Abstract
We recently found that the mRNA expression of Slc25a25, a Ca2+-sensitive ATP carrier in the inner mitochondrial membrane, fluctuates in a circadian manner in mouse skeletal muscle. We showed here that the circadian expression of muscle Slc25a25 was damped in Clock mutant, muscle-specific Bmal1-deficient, and global Bmal1-deficient mice. Furthermore, a ketogenic diet (KD) that induces time-of-day-dependent hypothermia (torpor), induced Slc25a25 mRNA expression in skeletal muscle. Hypothermia induced by KD did not affect thermogenic genes such as Sarcolipin and Pgc1a in muscles and Ucp1 in adipose tissues. Sciatic denervation abolished circadian and KD-induced Slc25a25 expression, suggesting that the circadian clock regulates muscle Slc25a25 expression via neural pathways. We measured body temperature (Tb) in sciatic denervated mice fed with KD to determine the functional role of KD-induced Slc25a25 expression. Sciatic denervation abolished Slc25a25 expression and augmented KD-induced hypothermia compared with sham-operated mice, but did not affect Tb in mice given a normal diet. These findings suggest that KD feeding induces expression of the muscle circadian gene Slc25a25 via neural pathways, and that SLC25A25 might be involved in muscle thermogenesis under KD-induced hypothermia in mammals.
Similar content being viewed by others
Introduction
Continuous interplay between the circadian clock system and homeostatic mechanisms regulates core body temperature (Tb) within a narrow range in homeothermic animals including mammals, and maintains it independently of the environmental temperature. The circadian rhythm of Tb that increases during activity and declines during rest is governed by the master clock located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus1, 2. Neurons located in the preoptic area of the hypothalamus are believed to contain the central thermostat for the homeostatic control of Tb. It receives and integrates information about peripheral (cutaneous and visceral) and local brain temperatures and provides appropriate command signals to peripheral thermoregulatory effectors that control heat dissipation and production3,4,5.
Adaptive thermogenesis is defined as heat production in response to environmental temperature or diet6. Neurons in the preoptic area trigger thermogenesis during exposure to cold by activating descending signals through hypothalamic and medullary sites to drive repetitive contractions of skeletal muscle (shivering)4. However, continuous muscle shivering leads to exhaustion and muscle damage. Therefore, non-shivering thermogenesis is activated during chronic cold exposure to sustain heat production through descending output from the hypothalamus to the raphe pallidus nucleus that in turn innervates sympathetic preganglionic neurons3. Brown adipose tissue (BAT) is extensively innervated by sympathetic fibers and thus is an important site of non-shivering thermogenesis in most mammals6. The important molecule involved in cold-induced thermogenesis in BAT is UCP1, which is a mitochondrial inner-membrane protein that uncouples proton entry from ATP synthesis6. Feeding acutely increases metabolic rates in mammals, and thus diet is also a potent regulator of adaptive thermogenesis. On the contrary, when faced with a harsh climate with inadequate food, some mammals periodically turn down their internal thermostat and enter torpor (controlled decrease of the metabolic rate, Tb and physical activity). Torpor is considered a means of survival during periods of low food availability. When food becomes available, such animals can rewarm to return to a normal level of activity7. Although the metabolic rate during torpor is strikingly decreased, Tb is regulated above a species-specific minimum by a proportional increase in heat production that compensates for heat loss8. The circadian system controls torpor timing that allows some mammals to stay entrained with the light-dark cycle, which facilitates continued foraging9. We previously reported that chronic feeding with a ketogenic diet (KD) comprising high fat with low carbohydrate and protein contents induces torpor and significantly decreases core Tb in mice10. We also found that KD induces a decrease in Tb particularly late in the dark (active) period, and increases Tb during the light-to-dark transition to a level like that of a normal diet in mice10. Several molecules such as PPARα and FGF21 might regulate time-dependent torpor11, although we found that both KD and fasting induce hypothermia in FGF21-deficient as well as in wild-type mice12. The underlying mechanism that regulates Tb under hypothermia induced by KD remains unknown, although BAT has been considered a major thermogenic organ against exposure to cold.
The circadian oscillator in the SCN is driven by transcription/translation-based autoregulatory feedback loops consisting of the periodic expression of clock genes2. Studies of clock genes in mammals have revealed that oscillatory mechanisms function in various peripheral tissues such as the heart, lungs, liver, kidneys, adipose tissues and skeletal muscles, and that they are entrained to the SCN by systemic time cues including neural, humoral and other signals such as feeding and body temperature2. Hundreds of circadian clock-controlled genes that regulate a remarkable diversity of biological processes have been identified in peripheral tissues including skeletal muscle using DNA microarray technology13,14,15,16. The significance of clock and clock-controlled genes in the skeletal muscle has been demonstrated in animal models of molecular clock disruption. For example, muscle force is reduced, mitochondria are dysfunctional and myofilament architecture is disrupted in Clock mutant and Bmal1-deficient mice14, 17. Muscle-specific Bmal1 knockout (KO) mice have muscle fibrosis18 and impaired glucose uptake19. These facts suggest that the molecular clock can modify skeletal muscle physiology. Here, we focused on the physiological role of Slc25a25 among 478 circadian genes in muscle16. SLC25A25 is a Ca2+-sensitive ATP-Mg2+/Pi carrier in the inner membranes of mitochondria that might be associated with thermogenesis in mice20, 21. We assessed the circadian regulatory mechanisms of Slc25a25, and its putative role in muscle thermogenesis during torpor induced by a KD.
Results
Systemic circadian clock regulates rhythmic expression of Slc25a25 via neural signals
Among 478 genes in the gastrocnemius muscles of mice that fluctuate in a circadian manner between day and night16, 313 lost rhythmicity after sciatic denervation and among these, we investigated Slc25a25 that encodes a Ca2+-sensitive ATP-Mg2+/Pi carrier in the inner membranes of mitochondria20. We initially assessed the effect of sciatic denervation on the circadian expression of Slc25a25 mRNA in mouse gastrocnemius muscles. The mRNA expression of Slc25a25 fluctuated in a circadian manner that peaked at zeitgeber time (ZT) 14 in intact and contralateral skeletal muscle (both P < 0.001; one-way ANOVA) (Fig. 1a). The circadian amplitude of Slc25a25 expression was decreased by 85% in the gastrocnemius muscle of mice with sciatic denervation relative to that in intact muscle (Fig. 1a), although the rhythmic expression of Slc25a25 was retained (P = 0.003; one-way ANOVA). We investigated the temporal expression profiles of Slc25a25 in homozygous Clock mutant (Clk/Clk), muscle-specific Bmal1 (M-Bmal1) KO, and global Bmal1 (G-Bmal1) KO mice to assess whether or not the molecular clock is involved in the circadian regulation of Slc25a25 expression. The circadian amplitude of Slc25a25 mRNA expression was decreased by 60% in the skeletal muscle of Clk/Clk compared with that of WT mice (Fig. 1b), although its mRNA levels remained rhythmic (P < 0.001; one-way ANOVA). The muscle-specific deletion of Bmal1 slightly decreased the peak level of Slc25a25 expression, but day/night expression was essentially retained (WT, P = 0.016; M-Bmal1 KO, P = 0.002; t-test) (Fig. 1c). The peak expression level of Slc25a25 was significantly lower in G-Bmal1 KO, than in WT mice, and day/night oscillation was abolished in G-Bmal1 KO (WT, P < 0.001; G-Bmal1 KO, P = 0.087; t-test; Fig. 1d).
Slc25a25 is a circadian gene in skeletal muscle. (a) Circadian expression of Slc25a25 in skeletal muscle seven days after sciatic denervation. Contralateral innervated muscles of same mice and muscles of intact mice served as controls. Gray shading indicates dark period. Data are expressed as means ± SEM (n = 5–6 per group). Maximal value for intact mice is expressed as 1.0. * P < 0.05 and ** P < 0.01 for intact vs. denervated muscle at corresponding Zeitgeber time (ZT). (b) Temporal expression profiles of Slc25a25 mRNA in skeletal muscle of Clock mutant (Clk/Clk) mice. Gray shading indicates dark period. Data are expressed as means ± SEM (n = 4–5 per group). * P < 0.05 for WT vs. mutant mice at corresponding ZT. (c,d) Muscle-specific (M-Bmal1 KO) or global (G-Bmal1 KO) Bmal1 knockout mice. Gray shading indicates dark period. Data are expressed as means ± SEM (n = 4–5 per group). Maximal value for wild-type (WT) mice is expressed as 1.0. * P < 0.05 for WT vs. mutant mice. † P < 0.05 and †† P < 0.01 for ZT2 vs. ZT14. Supplemental Tables 2 and 3 show results of statistical analysis.
Ketogenic diet induces Slc25a25 mRNA expression in skeletal muscle, but not in brown and white adipose tissues
A previous study has shown that SLC25A25 might contribute to a thermogenic pathway in mice with defective BAT thermogenesis, because Slc25a25 mRNA expression is induced during adaptation to cold stress in the skeletal muscle of Ucp1 KO mice21. On the other hand, normal Tb was maintained in global Slc25a25 KO mice after acute exposure to cold stress, suggesting that SLC25A25 is not essential for the regulation of Tb during exposure to cold21. We investigated the mRNA expression of Slc25a25 in sciatic denervated and sham-operated mice after seven days on a ketogenic diet (KD) that induces time-of-day-dependent hypothermia (torpor)10. In addition to Slc25a25, we also measured the mRNA expression of other thermogenic genes such as those for Sarcolipin (Sln), Pgc1a, Ucp2, and Ucp3. Sarcolipin regulates sarcoplasmic reticulum Ca2+-ATPase (SERCA) and it is involved in non-shivering muscular thermogenesis during cold stress22. The thermogenic molecule PGC1α functions as a transcriptional co-activator of PPARγ that modulates the expression of Ucp1 and thermogenesis in brown fat23. The KD upregulated mRNA expression of Slc25a25 and Ucp3 by 2.4-fold and 2.7-fold, respectively, in muscle from sham-operated mice compared with mice fed with a normal diet (ND) (Fig. 2a,g). However, the KD did not induce the mRNA expression of Slc25a25 isoforms such as Slc25a23 and Slc25a24 (Fig. 2b,c). The KD also did not affect the mRNA expression of Sln, Pgc1a and Ucp2 in muscle from sham-operated mice (Fig. 2d–f). On the other hand, sciatic denervation gene-specifically affected the mRNA expression of these genes; it significantly decreased the mRNA expression levels of Slc25a25, Pgc1a, and Ucp3, but increased those of Slc25a24 and Sln. The expression levels of Sln were increased about 400-fold by denervation independently of the diet (Fig. 2d). Among the thermogenic genes measured herein, KD increased Slc25a25 and Ucp3 expression in sham-operated muscle, and only Slc25a25 abolished the response to KD by denervation. The mRNA expression levels of Nr1d1 that inhibits the thermogenic activity of BAT24 were not affected by either the KD or denervation (Fig. 2h).
Prolonged ketogenic diet feeding upregulates Slc25a25 expression in skeletal muscle. Messenger RNA expression of thermogenic gene in skeletal muscle of mice fed with ketogenic (KD) or normal (ND) diets for seven days starting from 10 days after sciatic denervation or sham-operation. Data are expressed as means ± SEM (n = 5 per group). Value for sham-operated mice fed with ND is expressed as 1.0. * P < 0.05 and ** P < 0.01 for sham-operated vs. denervated. † P < 0.05 and †† P < 0.01 for ND vs. KD. Supplemental Table 4 shows results of statistical analysis.
Brown adipose fat is thought to be the primary contributor to thermogenesis through the function of UCP16. We examined the effects of the KD and sciatic denervation on the expression of thermogenic genes in BAT, and found that neither the KD, nor sciatic denervation significantly affected Slc25a25 expression (Fig. 3a). Furthermore, the mRNA expression of the thermogenic genes, Ucp1, Cidea, Ucp3 and Nr1d1 did not significantly differ among all groups (Fig. 3b,c,f,g). Sciatic denervation slightly upregulated Pgc1a mRNA expression, whereas the KD did not (Fig. 3d), although it increased Ucp2 expression in denervated mice (Fig. 3e).
Messenger RNA expression of thermogenic gene in brown adipose tissue. Prolonged ketogenic diet (KD) feeding minimally affects thermogenic gene expression in brown adipose tissues of mice fed with KD or normal diet (ND) for seven days starting at 10 days after sciatic denervation or sham-operation. Data are expressed as means ± SEM (n = 5 per group). Value for sham-operated mice fed with ND is expressed as 1.0. * P < 0.05 for sham-operated vs. denervated. † P < 0.05 for ND vs. KD. Supplemental Table 4 shows results of statistical analysis.
We evaluated the mRNA expression of thermogenic genes in white adipose tissue (WAT), because beige cells in WAT are involved in adaptation to a chronically cold environment25,26,27,28. Levels of Slc25a25, Cidea and Pgc1a gene expression did not significantly differ among all groups (Fig. 4a,c,d). The KD slightly induced the mRNA expression of Ucp1 in denervated and sham-operated mice (Fig. 4b).
Messenger RNA expression of thermogenic genes in white adipose tissue. Prolonged ketogenic diet (KD) feeding minimally affects thermogenic gene expression in white adipose tissues of mice fed with KD or normal diet (ND) for seven days starting at 10 days after sciatic denervation or sham-operation. Data are expressed as means ± SEM (n = 5 per group). Value for sham-operated mice fed with ND is expressed as 1.0. † P < 0.05 for ND vs. KD. Supplemental Table 4 shows results of statistical analysis.
Sciatic denervation augments hypothermia induced by a ketogenic diet
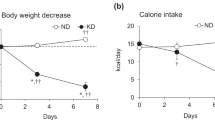
We assessed the effect of the KD on circadian Tb rhythm in sciatic denervated and sham-operated mice to determine the functional role of the skeletal muscle-specific induction of Slc25a25 mRNA expression in mice fed with the KD. The circadian fluctuation of Tb that peaked during the early night was identical between denervated and sham-operated groups fed with the ND (Fig. 5a). The KD obviously reduced Tb during the latter half of the dark period as described10 in both denervated and sham-operated mice (Supplemental Fig. 1). Notably, Tb was significantly lower during the early night in denervated, than in sham-operated mice fed with the KD (Fig. 5b), although denervation did not affect the Tb rhythm in mice fed with a normal diet (Fig. 5a). We compared the effect of KD on peak Tb between denervated and sham-operated mice, and found that sciatic denervation obviously augmented KD-induced hypothermia (Fig. 5c). Fourteen days of feeding with the KD decreased peak Tb from 38.1 ± 0.13 °C (on the day before KD feeding) to 37.5 ± 0.09 °C in sham-operated, and from 38.2 ± 0.11 °C to 36.9 ± 0.14 °C in denervated mice (Fig. 5c). These results indicate that skeletal muscle plays an important role in maintaining Tb while under feeding with a KD, although it does not seem essential under normal diet feeding.
Bilateral sciatic denervation exacerbates hypothermia during prolonged ketogenic diet feeding. Core body temperature rhythms for 24 h in mice fed with ketogenic (KD) or normal (ND) diets for two weeks starting at 10 days after sham operation or bilateral sciatic nerve transection. Hourly averaged values of body temperature on the day before starting (a) and after two weeks (b) on ketogenic diet. Gray shading indicates dark period. (c) Peak values of body temperature during experimental period. Body temperatures were averaged every two hours from ZT13 to ZT15. Data are expressed as means ± SEM (n = 5–7 per group). * P < 0.05 and ** P < 0.01 for sham-operated vs. denervated. ZT, zeitgeber time. Supplemental Tables 5 and 6 show results of statistical analysis.
Aging affects thermogenic gene expression in skeletal muscle
We assessed the thermogenic gene expression in skeletal muscle to determine the functional role of Slc25a25 on the decrease in Tb induced by aging. The levels of Slc25a25 and Sln mRNA expression were significantly decreased and increased, respectively, in the skeletal muscle of aged mice (Fig. 6a and b). Levels of Pgc1a and Ucp3 expression were essentially identical between adult and aged mice (Fig. 6c and d). We also examined the effect of chronic exercise on Slc25a25 expression in skeletal muscle. We found that four weeks of voluntary wheel running29 increased the mRNA expression of Slc25a25 (Fig. 6e), but not of Ucp3 (Supplemental Fig. 2) in skeletal muscle compared with that in sedentary mice.
Aging gene-dependently affects thermogenic gene expression. (a–d) Messenger RNA expression of thermogenic gene in skeletal muscles of adult and aged mice (aged 7–8 and 23–24 months, respectively). Data are expressed as means ± SEM (n = 6–10 per group). Maximal value for adult mice is expressed as 1.0. * P < 0.05 for adult vs. aged mice. (e) Effects of voluntary running on Slc25a25 mRNA expression in skeletal muscle. Mice were individually housed in cages with or without (to mimic sedentary conditions) running-wheels for four weeks. Gray shading indicates dark period. Data are shown as means ± SEM (n = 4–5). Maximal value for sedentary mice is expressed as 1.0. * P < 0.05 for sedentary vs. running-wheel. Supplemental Tables 7 and 8 show results of statistical analysis.
Discussion
The present study aimed to elucidate the functional role of circadian Slc25a25 expression in mouse skeletal muscle. We found that the circadian amplitude of Slc25a25 expression was decreased by 85% in denervated, compared with intact muscle. The circadian amplitude of muscle Slc25a25 expression was decreased by 60%, 38% and 70% in Clk/Clk, M-Bmal1 KO, and G-Bmal1 KO mice, respectively. These findings suggest that the circadian expression of Slc25a25 is largely dependent on neural signals in a molecular clock-dependent manner. A previous study found that SLC25A25 might contribute to a thermogenic pathway in mice with defective BAT thermogenesis, because Slc25a25 mRNA expression was induced during adaptation to cold stress in the skeletal muscle of Ucp1 KO mice21. On the other hand, that study also found that Tb remained normal in global Slc25a25 KO mice under acute exposure to cold stress, suggesting that SLC25A25 is not essential for Tb regulation during cold exposure21. The present study evaluated the functional role of Slc25a25 on adaptive thermogenesis in mice fed with a KD, which induces time-of-day dependent hypothermia (torpor). The KD significantly increased the mRNA expression of Slc25a25 in muscle. This was probably muscle-specific and governed by neural signals, because the KD did not induce Slc25a25 mRNA in other tissues such as BAT, WAT and the liver (Figs 3a, 4a, and Supplemental Fig. 3), and sciatic denervation abolished KD-induced mRNA expression. The mRNA expression of typical thermogenic molecules such as Ucp1 in BAT and WAT, and of Sln and Pgc1a in skeletal muscles was not affected by the KD. To evaluate the functional role of the skeletal muscle-specific induction of Slc25a25 mRNA expression in mice fed with the KD, we investigated the effects of the KD on circadian Tb rhythms in sciatic denervated and sham-operated mice. The Tb was significantly lower in sciatic denervated mice fed with the KD compared with sham-operated control mice, whereas it remained essentially identical between denervated and sham-operated mice fed with the ND, suggesting that muscle thermogenesis is involved in the maintenance of core Tb under KD feeding. The present findings suggest that both the central clock and energy homeostasis regulate muscle Slc25a25 expression via neural pathways, and that SLC25A25 may be involved in muscle thermogenesis under hypothermia induced by KD in mammals.
We assessed the temporal expression profiles of Slc25a25 and the typical clock gene, Per2, in C2C12 myotubes to determine the involvement of peripheral clocks in the rhythmic expression of Slc25a25 mRNA. However, we did not find rhythmic Slc25a25 expression in C2C12 myotubes, although the circadian expression of Per2 was robust (Supplemental Fig. 4). These findings suggest that molecular clock components are involved in the circadian expression of Slc25a25 in a systemic manner. On the other hand, day/night fluctuation of Slc25a25 expression was slight, but significantly retained in denervated (P < 0.001; one-way ANOVA), Clk/Clk (P < 0.001; one-way ANOVA), or M-Bmal1 KO mice (P = 0.002; t-test), but not in G-Bmal1 KO mice (P = 0.087; t-test). All experiments in the present study proceeded under light-dark cycles. Day/night locomotor activity rhythm was evident in denervated, Clk/Clk, and M-Bmal1 KO mice as well as WT animals, the result of a direct masking effect (Supplemental Fig. 5). Day/night behavioral and physiological rhythms including feeding and body temperature might, at least in part, be involved in the circadian regulation of Slc25a25 expression in muscle.
Muscle mRNA levels of Slc25a25 robustly fluctuated in a circadian manner that peaked at the day-to-night transition and were positively regulated by CLOCK and BMAL1 in a systemic manner through a neural pathway. However, the mRNA levels of Slc25a25 remained almost constant throughout the day in the liver, and upregulated in Clk/Clk mice (Supplemental Fig. 6a). These findings suggest that Slc25a25 transcription is tissue-specifically regulated and that muscle SLC25A25 plays an important role during the active onset.
We exposed mice to time-restricted feeding (diet available for 8 h during either daytime or nighttime) for one week30 to determine whether the circadian feeding schedule is involved in the circadian regulation of muscle Slc25a25 expression. This feeding schedule did not affect the circadian phase of Slc25a25 mRNA expression, which peaked at ZT14 in skeletal muscle (Supplemental Fig. 7a). In contrast, the reversed feeding schedule obviously affected the temporal expression profile of Slc25a25 mRNA in both the liver and WAT of mice (Supplemental Fig. 7b,c). The acrophase of mRNA expression in the liver and WAT corresponded to the feeding phase. These results suggest that feeding-derived signals are important to enforce the rhythmicity of Slc25a25 expression in the liver and WAT, but not in skeletal muscle. Feeding schedules do not affect the central clock in the SCN31. Therefore, neural signals from the SCN clock might be involved in the circadian regulation of Slc25a25 expression in skeletal muscle.
We analyzed the effects of a Clock gene mutation on Slc25a25 expression induced by the KD (Supplemental Fig. 8). We found that the KD-induced Slc25a25 expression in Clk/Clk mice, although to a lesser extent than in WT mice. These findings suggest that the molecular clock is dispensable, but partly involved in the KD-induced Slc25a25 expression in muscle.
We did not identify an effect of KD feeding on Ucp1 expression in either muscle or BAT, although previous studies have demonstrated KD-induced Ucp1 expression in BAT32, 33. We analyzed the effects of short-term (7 days) KD feeding, whereas Kennedy et al. and Srivastava et al. assessed the long-term effects (5 and 4 weeks, respectively)32, 33. The effects of KD on metabolic tissues might vary dependently on the experimental period.
The present study found that sciatic denervation significantly decreased nighttime Tb in mice fed with KD, but did not affect Tb in mice fed with a normal diet. Sciatic denervation did not affect starvation-induced endocrine systems since the KD similarly increased plasma FGF21 concentrations in both denervated and sham-operated mice (Supplemental Fig. 9). Importantly, expression levels of the thermogenic genes, Ucp1 and Pgc1a in BAT and WAT were insensitive to the KD, although Tb was extremely decreased. These observations suggest that muscle thermogenesis plays an important role in maintaining Tb under KD feeding. Muscle thermogenesis has been explained as burst contractions of skeletal muscle (shivering) in an immediate response to acute cold stress34, 35. However, shivering is not a long-term continuous thermogenic response but rather a transient thermogenic response to maintain the Tb for a few hours36. The KD did not affect the mRNA expression of Sln in muscle, although Sln is thought to be involved in non-shivering muscular thermogenesis during cold stress22. Therefore, SLN does not seem to be a critical contributor to the maintenance of Tb under a KD. Denervation remarkably increased the levels of Sln mRNA expression in muscle. These findings suggest that neural signals negatively and positively regulate the expression of Sln and of Slc25a25, respectively, in muscle.
Circadian amplitude and mean Tb decreases in elderly persons37,38,39. Changes in Tb rhythms associated with aging are frequently associated with a reduction in nighttime sleep quality40 and cerebral blood flow39. Levels of Slc25a25 mRNA expression were significantly decreased in the skeletal muscle of aged, compared with adult mice (Fig. 6a), whereas the levels of Sln mRNA expression were significantly increased (Fig. 6b). Several studies have found denervation in aging muscles41, including a progressive reduction in the number of motoneurons in the spinal cord beginning around the age of 60 years42, a loss of motoneurons in the periphery43, 44, degeneration of neuromuscular junctions45 and loss of motor units46. Aging-induced denervation might cause the downregulation of Slc25a25 in elderly persons, subsequent to a lower Tb. Meanwhile, Sln mRNA expression was significantly upregulated in aged muscle of mice (Fig. 6b). We speculated that aging-induced denervation increases Sln mRNA expression, and that the thermogenic function of Sln seems independent of aging-induced hypothermia. We also examined the effect of chronic exercise on Slc25a25 expression in skeletal muscle, because exercise is considered an effective approach to improve denervation in elderly persons47. We found that four weeks of voluntary wheel running29 increased Slc25a25 mRNA expression in the skeletal muscles of mice (Fig. 6e). Thus, elderly persons should maintain muscle thermogenic activity to retain Slc25a25 expression and prevent an age-related decrease in Tb. Regular exercise might be a useful approach to maintain muscle thermogenesis through induction of the Slc25a25 gene.
We could not evaluate the effects of aging on KD-induced Slc25a25 expression in mice because such experiments are very time-consuming. Further studies are needed to understand the relationships between aging and decreased Slc25a25 expression, including the effect of KD feeding and sciatic denervation.
The present findings suggest that chronic KD feeding induces expression of the circadian gene, Slc25a25, via a neural pathway in muscle. Moreover, muscle non-shivering thermogenesis seemed important for maintaining Tb under KD feeding, and Slc25a25 and Ucp3 might be involved in muscle thermogenesis (Fig. 7). This is the first report to suggest that thermogenesis derived from skeletal muscle is involved in the maintenance of core Tb under metabolic hypothermia. Sciatic denervation affects the expression of many metabolic genes as we have previously shown16. We believe that several genes are associated with muscle thermogenesis under metabolic hypothermia such as ketogenic conditions. In fact, the expression profiles of Ucp3 and Slc25a25 in skeletal muscle in response to the KD and sciatic denervation were similar. Further studies are needed to uncover the molecular mechanism of muscle thermogenesis under metabolic hypothermia.
Summary of present study. Mechanism of muscle thermogenesis starts with shivering as first defense against exposure to cold. Thereafter, Sln 22, 50 and Ucp1 51, 52 are respectively driven to elicit non-shivering thermogenesis in skeletal muscle and BAT. Exposure to cold also induces emergence of brown adipocytes in WAT by increasing expression of Ucp1, Cidea, and Pgc1a genes52, 53. Meanwhile, the present study found induced circadian Slc25a25 gene expression in skeletal muscle, but not in BAT and WAT of mice fed with ketogenic diet (KD), which induces torpor. Messenger RNA expression of thermogenesis-related genes such as Ucp1 was not affected by KD in BAT and WAT. Sciatic denervation abolished Slc25a25 expression and augmented KD-induced hypothermia. These results suggest that skeletal muscle is involved in thermogenesis during KD feeding, and that Slc25a25 and Ucp3 are candidates for muscle thermogenesis. Less Slc25a25 was expressed in skeletal muscle of aged, compared with adult mice, whereas Ucp1 expression was not altered by aging at thermoneutrality54. SLC25A25 might help to maintain Tb in aged mammals.
Methods
Animal care and surgical procedures
All animal experiments proceeded according to the guidelines for animal experiments of the National Institute of Advanced Industrial Science and Technology (AIST). The Animal Care and Use Committees at AIST approved all the experimental protocols described herein (Permission # 2016-166).
Circadian expression of the Slc25a25 gene was measured in C57BL/6J mice seven days after sciatic denervation. The contralateral innervated (sham-operated) muscles of the same animals and the muscles of intact animals served as controls, as described16. Homozygous Clock mutant mice were generated as described48. Global Bmal1 KO mice, muscle-specific Bmal1 KO mice and their control littermates generated as described49 were housed with access to a standard diet (CE2: CLEA Japan Inc., Tokyo, Japan) and water ad libitum under a 12 h light-12 h dark cycle (LD12:12); lights on at Zeitgeber time (ZT) 0 and lights off at ZT12. After sacrifice at the indicated times, the gastrocnemius muscles were dissected.
We assessed the effect of KD feeding on the expression of thermogenic genes and Tb in nine week-old male Jcl:ICR mice (Japan SLC Inc., Shizuoka, Japan) that were housed and fed ad libitum for two weeks under a 12 h light-12 h dark cycle. The mice were randomly assigned to receive muscle-denervation or a sham operation. The sciatic nerve was bilaterally transected under anesthesia. Sham-operated mice underwent identical dissection without transection. Ten days later, the denervated and sham-operated mice were each divided into two experimental groups and fed with either the AIN-93G (Oriental Yeast Co. Ltd., Tokyo, Japan) normal diet (ND) or the modified AIN-93G ketogenic (KD) diet (73.9% fat, 8.3% protein and 0.73% carbohydrate, w/w; Oriental Yeast Co. Ltd.) for two weeks. The proportions of calories derived from fat, carbohydrate and protein were ND: 12.6%, 58.3% and 29.3%; KD: 94.8%, 0.1% and 4.8%, respectively. The mice were sacrificed at ZT14 and the gastrocnemius muscle, white (WAT) and brown (BAT) adipose tissues were dissected, weighed and frozen in liquid nitrogen.
Mice aged 7–8 (adult) and 23–24 months (aged) were sacrificed to evaluate the effects of aging on thermogenic gene expression in the gastrocnemius muscles.
Seven-week-old male mice were individually housed in cages without running-wheels to mimic sedentary conditions or with running-wheels for four weeks to evaluate the effects of chronic exercise on Slc25a25 mRNA expression in the gastrocnemius muscles.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using guanidinium thiocyanate followed by RNAiso Plus (Takara Bio Inc., Otsu, Japan). Single-stranded cDNA was synthesized using PrimeScript™ RT reagent kits with gDNA Eraser (Takara Bio). Real-time RT-PCR proceeded using SYBR® Premix Ex Taq™ II (Takara Bio) and a LightCycler™ (Roche Diagnostics, Mannheim, Germany). The amplification conditions comprised 95 °C for 10 s followed by 45 cycles of 95 °C for 5 s, 57 °C for 10 s and 72 °C for 10 s. Supplemental Table 1 shows the primer sequences. Amounts of target mRNA were normalized relative to that of Actb.
Monitoring core body temperature
Mice were surgically implanted intra-abdominally with TempDisk TD-LAB data loggers (Labo Support Co. Ltd., Suita, Osaka, Japan) that were programmed to record body temperature (Tb) ± 0.1 °C every 15 min. Data obtained from each logger were analyzed using RhManager Ver.2.09 (KN Laboratories Inc., Ibaraki, Osaka, Japan) and hourly Tb values were averaged. We measured two-hour averaged Tb values between ZT13 and 15 during the experimental period to determine variations in peak Tb.
Statistical analysis
All values are expressed as means ± SEM. Levels of mRNA expression in denervated and sham-operated mice fed with a normal diet or KD were statistically evaluated using a two-way analysis of variance (ANOVA) and the Tukey multiple comparison test using Excel-Toukei 2010 software (Social Survey Research Information Co. Ltd., Osaka, Japan). The value of Tb in mice fed with the KD and the mRNA levels in clock gene-mutant mice at corresponding ZT were compared between groups using Student’s t-test. The mRNA levels of Slc2a25 in sciatic denervated mice and intact mice at corresponding ZT were compared between groups using one-way ANOVA. The effects of time on Slc25a25 mRNA expression were determined by one-way ANOVA in denervated and Clk/Clk mice, and by Student’s t-test in M-Bmal1 KO and G-Bmal1 KO mice. The circadian amplitude of Slc25a25 expression was set at half of the total peak-trough variation. Differences were considered significant at P < 0.05. Supplemental Tables 2–8 show the results of the statistical analysis.
References
Refinetti, R. The circadian rhythm of body temperature. Front Biosci (Landmark Ed). 15, 564–594, doi:10.2741/3634 (2010).
Buhr, E. D. & Takahashi, J. S. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol. 3–27, doi:10.1007/978-3-642-25950-0_1 (2013).
Saper, C. B. & Lowell, B. B. The hypothalamus. Curr Biol. 24, R1111–1116, doi:10.1016/j.cub.2014.10.023 (2014).
Nakamura, K. & Morrison, S. F. Central efferent pathways for cold-defensive and febrile shivering. J Physiol. 589, 3641–3658, doi:10.1113/jphysiol.2011.210047 (2011).
Morrison, S. F. & Nakamura, K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 16, 74–104, doi:10.2741/3677 (2011).
Lowell, B. B. & Spiegelman, B. M. Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660, doi:10.1038/35007527 (2000).
Melvin, R. G. & Andrews, M. T. Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol Metab. 20, 490–498, doi:10.1016/j.tem.2009.09.005 (2009).
Geiser, F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 66, 239–274, doi:10.1146/annurev.physiol.66.032102.115105 (2004).
Ruf, T. & Geiser, F. Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc. 90, 891–926, doi:10.1111/brv.2015.90.issue-3 (2015).
Oishi, K., Yamamoto, S., Uchida, D. & Doi, R. Ketogenic diet and fasting induce the expression of cold-inducible RNA-binding protein with time-dependent hypothermia in the mouse liver. FEBS Open Bio 3, 192–195, doi:10.1016/j.fob.2013.03.005 (2013).
Inagaki, T. et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425, doi:10.1016/j.cmet.2007.05.003 (2007).
Oishi, K. et al. FGF21 is dispensable for hypothermia induced by fasting in mice. Neuro Endocrinol Lett. 31, 198–202 (2010).
Miller, B. H. et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104, 3342–3347, doi:10.1073/pnas.0611724104 (2007).
McCarthy, J. J. et al. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31, 86–95, doi:10.1152/physiolgenomics.00066.2007 (2007).
Hodge, B. A. et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 5, 17, doi:10.1186/s13395-015-0039-5 (2015).
Nakao, R. et al. Atypical expression of circadian clock genes in denervated mouse skeletal muscle. Chronobiol Int. 32, 486–496, doi:10.3109/07420528.2014.1003350 (2015).
Andrews, J. L. et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 107, 19090–19095, doi:10.1073/pnas.1014523107 (2010).
Schroder, E. A. et al. Intrinsic muscle clock is necessary for musculoskeletal health. J Physiol. 593, 5387–5404, doi:10.1113/JP271436 (2015).
Dyar, K. A. et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 3, 29–41, doi:10.1016/j.molmet.2013.10.005 (2014).
Satrustegui, J., Pardo, B. & Del Arco, A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 87, 29–67, doi:10.1152/physrev.00005.2006 (2007).
Anunciado-Koza, R. P. et al. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. J Biol Chem. 286, 11659–11671, doi:10.1074/jbc.M110.203000 (2011).
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 18, 1575–1579, doi:10.1038/nm.2897 (2012).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839, doi:10.1016/S0092-8674(00)81410-5 (1998).
Gerhart-Hines, Z. et al. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 503, 410–413, doi:10.1038/nature12642 (2013).
Young, P., Arch, J. R. & Ashwell, M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167, 10–14, doi:10.1016/0014-5793(84)80822-4 (1984).
Loncar, D., Afzelius, B. A. & Cannon, B. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J Ultrastruct Mol Struct Res. 101, 109–122, doi:10.1016/0889-1605(88)90001-8 (1988).
Loncar, D., Afzelius, B. A. & Cannon, B. Epididymal white adipose tissue after cold stress in rats. II. Mitochondrial changes. J Ultrastruct Mol Struct Res. 101, 199–209, doi:10.1016/0889-1605(88)90010-9 (1988).
Cousin, B. et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 103(Pt 4), 931–942 (1992).
Yasumoto, Y., Nakao, R. & Oishi, K. Free access to a running-wheel advances the phase of behavioral and physiological circadian rhythms and peripheral molecular clocks in mice. PLoS One 10, e0116476, doi:10.1371/journal.pone.0116476 (2015).
Yasumoto, Y. et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 65, 714–727, doi:10.1016/j.metabol.2016.02.003 (2016).
Schibler, U., Ripperger, J. & Brown, S. A. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms 18, 250–260, doi:10.1177/0748730403018003007 (2003).
Kennedy, A. R. et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 292, E1724–39, doi:10.1152/ajpendo.00717.2006 (2007).
Srivastava, S. et al. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 65, 58–66, doi:10.1002/iub.1102 (2013).
Rowland, L. A., Bal, N. C. & Periasamy, M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev CambPhilos Soc. 90, 1279–1297, doi:10.1111/brv.2015.90.issue-4 (2015).
Hemingway, A. Shivering. Physiol Rev. 43, 397–422 (1963).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol Rev. 84, 277–359, doi:10.1152/physrev.00015.2003 (2004).
Falk, B., Bar-Or, O., Smolander, J. & Frost, G. Response to rest and exercise in the cold: effects of age and aerobic fitness. J Appl Physiol (1985) 76, 72–78 (1994).
Elia, M., Ritz, P. & Stubbs, R. J. Total energy expenditure in the elderly. Eur J Clin Nutr. 54(Suppl 3), S92–103, doi:10.1038/sj.ejcn.1601030 (2000).
Van Someren, E. J., Raymann, R. J., Scherder, E. J., Daanen, H. A. & Swaab, D. F. Circadian and age-related modulation of thermoreception and temperature regulation: mechanisms and functional implications. Ageing Res Rev. 1, 721–778, doi:10.1016/S1568-1637(02)00030-2 (2002).
Myers, B. L. & Badia, P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci Biobehav Rev. 19, 553–571, doi:10.1016/0149-7634(95)00018-6 (1995).
Rowan, S. L. et al. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One 7, e29082, doi:10.1371/journal.pone.0029082 (2012).
Tomlinson, B. E. & Irving, D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 34, 213–219, doi:10.1016/0022-510X(77)90069-7 (1977).
Hashizume, K. & Kanda, K. Differential effects of aging on motoneurons and peripheral nerves innervating the hindlimb and forelimb muscles of rats. Neurosci Res. 22, 189–196, doi:10.1016/0168-0102(95)00889-3 (1995).
Hashizume, K., Kanda, K. & Burke, R. E. Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol. 269, 425–430, doi:10.1002/cne.902690309 (1988).
Gutmann, E. & Hanzlikova, V. Motor unit in old age. Nature 209, 921–922, doi:10.1038/209921b0 (1966).
McNeil, C. J., Doherty, T. J., Stashuk, D. W. & Rice, C. L. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31, 461–467, doi:10.1002/mus.20276 (2005).
Valdez, G. et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA 107, 14863–14868, doi:10.1073/pnas.1002220107 (2010).
Oishi, K., Miyazaki, K. & Ishida, N. Functional CLOCK is not involved in the entrainment of peripheral clocks to the restricted feeding: entrainable expression of mPer2 and BMAL1 mRNAs in the heart of Clock mutant mice on Jcl:ICR background. Biochemical Biophys Res Comm. 298, 198–202, doi:10.1016/S0006-291X(02)02427-0 (2002).
Shimba, S. et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One 6, e25231, doi:10.1371/journal.pone.0025231 (2011).
Pant, M., Bal, N. C. & Periasamy, M. Cold adaptation overrides developmental regulation of sarcolipin expression in mice skeletal muscle: SOS for muscle-based thermogenesis? J Exp Biol. 218, 2321–2325, doi:10.1242/jeb.119164 (2015).
Enerback, S. et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94, doi:10.1038/387090a0 (1997).
Barbatelli, G. et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 298, E1244–1253, doi:10.1152/ajpendo.00600.2009 (2010).
Sidossis, L. & Kajimura, S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 125, 478–486, doi:10.1172/JCI78362 (2015).
Florez-Duquet, M., Horwitz, B. A. & McDonald, R. B. Cellular proliferation and UCP content in brown adipose tissue of cold-exposed aging Fischer 344 rats. Am J Physiol. 274, R196–203 (1998).
Acknowledgements
This study was supported by operational subsidies from AIST, and JSPS KAKENHI to R. Nakao (JP15K16499) and K. Oishi (JP16K00940) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan. We thank Dr. Hideaki Oike (National Agriculture and Food Research Organization) and Dr. Tetsuya Shiuchi (The University of Tokushima) for valuable comments. We are also grateful to Ms. Saori Yamamoto, Ms. Sayaka Higo-Yamamoto (AIST), Mr. Yuki Yasumoto (AIST, Tokyo University of Science), Ms. Haruka Yamazaki (AIST, Nihon University), and Mr. Hiroki Okauchi (AIST, Tokyo University of Science) for technical assistance.
Author information
Authors and Affiliations
Contributions
R.N. and K.O. designed the study protocol. R.N., S.S., and K.O. conducted experiments. S.S. derived the Bmal1 KO mouse strain. R.N. and K.O. analyzed data. R.N. and K.O. contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakao, R., Shimba, S. & Oishi, K. Ketogenic diet induces expression of the muscle circadian gene Slc25a25 via neural pathway that might be involved in muscle thermogenesis. Sci Rep 7, 2885 (2017). https://doi.org/10.1038/s41598-017-03119-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03119-8
- Springer Nature Limited
This article is cited by
-
Ketogenic nutritional therapy (KeNuT)—a multi-step dietary model with meal replacements for the management of obesity and its related metabolic disorders: a consensus statement from the working group of the Club of the Italian Society of Endocrinology (SIE)—diet therapies in endocrinology and metabolism
Journal of Endocrinological Investigation (2024)
-
The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia
Nature Communications (2020)
-
Molecular changes in transcription and metabolic pathways underlying muscle atrophy in the CuZnSOD null mouse model of sarcopenia
GeroScience (2020)
-
Ketogenic diet induces skeletal muscle atrophy via reducing muscle protein synthesis and possibly activating proteolysis in mice
Scientific Reports (2019)
-
The Influence of Short-term Fasting on Muscle Growth and Fiber Hypotrophy Regulated by the Rhythmic Expression of Clock Genes and Myogenic Factors in Nile Tilapia
Marine Biotechnology (2018)