of GSCs in Drosophila testis, we conducted a screen for male sterile mutants with P-element insertion, available from the Bloomington Stock Center. We isolated a line with a P-element insertion in the third chromosome15, P{PZ}gish 04895, which resulted in small testis and reduction in germ-cell number in homozygous mutant males. To thoroughly analyze the behavior of germ cells in gish 04895 mutant, we used anti-Fas III and anti-Vasa antibodies to visualize hub and germ cells17, 18, respectively (Fig. 1b). Hub is a cluster of somatic cells found at the tip of the adult testis which could be characterized by anti-Fas III antibody staining in Drosophila. As shown in Fig. 1b, we visualized GSCs and germ cells with an anti-Hts antibody19. A spherical fusome (also called the “spectrosome”) is the marker of GSCs and GB. GB undergoes 4 rounds of cell division to produce a 16-cell germline cluster, in which branched fusomes are visualized by the anti-Hts antibody (Fig. 1a,b).
In the tip of wild-type testis, 6–10 GSCs that were directly adjacent to the hub were recognized by an anti-Vasa antibody (Fig. 1b)17. In contrast, in the 10-day-old testes from gish 04895 homozygous males, about 30–40% of mutant testes (n > 100) contained 3–4 GSCs attached to the hub. This finding suggests that gish may plays a key role in the maintenance of GSCs. To explore whether gish is involved in the GSC maintenance in other relevant genetic background, we next performed phenotypic analyses with gish removal in three allelic combinations (Table 1). Using the methods described in the previous study17, we counted the number of GSCs in gish allelic combinations at days 1, 10 and 20 after eclosion. Compared to wild-type and gish/+ heterozygotes, the newly eclosed gish mutants (gish 04895/gish KG03891, gish 04895/gish MI08417, gish KG03891/gish MI08417) contained an average of 6.7, 6.5 and 6.3 GSCs/testis (Fig. 1c and Table 1). When these three gish mutants were cultured at room temperature for 10 days, the testes had an average of 5.4, 5.7 and 5.8 GSCs/testis respectively (Fig. 1d and Table 1), whereas the testes from wild-type contained a normal average of 7.7 GSCs/testis. At day 20, the average number of GSCs was dramatically reduced to 3.9, 3.7 and 3.5 GSCs/testis respectively (Fig. 1e,f and Table 1), compared to the average number of wild-type maintained at the normal level (6.0 GSCs/testis) (Table 1). The testes from gish 04895/Df and gish KG03891/Df exhibited a similar loss of GSCs with ageing (Table 1). These statistical data indicate that the deficiency of gish leads to a progressive loss of GSCs with ageing.
To test whether the GSC loss phenotype is due to a reduced gish expression level in gish mutant testis, we performed real-time quantitative polymerase chain reaction (qPCR) experiments to compare the mRNA level between the wild-type and mutant fly testis20. The gish gene totally has thirteen predicted mRNA splicing variants (A, B, C, D, E, F, G, H, I, J, K, L and M) derived from the Drosophila database (www.flybase.org), but their corresponding encoded proteins share a conserved kinase domain. Based on this, the qPCR primers were designed (see the materials and methods) to target the conserved cDNA region using the online Primer 3.0 version software to detect the whole mRNA expression level of gish. We extracted total RNA from Drosophila testes, performed reverse-transcription (RT), and conducted qPCR experiments to measure the whole gish mRNA level with the rp49 gene as reference21. Compared to wild-type, the total gish mRNA expression levels in gish mutant testes (gish 04895/gish KG03891, gish KG03891/gish MI08417and gish 04895/gish MI08417) were severely reduced (Fig. 1g). These results strongly suggest that Gish is reduced in these gish mutants’ testes, and also imply that the Gish protein is responsible for the loss of GSCs phenotype in gish mutant flies.
To further confirm the role of gish in GSC maintenance, we next performed a gish rescue assay using gish cDNA. The gish gene contains numerous splicing variants as mentioned above. To explore which isoforms are expressed in Drosophila testis, we designed a dozen of primer pairs (Supplementary Table S1), each for each known gish transcript, and performed RT-PCR analysis22 using total RNAs isolated from wild-type fly testis. We detected transcripts gishF and B in testis (Supplementary Fig. S1). Based on these findings, we generated a transgene of P{gishP-gishF}, in which the gishF cDNA was placed under the control of a 5.0 kb gish promoter. We found that the GSC loss phenotypes in three gish allelic mutants were fully rescued by the transgenic line of P{gishP-gishF} (Fig. 1h and Supplementary Table S2). Taken together, our findings demonstrate that the gish gene plays an essential role in GSC maintenance.
Previous study has reported that gish mutant germ cells contain abnormal actin cones in individualizing spermatid cysts15, this result implies that gish possibly affects the F-actin-mediated cell adhesion junctions. To explore whether the gish mutant GSCs lose adhesion to the hub, we visualized F-actin with phalloidin15 and labeled GSCs with anti-Vasa antibody17. We found that there was no difference in F-actin-based GSCs adhesion to hub cells between wild-type control and gish KG03891/gish MI08417 mutant testes (n > 80) at day 14 after eclosion (Supplementary Fig. S2), just as the wild-type, GSCs from gish mutant testes contacted directly to hub cells. Similar phenotype was founded in gish 04895/gish MI08417 mutant testes (n > 70). These results manifest that the mutation of gish gene doesn’t affect cell-cell (GSC and hub cell) adhesions in fly testes, suggesting some other mechanisms maybe responsible for the GSCs loss phenotype.
To address whether the loss of GSCs in gish mutants was due to premature differentiation or cell death of GSCs, we measured the rate of apoptosis in the gish mutant GSCs by TUNEL assays23. We found that there was no difference in apoptosis between wild-type control and gish mutant GSCs (Supplementary Table S3 and Supplementary Fig. S3). These results suggest that mutant GSCs may have deficiency in GSC self-renewal and/or switch to pre-differentiation.
The gish gene is intrinsically required for GSC maintenance
Previous studies have shown that the maintenance of GSCs is regulated through intrinsic and extrinsic signaling pathways in testis24, 25. To explore the role of gish in GSC maintenance, we generated a transcriptional reporter P{gishP-GFP}, in which the gfp expression pattern represents that of gish gene26. As shown by immunofluorescent staining assays (Fig. 2a and Supplementary Fig. S4), GFP was ubiquitously expressed in all cell types including somatic cells (e.g. hub) and germline cells (e.g. GSCs and GBs) in transgenic fly testes (n > 100), suggesting a broad transcription activity of the gish promoter.
Gish is required intrinsically for GSC maintenance. (a) Testis bearing a transgene P{gishP-GFP} was stained with anti-Fas III antibody (red) to label the hub (a circle), anti-Hts antibody (red) to visualize fusomes, and anti-GFP antibody (green) to show the gish expression pattern. The gene gish expresses (green) both in GSCs (broken lines, arrowhead) and in hub cells (a circle, arrow). (b–f) GSC clones were induced in testes by FLP/FRT-mediated mitotic recombination in adult flies. Testes from FRT control flies (b,c) and FRT, gish flies (d–f) were dissected at different days after heat-shock treatment, then stained with anti-GFP (green), anti-Fas III (red) anti-Hts (red) antibodies and DAPI (blue). Hubs were marked by asterisks. GSC clones (indicated by broken lines) and spermatogonium clones (noted by circles) were identified by lack of GFP expression. (g–j) Testes from 20-day-old flies were stained with anti-Fas III antibody to label the hubs (red, indicated by asterisks), anti-Hts antibody to label the fusomes (red), and anti-Vasa antibody to label germ cells (green). GSCs were highlighted by white dots. (g) gish 04895/gish MI08417 testis showing only one GSC remains. (h) nosP-gishF; gish 04895/gish MI08417 testis showing normal GSCs number (8 GSCs). (i) C587; UASp-gishF; gish 04895/gish MI08417 testis. Only two GSCs close to the hub. (j) nosP-gal4; UASp-gishF; gish 04895/gish MI08417 testis showing the restored GSC number (10 GSCs). Scale bars: 10 μm. (k) Percentage of the negatively-marked GSC clones (lack of GFP expression, GFP-) in FRT control and two FRT, gish alleles at days 2, 10 and 20 after heat-shock treatment. The percentage of the negatively-marked GSCs (GFP-) lacking gish was reduced strongly, compared with the negatively-marked GSCs (GFP-) in FRT control.
To determine whether gish acts as an intrinsic or extrinsic regulator, we then generated gish mutant GSC clones using the FLP/FRT-mediated mitotic recombination technique27,28,29. The gish mutant GSCs were negatively-marked after several days of heat-shock treatments. We counted and compared the loss rate of marked GSC clones, as described previously17, 23, between the FRT control (hs-flp; FRT82B/FRT82B, ubi-gfp) and the gish mutant genotype (hs-flp; FRT82B, gish/FRT82B, ubi-gfp), at days 2, 10 and 20 after heat-shock treatments (AHST). As shown in Fig. 2, the rates of marked GSC clones from FRT control decreased weakly from the initial of 72.0% (n = 254) at day 2 to the last point of 59.9% (n = 197) at day 20 AHST (Fig. 2b,c and k). Only 16.8% of the marked GSCs were lost during the 20-day AHST period. By contrast, under the same experimental conditions, the initial marked gish 04895 and gish MI08417 mutant GSCs clones were 66.6% (n = 213) and 66.4% (n = 188) respectively at day 2 AHST, whereas they reduced to the rates of 21.2% (n = 189) and 24.4% (n = 279) respectively at day 20 AHST (Fig. 2d–f and k). These results revealed that 68.2% and 63.3% of marked gish 04895 and gish MI08417 mutant GSCs clones were lost during the 20-day AHST. Taken together, these findings suggest that gish plays an essential role for GSC maintenance through an intrinsic mechanism. To further confirm this point, we generated a transgene, P{nosP-gishF}, in which the gishF coding sequence was placed under the control of the promoter of the gene nanos that possesses a high expression level in germline cells. We found that the GSCs loss phenotype was fully rescued in gish mutant flies carrying the P{nosP-gishF} transgene (Fig. 2g,h and Supplementary Table S4). This result supports that Gish functions as an intrinsic factor.
To further test whether gish also plays an extrinsic role in GSC self-renewal, we generated a new transgenic fly strain carrying P{UASp-gishF}, in which the gishF coding sequence is under the control of the UASp promoter30. Using the Gal4-UAS system, we expressed the Gish protein in somatic hub cells and somatic stem cells by the c587-gal4-driven UASp-gishF expression17. We found that the GSCs loss phenotype was not rescued in gish mutant testes carrying the c587-gal4 and UASp-gishF transgenes (Fig. 2i and Supplementary Table S5). We then expressed gish specifically in germ cells that carried transgenes of P{UASp-gish} and P{nosP-gal4}, in which a germline-specific nanos-gal4 driver can express the target gene under the control of UASp promoter31. We found that the gish phenotype was fully rescued (Fig. 2j and Supplementary Table S6). This result is consistent with the above observation on the rescuing effect of P{nosP-gishF}. Taken together, these data strongly indicate that gish is an intrinsic factor that regulates GSC self-renewal.
gish is not required for Dpp/Gbb signaling silencing of bam
A Previous study has shown that two BMP members, Dpp and Gbb function cooperatively to maintain GSCs in Drosophila testis by repressing bam transcription in GSCs17. To explore whether gish is involved in Dpp/Gbb-dependent bam silencing, we examined the bam expression pattern in gish mutant testes through the expression of bam transcriptional reporter P{bamP-GFP}32. As shown in Fig. 3, the germ cells in testes from 5-day-old flies after eclosion were labelled with anti-GFP antibody and DAPI staining. We found that 88.5% of GSCs and 83% of GBs (n = 65 testes) from the male wild-type flies carrying P{bamP-GFP} reporter exhibited a completely negative GFP pattern (Fig. 3a). Similar phenotypes were observed in gish mutant testes, 89% of GSCs and 86.5% of GBs (n = 70 testes) showed to be negative for GFP (Fig. 3b). These data strongly suggest that gish is not required for bam silencing.
Gish has no affect the expression patterns of bam and Dad. The testes in (a–d) were marked with Fas III (red, hub with asterisk), Hts (red, fusomes), GFP (green) and DAPI (blue). GSCs and GBs were highlighted by circles (arrows) and broken lines (arrowheads), respectively. (a,b) gish is not required for Dpp/Gbb signaling silencing of bam. Testes from bamP-gfp (a) and bamP-gfp; gish 04895/gish MI08417 (b) male flies show negative GFP expression in either GSCs (indicated by arrows) or GBs (indicated by arrowheads). (c–d) gish is dispensable for Dpp/Gbb signaling-dependent Dad expression. Testes from DadP-gfp (c) and DadP-gfp; gish 04895/gish MI08417 (d) exhibit GFP expression in both GSCs (indicated by arrows) and GBs (indicated by arrowheads). Scale bars: 10 μm.
Gish is dispensable for dpp/gbb signaling-dependent Dad expression
It has been reported that dpp signaling is necessary for Dad expression, whereas Dad negatively modulates dpp signaling, forming a negative-feedback loop in Drosophila wing development33, 34. Another study shows that Dad is a gbb-responsive gene that negatively regulates gbb signaling in GSCs and GBs in Drosophila testis17. To test whether mutation of gish affects Dpp/Gbb signaling-dependent Dad expression in GSCs, we examined the expression of Dad transcriptional reporter P{DadP-GFP} in the gish mutant background. After gish mutant males were cultured at 25 °C for five days, 93% of GSCs and 94.5% of GBs (n = 65 testes) of wild-type flies carrying P{DadP-GFP} reporter showed positive for GFP (Fig. 3c). Similarly, 95% of GSCs and 90% of GBs (n = 68 testes) from gish mutant testes exhibited a GFP-positive pattern (Fig. 3d). These results convincingly suggest that gish is dispensable for Dad expression.
Ectopic gish expression had no effects on the number of GSC
Loss of function of gish contributed to testis GSC loss without involvement of apoptosis, it is likely that overexpressed gish in testis may delay GSCs/GBs differentiation. To test this possibility, we isolated GSCs from somatic cells and differentiated germ cells by using anti-Hts, anti-fas III and anti-Vasa antibodies to visualize fusomes, hub cells and germ cells, respectively17, 18. The spectrosome, a spherical fusome, is the marker of GSCs and their early progeny (also known as GB). When a late-stage GB divides to 2-cell, the fusome is visualized as a short-bar connecting two GB cells. GB undergoes 2, 3 and 4 rounds of synchronous cell division to produce a 4, 8 and 16-cell germline cluster, in which branched fusomes were visualized by anti-Hts antibody (Fig. 1a). We counted the number of germ cells carrying spectrosomes in testes from wild-type, and P{nosP-gishF} transgenic flies 7 days after eclosion. We observed the averages of 11.3 spectrosome-contained GSC/GBs (n = 67 testes) per testis in wild-type and 12.5 spectrosome-contained GSC/GBs (n = 70 testes) per testis in P{nosP-gishF} flies (Table 2 and Fig. 4a and b). These results suggest that there is no significant difference in the number of spectrosome-contained germ cells among wild-type and gish-overexpression transgenic flies.
Ectopic gish expression had no effect on the number of GSC. The testes in (a–d) were stained with anti-Fas III antibody (red, hub with asterisk), anti-Hts antibody (red, fusomes), and anti-Vasa antibody (green, germ cells). (a,b) Testes were collected from wild-type (a) and P{nosP-gishF} (b) male flies. (c,d) Testes were dissected from P{hsP-gishF} male flies that were cultured at 25 °C (c) and at 37 °C (d) for 1.5 hours three times per day, respectively. Spectrosomes-containing GSCs and GBs are indicated by arrows. Scale bars: 10 μm.
To substantiate this result, we generated transgenic flies carrying P{hs-gishF}, in which a gishF cDNA was placed under the control the heat-shock promoter35. We overexpressed gishF in testes by applying heat-shock treatment at 37 °C for 1.5 hours three times each day, counted the number of germ cells carrying spectromes in testes 7 days after heat-shock treatment with P{hs-gishF} flies cultured at 25 °C as control. We found an average of 11.6 GSC/GBs carrying spectrosomes per testis (n = 69 testes) in control flies (Table 2 and Fig. 4c). In contrast, in testes with ectopic gishF expression, the number of the germ cells containing spectrosomes was increased to an average of 14.8 cells per testis (n = 72 testes) (Table 2 and Fig. 4d). These results also show that there is no obvious increase in the numbers of spectrosome-containing germ cells after ectopic gish expression in P{hs-gishF} transgenic flies. Taken together, these data imply that increased Gish activity is not sufficient to block GSC/GB differentiation.
Discussion
Past research has demonstrated that male flies with homozygous gish of a P element insertion (P{ry+t7.2 = PZ}gish 04895) exhibited sterile and defective spermatid individualization in Drosophila testes13. Our previous observation found that the testis became very small in size in gish 04895 mutant fly. These thinned testes prompted us to explore whether gish is involved in the maintenance of germline stem cells in Drosophila testis. Combining germline clonal analysis and rescue tests, we showed that gish plays an intrinsic role in GSC self-renewal, suggesting that gish is necessary to regulate GSCs’ fate. In addition, we found that ectopic gish expression only slightly increased the number of GSC-like cells. These results suggest that overexpression of gish is not sufficient to repress GSCs/GBs differentiation. Previous studies demonstrated that mutation in gish gene led to abnormal nuclei and altered structure of actin cones in the individualizing spermatid cyst15, 36, but no further information is available during the early process of GSCs maintenance or switch to differentiation. Our results suggest that gish plays a novel role as a GSC intrinsic maintenance factor, but has no roles in the differentiation of GSC/GB in Drosophila testis.
Previous research has manifested that Bmp signals from somatic cells, Dpp and Gbb, are essential for the maintenance of GSCs in the Drosophila testis17. Both Gbb and Dpp function as short-range signals in the tip of the Drosophila testis, and their signaling activities are restricted to GSCs and GBs17, 33, 34. Interestingly, Dad is expressed in GSCs and GBs, whereas bam is not expressed in either kind of cell. Both of Dpp and Gbb are essential for maintenance of GSC number17. Thus, we checked the bam and Dad expression pattern using bam-GFP and Dad-GFP transgene. The results show that the mutation in gish has no effect on the expression pattern of bam and Dad in GSCs and GBs in Drosophila testes. These data suggest that gish functions downstream of or parallel to Dad/bam, and is independent of Gbb/Dpp-bam signaling pathway.
Conclusion
In this study, using genetic strategies, we identified and characterized that Drosophila gish, encoding a casein kinase 1 protein, as a key player in the regulation of GSCs’ fate. Our results from FLP/FRT-mediated mitotic recombination analyses and rescue assays demonstrate that gish functions as an intrinsic factor for maintenance of the number of GSCs in Drosophila testis. The action of Gish is dispensable for bam silencing or the expression pattern of Dad. Our results reveal a new role for the casein kinase 1 (CK1) protein in the fate determination of stem cells.
Materials and Methods
Drosophila stocks
Oregon-R was used as a wild-type strain. The w 1118 strain was used as the host for all P-element-mediated transformations37. The following strains were also used for experimentation: (1) gish 04895, gish MI08417 and gish KG03891 alleles (Bloomington Stock Center); (2) P{bamP-GFP}38; (3) P{nosP-gal4}, which was described previously31. P{dadP-GFP}, neoFRT82B/TM3 and hs-FLP; FRT82B, Ubi-GFP/TM 3 was a generous gift from Dr. Dahua Chen. Fly stocks used in this study were maintained at room temperature on a standard medium.
Histochemistry and microscopy
Testes were prepared for immunostaining as previously described17, 23. Primary antibodies were diluted as follows: rabbit anti-GFP (1:500, Invitrogen); mouse monoclonal anti-Hts antibody (1:100, DSHB); rabbit anti-Vasa (1:500, Santa Cruz). Secondary antibodies goat anti-rabbit Alexa 488; goat anti-mouse Alexa 555 (Molecular Probes) were used at a 1:1000 dilution. FITC-conjugated Phalloidin (1:200, Beyotime) was used to visualize F-actin. All samples were examined using a Leica fluorescent microscope, and micrographs were taken using an Olympus confocal FV3000 microscope.
Generation and Analysis of GSC Clones
Mutant GSC Clones were generated by FLP/FRT-mediated mitotic recombination, as described previously28, 39. To generate stocks for stem cell clonal analyses, males of hs-FLP; FRT82B,Ubi-GFP/FRT82B,gish 04895 and hs-FLP; FRT82B,Ubi-GFP/FRT82B,gish MI08417 genotypes (hs-FLP; FRT82B, Ubi-GFP/FRT82B as the wild-type control) were produced by standard genetic crosses. 2-day-old adult males were heat-shocked for 60 minutes at 37 °C three times per day. Five days after heat-shock treatment, testes were dissected for antibody staining at days 2, 10, 20 after last heat-shock treatment. GSC clones were identified and quantified by the lack of GFP expression, as well as their attachment position to the hub cells.
Detecting gene expression in Drosophila testis using RT-PCR and qPCR
Total RNA was extracted from wild-type fly testes using Trizol reagent (Sangon), then the amount of 1 µg was incubated with PrimeScript RTase(50 U/µl) to transcribe cDNA, which was used as the template in PCR reactions, according to the manufactures’ protocol (PrimeScript High Fidelity RT-PCR Kit, Takara). The PCR primers were designed to explore the different gish mRNA splicing variants (S1 Table). Total RNAs of fly testes were independently isolated from each phenotype (wild-type and gish mutant) using the Trizol reagent (Sangon) and reverse transcribed into cDNA according to the manufactures’ protocol (PrimeScript RT reagent Kit with gDNA Eraser, Takara). For each independent cDNA sample, quantitative PCR was run on CFX96 Touch (BioRad) to measure total gish mRNAs with rp49 as reference according to the manufactures’ protocol (SYBR Premix EX TaqTM II qPCR Kit, Takara). The following primers were used in this study: gish, 5′-GCCGGTGGTAAAAGCTCAAG-3′ (sense) and 5′-CGCCAAAATTACCACAGCCA-3′ (antisense); rp49, 5′-CACTTCATCCGCCACCAGTC-3′ (sense) and 5′-CGCTTGTTCG ATCCGTAACC-3′ (antisense).
References
Weissman, I. L. Stem cells: units of development, units of regeneration, and units in evolution. cell 100, 157–168 (2000).
Kørbling, M. & Estrov, Z. Adult stem cells for tissue repair - a new therapeutic concept? New England Journal of Medicine 349, 570–582 (2003).
Barrilleaux, B., Phinney, D. G., Prockop, D. J. & O’Connor, K. C. Review: ex vivo engineering of living tissues with adult stem cells. Tissue Engineering 12, 3007–3019 (2006).
Morrison, S. J., Shah, N. M. & Anderson, D. J. Regulatory Mechanisms in Stem Cell Biology. Cell 88, 287–298 (1997).
Xie, T. & Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
Allan, S., Fuller, M. T., Braun, R. E. & Shosei, Y. Germline stem cells. Cold Spring Harbor Perspectives in Biology 3 (2011).
Fuller, M. T. & Spradling, A. C. Male and female Drosophila germline stem cells: two versions of immortality. Science 316, 402–404 (2007).
Matunis, E. L., Stine, R. R. & Cuevas, M. D. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis 2, 137–144 (2012).
Schulz, C., Wood, C. G., Jones, D. L., Tazuke, S. I. & Fuller, M. T. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523–4534 (2002).
Knippschild, U. et al. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cellular Signalling 17, 675–689 (2005).
Castrillon, D. H. et al. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics 135, 489–505 (1993).
Hummel, T., Attix, S., Gunning, D. & Zipursky, S. L. Temporal control of glial cell migration in the Drosophila eye requires gilgamesh, hedgehog, and eye specification genes. Neuron 33, 193–203 (2002).
Schulz, C. et al. A Misexpression Screen Reveals Effects of bag-of-marbles and TGFβ Class Signaling on the Drosophila Male Germ-Line Stem Cell Lineage. Genetics 167, 707–723 (2004).
Davidson, G. et al. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438, 867–872 (2005).
Nerusheva, O. O., Dorogova, N. V., Gubanova, N. V., Yudina, O. S. & Omelyanchuk, L. V. A GFP trap study uncovers the functions of Gilgamesh protein kinase in Drosophila melanogaster spermatogenesis. Cell Biology International 33, 586–593 (2009).
Tan, Y., Yu, D., Pletting, J. & Davis, R. L. Gilgamesh is required for rutabaga-independent olfactory learning in Drosophila. Neuron 67, 810–820 (2010).
Kawase, E., Wong, M. D., Ding, B. C. & Xie, T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131, 1365–1375 (2004).
Sheng, X. R. et al. Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Developmental Biology 334, 335–344 (2009).
Wang, H. et al. Rap-GEF Signaling Controls Stem Cell Anchoring to Their Niche through Regulating DE-Cadherin-Mediated Cell Adhesion in the Drosophila Testis. Developmental cell 10, 117–126 (2006).
Bustin, S. A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. Journal of Molecular Endocrinology 29, 23–39 (2002).
Lhocine, N. et al. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host & Microbe 4, 147–158 (2008).
Gupta, R. K. & Prasad, S. Optimization of multiplex RT-PCR for M1, M23, and M23X splice variants of AQP4 and β-actin transcripts in Dalton’s lymphoma mouse tissues. Turkish Journal of Biology 39, 567–574 (2015).
Chen, D. et al. Effete-mediated degradation of Cyclin A is essential for the maintenance of germline stem cells in Drosophila. Development 136, 4133–4142 (2009).
Tran, J., Brenner, T. J. & Dinardo, S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature 407, 754–757 (2000).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Ferrandon, D. et al. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. The EMBO Journal 17, 1217–1227 (1998).
Xu, T. & Rubin, G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993).
Xie, T. & Spradling, A. C. decapentaplegic Is Essential for the Maintenance and Division of Germline Stem Cells in the Drosophila Ovary. Cell 94, 251–260 (1998).
Tulina, N. & Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546–2549 (2002).
Rørth, P. Gal4 in the Drosophila female germline. Mechanisms of Development 78, 113–118 (1998).
Van, D. M., Williamson, A. L. & Lehmann, R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Current Biology 8, 243–246 (1998).
Chen, D. & Mckearin, D. M. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130, 1159–1170 (2003a).
Tsuneizumi, K. et al. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389, 627–631 (1997).
Inoue, H. et al. Interplay of signal mediators of decapentaplegic (Dpp): molecular characterization of mothers against dpp, Medea, and daughters against dpp. Molecular Biology of the Cell 9, 2145–2156 (1998).
Ohlstein, B. & Mckearin, D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development 124, 3651–3662 (1997).
Nerusheva, O. O., Dorogova, N. V., Gubanova, N. V. & Omel’Yanchuk, L. V. The role of Gilgamesh protein kinase in Drosophila melanogaster spermatogenesis. Russian Journal of Genetics 44, 1049–1053 (2008).
Spradling, A. C. & Rubin, G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218, 341–347 (1982).
Chen, D. & Mckearin, D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Current Biology 13, 1786–1791 (2003b).
Jiang, X. et al. Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Developmental cell 14, 494–506 (2008).
Acknowledgements
We thank Dahua Chen for kindly gifting fly strains and anti-Vasa antibody. We thank Qingchun Tong for critical reading of the manuscript and the valuable comments. We thank Xiao-Yan Ma and Hao Yan for their technical assistance in taking confocal pictures. This work was supported by grants from the Innovation Team of Scientific Research Platform in Anhui Universities (#20151105), the Key Project of Natural Science Foundation in Anhui Universities (#KJ2015A082), and the National Science Foundation of China (#31071266, #30871441).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: D.C. Performed the experiments: D.C., X.Z., L.Z., J.W., X.T., S.W., F.S., Z.H. and Y.G. Analyzed the data: D.C., X.Z., L.Z., J.W., X.T. and X.K. Contributed analysis tools: X.K. Wrote the paper: D.C. Obtained the funding: D.C.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, D., Zhu, X., Zhou, L. et al. Gilgamesh is required for the maintenance of germline stem cells in Drosophila testis. Sci Rep 7, 5737 (2017). https://doi.org/10.1038/s41598-017-05975-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05975-w
- Springer Nature Limited
This article is cited by
-
α-Tubulin Regulates the Fate of Germline Stem Cells in Drosophila Testis
Scientific Reports (2021)
-
G-protein signaling is required for increasing germline stem cell division frequency in response to mating in Drosophila males
Scientific Reports (2020)