Abstract
The effects of testosterone and flutamide on reproduction in Brachionus calyciflorus were studied. Asexual reproduction in B. calyciflorus was not affected by testosterone at different concentrations of flutamide. Flutamide in combination with 0, 25, 50, or 75 µg L−1 testosterone had a significant effect on mixis rate. The combination of 5 µg L−1 flutamide with 25 µg L−1 or 50 µg L−1 testosterone resulted in a mixis rate that was 2.2× lower than that with flutamide alone. Fertilization rate was significantly decreased by 7.5 µg L−1 flutamide in combination with 25, 50, or 75 µg L−1 testosterone. The number of resting eggs produced per mictic female was significantly lower at all concentrations of testosterone. A low concentration of flutamide in combination with testosterone resulted in antagonism, increasing the number of resting eggs produced. However, when testosterone was combined with a higher concentration of flutamide, resting egg production declined. Therefore, long-term exposure to either testosterone, flutamide, or a combination of these two compounds may significantly reduce resting egg production in rotifers. This implies that resting egg production is affected differently by hormone pathways.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Anthropogenic chemicals with the potential to perturb the function of endocrine systems are called endocrine disrupting chemicals (EDCs). Many EDCs can interfere with reproduction, development, survival, distribution, and cause disease or deformity in organisms and their offspring1. Previous studies have demonstrated that some anthropogenic chemicals can disrupt normal hormonal communication, producing harmful effects on reproduction in a wide variety of animals, including rotifers2,3,4. In respect to endocrine disruptors, rotifers seem to be particularly sensitive to androgenic and anti-androgenic substances5. And especially Brachionus calyciflorus, are ideal models for ecotoxicology studies because of their wide distribution, short life history, facility of culture, and the convenience of resting eggs6. Monogonont rotifer reproduction includes asexual and sexual periods. In asexual reproduction, an amictic female rotifer produces clones of herself via ameiotic parthenogenesis, whereas in sexual reproduction, females produce haploid eggs that develop into males if unfertilized or into resting eggs if fertilized. Dormant resting eggs ensure the survival of the species during adverse environmental conditions7. The initiation of sexual reproduction, therefore, is a critical phase of the rotifer life cycle that has important ecological and evolutionary consequences for the population. The adaptive features of resting eggs are outlined including their contribution to genetic variability through recombination, their provision for environmental escape by dormancy8. The rotifer life cycle, including both isolated parts and the full life-cycle, has been used for the development of toxicity tests to assess the hazards of anthropogenic chemicals in freshwater and marine environments9. Examples of the application of rotifers in toxicity testing include measurements of 24 h mortality for comparing the toxicity of chemicals10, the Triclosan exposure on the hatching rate during the formation period had a greater effects on those during the resting egg hatching period11, 2 d population growth rate (r) for assessing chronic toxicity12, life history parameters for the assessment of the toxicity13, full life cycle toxicity assessment using rotifer resting egg production, a 96-h B. calyciorus resting egg toxicity test was developed and used to estimate the toxicity of pentachlorophenol (PCP) and copper. Results were compared to a variety of acute and sublethal endpoints for both toxicants9, and mixis rate and hatchability of resting eggs for the detection of the effects of pesticide exposure14. Besides, rotifers may be more sensitive than the standard invertebrate test species Daphnia magna to fungicides15.
The effects of various hormones on rotifer reproduction have been recently investigated16. Batch cultures of Brachionus plicatilis treated with 0.05 and 0.5 mg L−1 juvenile hormone (JH) experienced a significant increase in mixis rate of approximately 30%, compared to a 10% mixis rate in the control. However, 50 mg L−1 JH decreased the mixis rate compared to the controls. Treatment of rotifers with 50 mg L−1 17β-estradiol increased the mixis rate to approximately twice that of the control, although lower concentrations had no effect. Growth hormone and gamma aminobutyric acid significantly stimulated population growth and mictic female production over control levels in B. plicatilis 17. Treatment of female rotifers with JH (5 and 50 mg L−1) resulted in a significantly higher mictic rate that increased from 4% in the controls to 8% in the F2 generation of females in B. plicatilis 18. Progesterone at 10 mg L−1 significantly increased the mixis rate (1.18 times higher than the control) of B. manjavacas 19. A depression of the fertilization rate in B. calyciflorus was reported following treatment with 1 µg mL−1 nonylphenol, 10 µg mL−1 testosterone, or 50 µg mL−1 flutamide4. In addition, population growth rate and the ratio of ovigerous to non-ovigerous females are both suitable endpoints for the detection of the reproductive disrupting effects of ethinylestradiol, nonylphenol, and testosterone20. Thus, rotifer is one of the promising tools for the assessment of the impact of potential endocrine disruptors on aquatic invertebrates21. However, many of these previous studies have only investigated the limited effects of single hormones in isolation on rotifer reproduction. The combined effects of these hormones on rotifer reproduction have not yet been studied.
In this study, testosterone (an androgen) and flutamide (an anti-androgen) were selected to test the hypothesis that these chemicals would have antagonistic effects on rotifer reproduction. To test this hypothesis, the effects of testosterone in the presence of different concentrations of flutamide on B. calyciflorus reproduction was investigated.
Materials and Methods
Test animals
Brachionus calyciflorus were obtained by hatching resting eggs which were donated by Prof. T.W. Snell (Georgia Institute of Technology). This rotifer strain was produced in a lab from a population originally collected in Gainesville, Florida, USA, in 1983. Hatching was performed in synthetic freshwater (EPA medium; 96 mg NaHCO3, 60 mg CaSO4·H20, 123 mg MgSO4, and 4 mg KCl in 1 L of deionized water, Table 1) adjusted to pH 7.5 at 25 °C under a light intensity of 2000 lux12, 22. After 18 h of hatching, cysts were inspected to ensure the collection of test animals within 2 h of hatching. Rotifers were fed with green Chlorella pyrenoidosa (Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China) of 3.3 × 106 cell per ml that was cultured in continuous culture using HB-4 medium23. Algae were grown at a high density and were centrifuged and diluted to a suitable density in EPA medium prior to feeding.
Test chemicals
Stock testosterone and flutamide solutions of 1000 mg L−1 (analytical standard 99.5% and 99.8%, respectively, Sigma–Aldrich chemical Chemie, Munich, Germany) were prepared by dissolution in 100% acetone (Analytical grade, 99.5%). For experimental exposures, testosterone and flutamide stock solutions were further diluted in EPA medium with a final acetone concentration less than 0.01%. This concentration of acetone was demonstrated to not have any significant influence on the rotifer survival, growth and reproduction parameters compared to the controls19.
Experiment design
A number of range-finding experiments were performed to determine the lowest observed effect concentrations of testosterone (100 μg L−1) and flutamide (10 μg L−1) on the population growth rate at 2 d12. Based on these results, exposure concentrations were selected for flutamide (F; 0, 2.5, 5.0, and 7.5 µg L−1) and testosterone (T; 0, 25, 50, and 75 µg L−1), and these were combined to obtain 16 treatment combinations: F0(0 + 0, 0 + 25, 0 + 50, 0 + 75 µg L−1), F1(2.5 + 0, 2.5 + 25, 2.5 + 50, 2.5 + 75 µg L−1), F2(5.0 + 0, 5.0 + 25, 5.0 + 50, 5.0 + 75 µg L−1), F3(7.5 + 0, 7.5 + 25, 7.5 + 50, 7.5 + 75 µg L−1).
Asexual population growth rate
Four young rotifers (less than 4 hours old) were introduced into 8 mL of EPA medium containing 3.0 × 106 cells mL−1 C. pyrenoidosa (freshwater) in a 16 × 150 mm glass test tube. There were five replicate tubes at each treatment combination for a total of 80 test tubes. The test tubes were placed on a rotator turning at 10–15 rph at 25 °C. After 48 h, the total number of rotifers in each tube was counted. After each count, the volume of each tube was restored to its original level. The population growth rate (r) was calculated according to the exponential growth equation: r = (ln Nt − ln N0)/t, where Nt = the final number of female rotifers in the tube, N0 = the initial number of rotifers in each tube, and t = time in days24. After counting, the rotifers were placed back into the tubes and cultured for two more days12.
After 4 d, the number of each type of female rotifer in the tubes was counted following the methods described by Preston4. From these counts, the mixis rate (MR) was calculated for each test tube as the proportion of mictic females among the ovigerous females, and the fertilization rate (FR) was calculated as the proportion of fertilized mictic females among the mictic females. The ratio of ovigerous fertilized females was calculated as fertilized females among all rotifers. After counting, rotifers and resting eggs were placed back into the original tubes and continuously cultured until 7 d4. At 7 d, the number of resting eggs in each test tube was counted to compare the effect of the treatments on resting egg production14.
Statistical analysis
All statistical analyses were performed using SPSS 16.025. The effects of testosterone and flutamide were compared using two-way analys is of variance (ANOVA), with concentrations as the independent variables, and population growth rate (r), mixis rate (MR), fertilization rate (FR), or RE as the dependent variable. Tukey’s test was used for pairwise comparisons of each treatment concentration relative to the control.
Results
Population growth rate
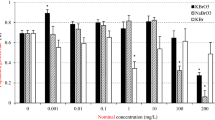
The addition of testosterone alone significantly affected the population growth rate (r) (F3,1 = 3.55, p = 0.039) (Fig. 1). Testosterone at 25 µg L−1 resulted in a significant increase in the population growth rate relative to the control (p < 0.05), while at 50 µg L−1 this effect was not significant (p > 0.05). With the addition of 2.5 µg L−1 flutamide, the population growth rate was significantly decreased with increases in the concentration of testosterone (F3,16 = 107.55, p = 0.000). Flutamide concentrations of 5.0 and 7.5 µg L−1 added to the different concentrations of testosterone significantly affected the population growth rate (F3,16 = 27.36, p = 0.000, F3,16 = 114.88, p = 0.000, respectively). The population growth rate was increased at a low concentration of flutamide in combination with testosterone, while it was reduced at higher concentrations of flutamide. This pattern suggest a hormetic effect26. Flutamide mixed with testosterone significantly decreased the population growth rate.
Treatment with testosterone or flutamide alone significantly affected the population growth rate (F = 31.35, p = 0.000, and F = 8.555, p < 0.05, respectively), but the combination of these treatments did not have any further significant effect on the population growth rate (F = 1.87, p > 0.05, Table 2).
Rotifer mixis rate at 4 d
The addition of testosterone significantly affected the mixis rate (MR) (F3,16 = 5.583, p = 0.008) (Fig. 2). Testosterone treatment significantly increased the MR relative to the control (p < 0.05), however, there were no significant differences between the different concentrations of testosterone. The addition of 2.5 µg L−1 flutamide to the 0, 25, 50, and 75 µg L−1 testosterone groups significantly affected the MR (F3,16 = 7.477, p = 0.002). The MR at 25 µg L−1 testosterone was the lowest, while at 50 and 75 µg L−1 testosterone the MR was similar to the control. Other groups showed similar results. However, 5 µg L−1 flutamide, in combination with 25 or 50 µg L−1 testosterone, resulted in the largest reduction in the MR. When rotifers were exposed to testosterone or flutamide alone, the MR was significantly affected (F = 89.41, p < 0.05, and F = 35.16, p < 0.05, respectively). However, there was also a significant interaction between the effects of testosterone and flutamide on the MR (F = 14.64, p < 0.05, Table 2).
Fertilization rate at 4 d
Flutamide at 2.5 or 5.0 µg L−1 in combination with different concentrations of testosterone did not have any significant effect on the Fertilization Rate (FR), but a significant effect was observed at 7.5 µg L−1 flutamide (F3,16 = 0.469, p = 0.708, F3,16 = 1.180, p = 0.186, F3,16 = 2.981, p = 0.063, and F3,16 = 10.55, p = 0.000 for 0, 2.5, 5.0, and 7.5 µg L−1, respectively) (Fig. 3). In addition, treatment with 2.5 µg L−1 flutamide tended to increase the Fertilization Rate (FR) relative to the control, however, higher concentrations of flutamide (5 and 7.5 µg L−1) resulted in a declining trend in FR. With two-way analysis of variance, testosterone or flutamide alone significantly affected the FR (F = 105.87, p < 0.005, and F = 15.04, p < 0.005, Table 2), however, the combined effect of these treatments had no further significant effect on the FR (F = 1.37, p > 0.05, Table 2).
Number of resting eggs produced at 7 d
The addition of testosterone significantly decreased the number of resting eggs produced in the absence of flutamide (F3,16 = 78.47, p = 0.000) (Fig. 4). At 2.5, 5.0, and 7.5 µg L−1 flutamide, the results were similar to the controls across the different testosterone concentrations. However, at 7.5 µg L−1 flutamide and 25 µg L−1 testosterone, the number of resting eggs produced was significantly lower than the control (p < 0.05). With two-way analysis of variance, testosterone alone, flutamide alone, and the combination these treatments significantly affected the number of resting eggs (F = 21.75, p = 0.000, F = 27.31, p = 0.000, and F = 3.43, p < 0.05, Table 2).
Discussion
In recent years, a new field of scientific inquiry has emerged against the backdrop of numerous reports of disrupted endocrine function in various groups of vertebrates. U.S Environmental Protection Agency (EPA) lists sixty kinds of the environmental hormone substances. These included testosterone and flutamide. It has not been reported in China in recent years. Rotifers were chosen to assess the impact of potential endocrine disruptors on invertebrate reproduction in this study. It was observed that low concentration of testosterone increased the rotifer population growth rate but a higher concentration reduced the population rate, which was similar to a previous study20. It is probably because a slight stress can increase the reproduction of many animals due to compensatory response as hormesis, while a severe stress might suppress it ref. 27. In another study, medroxyprogesterone had a different effect than progesterone on the population growth of B. manjavacas 19. Snell and Moffat (1992) reported that a 2 d population growth test with B. calyciflorus was often more sensitive than a 7 d Ceriodaphnia dubia reproductive test. In the present study, the results of the combined effect of flutamide and testosterone on B. calyciflorus also demonstrated that the 2 d population growth rate might be a sensitive parameter. A significant reduction in the number of resting eggs was observed after 7 d with increasing concentrations of testosterone in the current study. These results were similar to those reported by Marcial et al.14 who observed a decrease in resting egg production in B. plicatilis exposed to pesticides including diazinon, fenitrothion, methropone, and isoprothiolane. These results infer that testosterone exposure in the current study may have disrupted reproduction in B. calyciflorus resulting in a reduction in the formation of resting eggs, similar to that reported by Marcial et al.14. The reduced egg production following testosterone treatment in this study was antagonized by a low concentration of flutamide, which resulted in an increased resting egg production. However, when testosterone was combined with a higher concentration of flutamide, the resting egg production declined. This result was similar to that observed in B. manjavacas exposed to medroxyprogesterone and juvenile hormone, but different to that observed following progesterone exposure19.
From the results of the two-way ANOVA, it was apparent that testosterone and flutamide combined had antagonistic effects on the population growth rate of B. calyciflorus. A similar result was observed for the mixis rate following the combined exposure. However, testosterone and flutamide combined did not have antagonistic effects on the fertilization rate. These results suggest that the fertilization rate is not a sensitive parameter for the assessment of the effects of testosterone and flutamide on rotifers. Exposure to progesterone, combined with either testosterone or estradiol at 1000 μg L−1, resulted in a decrease in resting egg production B. calyciflorus 16. In the current study, the 2 d population growth rate and the fertilization rate at 4 d were more sensitive than the number of resting eggs produced after 7 d. In addition, Rotifers is a good model for studies of the effects of endocrine disruptors on reproduction. The observation that testosterone combined with flutamide resulted in a decreased resting egg production may be explained by the observation that B. calyciflorus may use oxidized sterols to regulate sexual reproduction16.
Rotifers may provide a better model than other animals for studies of the effects of endocrine disruptors on reproduction. The molecular mechanisms of steroid action have been investigated in rotifers. The presence of a steroid signaling cascade in B. manjavacas that regulates sexual reproduction was suggested, and exposure to 10 mg L−1 progesterone resulted in a 4.4× increase in resting egg production in this species19. The effects of anti-androgenic compounds on sexual reproduction in B. calyciflorus were investigated by Joaquim-Justo and Snell. In the current study, 25 µg L−1 testosterone resulted in a resting egg production that was lower than the control (1.8×), and exposure to higher concentrations of testosterone had a similar result. Exposure to high concentrations of flutamide and testosterone have been demonstrated to increase population growth of B. plicatilis 4, 17 demonstrated that flutamide and testosterone may interfere with endocrine signaling, causing a reduction in resting egg production in rotifers. In the current study, 7.5 µg L−1 flutamide combined with testosterone had a stronger effect on 7 d resting egg production than flutamide or testosterone alone, indicating that rotifer resting egg production may be the most sensitive endpoint28. Testosterone and flutamide may have their effects on reproduction in the rotifer in the same way, possibly through the same rotifer steroid receptor. In a previous study, the percentage of fertilized ovigerous female rotifers was reduced to 11% versus 26% in the controls following treatment with 10 µg L−1 testosterone, while 1 µg L−1 flutamide significantly reduced fertilization to 1% versus 14% in controls4. However, in the current study, testosterone up to 75 µg L−1 had no effect on the FR, and low concentrations of flutamide increased the FR while high concentrations reduced the FR. The reasons for the differences between these studies may be due to different culture and experimental methods.
The progesterone receptor has been described in female and male B. manjavacas and has been demonstrated to bind progesterone29. Existing evidence suggests that steroid hormones have a role in endocrine signaling in invertebrates26. Vertebrate-type steroids have been detected in several arthropod species30, but the functional role of these hormones is still not clear. A rotifer progesterone receptor has been discovered19, but an estrogen receptor has not been found in the rotifer transcriptome and there is only weak evidence for the presence of an androgen receptor. Transcriptome surveys have shown that steroid receptors, biosynthetic enzymes, and signal transduction pathway elements are present in brachionids. There is strong evidence that steroids similar to progesterone and androgens are employed as regulatory signals in rotifers, whereas estrogens seem less important31.
The effects of steroid hormones on reproduction in other invertebrates have been reported. Long-term testosterone exposure at concentrations ranging from 0.31 to 2.48 mg L−1 in Daphnia magna resulted in reduced fecundity and fertility, and the decrease in fecundity was associated with an increase in the number of aborted eggs32. Testosterone also functions as an anti-ecdysteroid, a mechanism of high relevance to arthropods33. Eastern mud snail (Ilyanassa obsoleta) express NR3C4-like (androgen) and NR3A-like (estrogen) receptors, and exhibit changes in reproductive tract development that are typically associated with androgen or estrogen signaling in vertebrates when exposed to EDCs33. The effects of a mixture of flutamide and testosterone on rotifer mixis rate, population growth rate, and resting egg production in the current study demonstrates that these compounds inhibit rotifer reproduction. These results also support the existence of androgen receptors in the endocrine system of rotifers. The ubiquity of certain androgen biotransformation processes in invertebrates reveals differences in the androgen metabolic pathway between this group and vertebrates30. Therefore, there might also be an unknown hormone receptor (androgen or otherwise) that binds to metabolites of testosterone and affects endocrine function in the rotifer.
Janer et al.34 found that vertebrate steroids were also presented in arthropods. And testosterone was presented as an anti-degrading steroid hormone, but the exact function was not clear32. Thornton et al. isolated the estrogen receptor (ER) of the invertebrates from the mollusks, and found that endocrine signaling pathways are also present in low-grade organisms from the perspective of systemic biology35. In addition to estrogen receptor ER, the androgen receptor (AR) also belongs to the nuclear receptor NR3C family from the point of phylogeny. The mechanisms of this receptor are as follows: androgen receptor binding, and the establishment of the target gene between the establishment of a direct signal connection, and ultimately produce the corresponding physiological and biochemical effects. The number of receptors present in other aquatic species (such as Ciona) and the NR3C gene repeats have been identified. However, it’s unknown in rotifers, which requires further study.
The presence of steroid hormone has been reported in the female and male individuals of B. manjavacas. Since the gene fragment of transcription group in rotifers is not complete. There is no report of the presence of male hormone receptors. At present, the specific mechanism of male hormone in rotifers is not clear. Snell isolated a protein, induced by sexual reproduction, with a molecular weight of 39 kDa from the B. plicatilus 36. Its N-terminal has 17 amino acid estrus, which is consistent with the steroid-induced protein isolated from human ovarian follicular fluid. And they speculate that the substance may be combined with membrane receptors to produce a series of biological effects, lead to the replacement of asexual reproduction by sexual reproduction. In this study, the concentration of testosterone and flutamide in the present experiment showed that antagonism was observed in the r value, MR, FR, and RE. It is speculated that there may also be a kind of male hormone receptor (AR) and male hormone interaction in the rotifer, which can act on the target gene by signal transduction, and finally interfere with the reproductive system. The existence of the receptor and the specific molecular structure should be studied further.
Conclusion
This study has demonstrated that testosterone has little effect on asexual reproduction, but has a significant effect on sexual reproduction, in B. calyciflorus. Flutamide was more potent than testosterone in the inhibition of reproduction in B. calyciflorus. This work also demonstrated that a mixture of flutamide and testosterone had an additive inhibitory effect on reproduction in B. calyciflorus. Long-term exposure to either testosterone, flutamide, or a combination of these two compounds may significantly reduce resting egg production in rotifers. This implies that resting egg production is affected differently by hormone pathways and may represent differences in the regulation of rotifer reproduction. The additive toxicity demonstrated in this study suggests a potential ecological risk that requires further study.
References
Mckinlay, R., Plant, J. A., Bell, J. N. & Voulvoulis, N. Endocrine disrupting pesticides: implications for risk assessment. Environ. Int. 34, 168–83 (2008).
Colborn, T., Saal, F. S. V. & Soto, A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health. Perspect. 101, 469–489 (1993).
Palma, P. et al. Effects of atrazine and endosulfan sulphate on the ecdysteroid system of Daphnia magna. Chemosphere 74, 676–681 (2009).
Preston, B. L., Snell, T. W., Robertson, T. L. & Dingmann, B. J. Use of freshwater rotifer Brachionus calyciflorus in screening assay for potential endocrine disruptors. Environ. Toxicol. Chem. 19, 2923–2928 (2000).
Dahms, H. U., Hagiwara, A. & Lee, J. S. Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquat. Toxicol. 101, 1–12 (2011).
Snell, T. W. & Janssen, C. R. Rotifers in ecotoxicology: a review. Hydrobiologia 313–314, 231–247 (1995).
Snell, T. W. & Carmona, M. J. Comparative toxicant sensitivity of sexual and asexual reproduction in the rotifer Brachionus calyciflorus. Environ. Toxicol. Chem. 14, 415–420 (1995).
Pourriot, R. & Snell, T. W. Resting eggs in rotifers. Hydrobiologia 104, 213–224 (1983).
Preston, B. L. & Snell, T. W. Full life-cycle toxicity assessment using rotifer resting egg production: implications for ecological risk assessment. Environ. Pollut. 114, 399–406 (2001).
Snell, T. W., Moffat, B. D., Janssen, C. & Persoone, G. Acute toxicity tests using rotifers. III. Effects of temperature, strain, and exposure time on the sensitivity of Brachionus plicatilis Environ. Toxic. Watter. 6, 63–75 (1991).
Zhang, L., Niu, J. & Wang, Y. Full life-cycle toxicity assessment on triclosan using rotifer Brachionus calyciflorus. Ecotox. Environ. Safe. 127, 30–35 (2016).
Snell, T. W. & Moffat, B. D. A 2-d Life cycle test with the rotifer Brachionus calyciflorus. Environ. Toxicol. Chem. 11, 1249–1257 (1992).
Ramírez-Pérez, T., Sarma, S. S. & Nandini, S. Effects of mercury on the life table demography of the rotifer Brachionus calyciflorus pallas (rotifera). Ecotoxicology 13, 535–544 (2004).
Marcial, H. S., Hagiwara, A. & Snell, T. W. Effect of some pesticides on reproduction of rotifer Brachionus plicatilis, Müller. Hydrobiologia 181, 569–575 (2005).
Moreira, R. M., Mansano, A. S., Rocha, O. & Daam, M. A. The use of rotifers as test species in the aquatic effect assessment of pesticides in the tropics. Hydrobiologia 35, 1–9 (2016).
Yang, J. X. & Snell, T. W. Effects of Progesterone, Testosterone, and Estrogen on Sexual Reproduction of the Rotifer Brachionus calyciflorus. Int. Rev. Hydrobiol. 95, 441–449 (2010).
Gallardo, W. G. et al. Effect of some vertebrate and invertebrate hormones on the population growth, mictic female production, and body size of the marine rotifer Brachionus plicatilis Müller. Hydrobiologia 358, 113–120 (1997).
Gallardo, W. G., Hagiwara, A. & Snell, T. W. Effect of juvenile hormone and serotonin (5-HT) on mixis induction of the rotifer Brachionus plicatilis Müller. J. Exp. Mar. Biol. Ecol. 252, 97–107 (2000).
Snell, T. W. & Desrosiers, N. J. D. Effect of progesterone on sexual reproduction of Brachionus manjavacas, (rotifera). J. Exp. Mar. Biol. Ecol. 363, 104–109 (2008).
Radix, P., Severin, G., Schramm, K. W. & Kettrup, A. Reproduction disturbances of Brachionus calyciflorus (rotifer) for the screening of environmental endocrine disrupters. Chemosphere 47, 1097–1101 (2002).
Snell, T. W. & Joaquim-Justo, C. Workshop on rotifers in ecotoxicology. Hydrobiologia 593, 227–232 (2007).
American Society for Testing and Materials. ASTM Standard guide for acute toxicity tests with the rotifer Brachionus. Annual book of ASTM standards, water and environmental technology, vol 1105, Biological effects and environmental fatesAnmol, New Delhi (2001).
Zhang, Z. S. & Huang, X. F. Method for Study on Freshwater Plankton. Science Press, Beijing (1991).
Wisdom, M. J. & Doak, D. F. Life stage simulation analysis: estimating vital-rate effects on population growth for conservation. Ecology 81, 628–641 (2000).
Norusis, M. SPSS 16.0 Guide to Data Analysis. Prentice Hall Press 49,397–400 (2008).
Janer, G., LeBlanc, G. A. & Porte, C. A comparative study on androgen metabolism in three invertebrate species. Gen. Comp. Endocr. 143, 211–221 (2005).
Snell, T. W. & Boyer, E. M. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller). J. Exp. Mar. Biol. Ecol. 124, 73–85 (1988).
Stout, E. P. et al. Conservation of progesterone hormone function in invertebrate reproduction. P. Natl. Aca. Sci. USA 107, 11859–11864 (2010).
Duft, M., Schulteoehlmann, U., Weltje, L., Tillmann, M. & Oehlmann, J. Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail potamopyrgus antipodarum 64(4), 437–449 (2003).
Snell, T. W. A review of the molecular mechanisms of Monogonont rotifer reproduction. Hydrobiologia 662, 89–97 (2011).
Barbosa, I. R., Nogueira, A. J. & Soares, A. M. Acute and chronic effects of testosterone and 4-hydroxyandrostenedione to the crustacean Daphnia magna. Ecotox. Environ. Safe. 71, 757–64 (2008).
Mu, X. & Leblanc, G. A. Developmental toxicity of testosterone in the crustacean Daphnia magna involves anti-ecdysteroidal activity. Gen. Comp. Endocr. 129, 127–133 (2002).
Sternberg, R. M., Hotchkiss, A. K. & LeBlanc, G. A. The contribution of steroidal androgens and estrogens to reproductive maturation of the eastern mud snail Ilyanassa obsoleta. Gen. Comp. Endocr. 156, 15–26 (2008).
Mattson, M. P. Mattson. hormesis defined. Ageing Res. Rev. 7, 1–7 (2008).
Thornton, J. W., Need, E. & Crews, D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science 301, 1714–1717 (2003).
Snell, T. W. et al. A protein signal triggers sexual reproduction in Brachionus plicatilis (rotifera). Mar. Biol 149, 763–773 (2006).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number: 31272388), the Natural Science Foundation of Jiangsu Province of China (Grant Number: BK2007225 to J.X.Y.).
Author information
Authors and Affiliations
Contributions
Jian T. performed research, analyzed the data, and wrote the manuscript; Jian T. and Yajie H. helped interpret the data and edited the manuscript; Yuanhao Y., Lulu L. and Sichen J. provide the rotifers and algae for experiment; Jiaxin Y. designed the research. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, J., Liu, L., Han, Y. et al. Effects of testosterone and flutamide on reproduction in Brachionus calyciflorus . Sci Rep 7, 6569 (2017). https://doi.org/10.1038/s41598-017-05517-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05517-4
- Springer Nature Limited
This article is cited by
-
Endocrine Disrupting Chemicals in Aquatic Ecosystem: An Emerging Threat to Wildlife and Human Health
Proceedings of the Zoological Society (2021)
-
Chronic Effects of Bromate on Sexual Reproduction of Freshwater Rotifer Brachionus calyciflorus
Bulletin of Environmental Contamination and Toxicology (2021)