Abstract
Sound vibration (SV), a mechanical stimulus, can trigger various molecular and physiological changes in plants like gene expression, hormonal modulation, induced antioxidant activity and calcium spiking. It also alters the seed germination and growth of plants. In this study, we investigated the effects of SV on the resistance of Arabidopsis thaliana against Botrytis cinerea infection. The microarray analysis was performed on infected Arabidopsis plants pre-exposed to SV of 1000 Hertz with 100 decibels. Broadly, the transcriptomic analysis revealed up-regulation of several defense and SA-responsive and/or signaling genes. Quantitative real-time PCR (qRT-PCR) analysis of selected genes also validated the induction of SA-mediated response in the infected Arabidopsis plants pre-exposed to SV. Corroboratively, hormonal analysis identified the increased concentration of salicylic acid (SA) in the SV-treated plants after pathogen inoculation. In contrast, jasmonic acid (JA) level in the SV-treated plants remained stable but lower than control plants during the infection. Based on these findings, we propose that SV treatment invigorates the plant defense system by regulating the SA-mediated priming effect, consequently promoting the SV-induced resistance in Arabidopsis against B. cinerea.
Similar content being viewed by others
Introduction
Plants have evolved themselves against environmental challenges with effective physiological and developmental modifications. For instance, plants undergo significant changes in their growth and development in response to mechanical stimuli (e.g., touch and wind), which is called thigmomorphogenesis1. It is a complex plant response that involves changes in growth, flowering time, senescence, pithiness, chlorophyll content, hormone level, biotic and abiotic stress resistance, and control of stomatal aperture1. Interestingly, recent studies have indicated that sound vibration (SV) may act as a pressure wave, triggering thigmomorphogenesis (reviewed in2). The available evidence indicates that plants are responsive towards natural sounds, thus suggesting an ecological and environmental relevance of plant-acoustic interaction. For instance, the ‘Buzz Pollinated’ plants release pollens from anthers when exposed to a specific frequency of sound produced by bee buzz3. A large number of plant species are known to possess such peculiar floral behavior3. It was identified that pretreatment with vibrations caused by chewing sound of caterpillar can induce the Arabidopsis chemical defense4. However, leafhopper song failed to prime the defense response in the same study. This finding suggests that plants evolved appreciably enough to selectively respond to particular SV. Moreover, the elevated levels of polyamines and oxygen uptake in Chinese cabbage after the exposure of green music (e.g. certain natural music like bird’s singing, cricket’s stridulating etc.) also substantiate the ecological significance5. Additionally, several other plausible ecological relevance of plant-acoustic interaction has been discussed by Mishra et al.2. These examples strongly suggest that plants have evolved sensitivity towards ecologically relevant SV, although the molecular mechanism is poorly understood.
The beneficial effects of synthetic SV on plant growth and development were studied in various plant species, including paddy, wheat, tomato, lettuce, spinach, cucumber and strawberry6. These beneficial effects include stimulation of seed germination and an increase in the number of fruits, plant height, tiller growth and crop yield. Furthermore, SV exposure with 1000 Hertz (Hz) caused various biochemical changes in Chrysanthemum, Dendrobium, and Actinidia such as induction of soluble sugar content, protein, antioxidant enzyme activity and optimized energy metabolism2, 6. Moreover, Chrysanthemum exposed to 1400 Hz SV showed accumulation of indole-3-acetic acid (IAA) and reduction of abscisic acid (ABA)7. Young roots of maize were grown toward acoustic emissions (220 Hz), showing thigmotropism or sound tropism8. These aforementioned studies reveal the beneficial effects of SV on plant, suggesting the existence of sophisticated molecular mechanisms for SV perception and signal transduction without any specialized sensory organs like ears. It is evident that external auditory organs are not obvious for SV perception in kingdom Animalia8. Nonetheless, how plants perceive SV remains elusive. It has been hypothesized that the cytoskeleton-plasma membrane-cell wall interface has an important role in SV perception9. Moreover, the expression analysis of SV-regulated genes after touch treatment hints at the possibility that SV is perceived as a stimulus distinct from touch, even though there is close resemblance between these two stimuli at molecular level10.
Further, SV can result in some molecular alterations that may enable plants to cope up with future stresses. Few reports suggested that SV also acts as a priming agent like other mechanical stimuli. For example, SV could induce disease resistance in strawberry11. Similarly, Arabidopsis was reported to perceive SV generated from insect herbivore and subsequently elicit systematic chemical defense4. Even pre-exposure of SV could increase drought stress tolerance in rice12. It is suggested that plant cells have the ability to get gradually primed when exposed to certain environmental or chemical challenges13. According to the priming effect hypothesis, repeated external stimulation can be imprinted as molecular-memory in the form of epigenetic marks or protein synthesis within plant cells, which then prepare the plant for sturdy response against future biotic or abiotic stresses14. This priming effect may explain how previous exposure to mild stress enables the plant to respond effectively to new stress factors. Such priming can be achieved by mechanical stimulation as well15. For instance, daily repetitive or dose dependent touch treatment on Arabidopsis leaves increased resistance against Botrytis cinerea 16, 17.
Considering that SV treatment might be an easy and inexpensive means to increase stress resistance in crops, herein, we investigated the effect of SV on biotic stress response. A better understanding of the SV-mediated priming mechanisms might have direct implications on the agriculture industry. As a step toward unraveling the mechanisms by which plants respond to SV and increase their resistance against biotic stresses, we investigated SV-mediated transcripts and hormonal changes during fungal infection. In this report, we described that SV pre-treatment plays a positive role in Arabidopsis defense against B. cinerea.
Results
SV treatment with 1000 Hz increases disease resistance against B. cinerea in A. thaliana
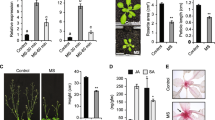
In the preliminary study, three different frequencies [500, 1000, and 3000 Hz] with constant amplitude [100 decibels (dB)] were separately applied (daily 3 h for 10 days) in Arabidopsis for the induction of disease resistance against B. cinerea (Supplementary Figs S1 and S2). Pre-treatment with 1000 Hz showed significant increase in resistance against B. cinerea (Fig. 1a). In the whole plant assay, significant difference in disease level was observed between control and treatment at 72 h post infection (hpi) (Fig. 1b). In the detached leaf assay, lesion diameter and disease progression were observed at 72 hpi (Fig. 1c and d). Detached leaf assay also confirmed the findings of the whole plant assay, showing that SV treatment increased disease resistance. Since 1000 Hz with 100 dB treatment induced the highest disease resistance against B. cinerea in both whole plants and detached leaves, we chose 1000 Hz for further study.
Reduction of disease symptoms after infection with B. cinerea in A.thaliana exposed to 1000 Hz sound vibration. (a) Percentage of disease level in whole plant assay. Percentage of disease level was calculated by counting fully senescent leaves at 72 h post inoculation (hpi). Bars represent mean and standard error of four biological replications. (b) The disease phenotype of whole plants at 72 hpi. Plants exposed to sound vibration (daily 3 h for 10 days, treatment) and control were inoculated with fungal spores and kept under growth room condition with high humidity. (c) Lesion diameter in detached leaf assay. Lesion diameter was measured at 72 hpi. Bars indicate the means of the lesion diameter with standard errors in 40 detached leaves. (d) The lesion phenotype of detached leaves. Detached leaves exposed to a 1000 Hz sound vibration (upper) and control (lower) at 72 hpi of B. cinerea spores. bar = 1 cm. P-value ranges are marked by asterisks: *** P < 0.01, ** 0.01 < P < 0.05, * P < 0.1.
SV treatment with 1000 Hz alters transcriptomic profiles in A. thaliana during B. cinerea infection
As SV pre-treatment increased the disease resistance of Arabidopsis against B. cinerea, we investigated the transcriptome changes using Affymetrix microarray. Samples from three different time points were analyzed through microarray: (1) right after the 10th day of 3 h SV treatment (0 h time point) to analyze the effect of SV exposure on gene expression, (2) after Botrytis spore inoculation (12 and 24 hpi time points) to compare the defense-related gene expression between SV-treated and control plants. A schematic view of SV treatment method, pathogen inoculation and sample harvesting time is shown in Supplementary Fig. S1.
The largest number of differentially expressed genes was observed at 24 hpi between control and SV-treated plants. The genes that were differentially expressed (P < 0.05 and fold change >1.5) were subjected to the gene ontology (GO) analysis through DAVID bioinformatics resources. List of these genes and details of the enrichment analysis (P < 0.05) of 3 GO classes (biological process, molecular function, and cellular component) are available in Supplementary Table S1. Enrichment analysis of biological process indicates that several defense-related and SA-responsive/signaling genes were up-regulated at 0 and 12 hpi in the SV-treated plants compared to control plants (Fig. 2a). Furthermore, the genes related to defense response, induced systemic resistance and cell wall organization were notably up-regulated at 24 hpi in the SV-treated plants. Interestingly, several genes related to abiotic stress response (like- osmotic, salt, high light intensity and heat) were down-regulated in the SV-treated plants after 24 hpi compared to control plants (Fig. 2a). The Venn diagrams indicate that not even a single gene was commonly up- or down- regulated at three different time points (Fig. 2b). However, there were few up- and down- regulated genes which were common between the two different time points (0 vs 12, 0 vs 24, and 12 vs 24 hpi). Totally, 22 up-regulated and 9 down-regulated genes were noted to be common while considering their expressions at least at two different time points, which were further analyzed through GO enrichment study (P < 0.05). Here too, the up-regulated common genes were enriched with SA responsive/signaling GO term (Fig. 2c). At 0, 12 and 24 hpi, a total of 7, 35 and 93 genes were up-regulated (fold change > 2) compared to control plants, respectively; while 14, 34 and 112 genes were down-regulated (fold change > 2), respectively (Supplementary Table S2). A total of 280 differentially expressed (2 fold as threshold) genes were hierarchically clustered with Z-score normalization. Seven clusters were identified based on the expression kinetics of these genes (Fig. 3). Simultaneously, GO enrichment study of each cluster was performed at P < 0.05. Details of the enrichment analysis of 3 GO classes are available in Supplementary Table S2. Defense-responsive and SA signaling pathway genes mainly belong to the cluster 3 and 4 (Fig. 3). Broadly, the genes of these two clusters were up-regulated at 12 hpi in the SV-treated plants compared to control plants. Systemic acquired resistance (SAR) and cell wall organization /biogenesis related genes belong to clusters 5 and 6 where the genes were broadly up-regulated at 24 hpi in the SV-treated plants (Fig. 3).
Gene ontology enrichment analysis of the differentially expressed genes after infection with B. cinerea in A. thaliana exposed to 1000 Hz sound vibration. (a) The differentially expressed genes (P < 0.05, fold change > 1.5) were categorized according to gene ontology (GO) biological process. The detailed analyses of all GO classes are available in Supplementary Table S1. (b) Venn diagram represents the number of overlapping genes between the analyzed time points. The time (0, 12, and 24 h) indicates h post inoculation (hpi). Up and down indicate the up- and down- regulated genes respectively at given time points. (c) Enriched GO of biological process within the list of genes found to be common at least in two different time points. Red and green color bars indicate up- and down- regulated genes, respectively. GO enrichment analysis was performed through DAVID bioinformatics resources at P < 0.05. Microarray analysis was performed with three biological replications.
Hierarchical clustering of differentially expressed genes after infection with B. cinerea in A. thaliana exposed to 1000 Hz sound vibration. A total of 280 differentially expressed (2 fold as threshold) genes were classified in seven clusters (1 to 7) through hierarchical clustering with Z-score normalization. Red and green colors indicate up- and down- regulated genes, respectively. Enriched gene ontology of biological process (P < 0.05) were mentioned in every cluster. Details of the genes and enrichment analysis of all GO classes are available in Supplementary Table S2. Line graphs at bottom indicate the fold change of the genes of each cluster. The time (0, 12, and 24 h) indicates h post inoculation (hpi).
Validation by qRT-PCR
Microarray results were validated using qRT-PCR in whole plant system (Fig. 4). A total of twenty genes (fold change >2) involved in disease resistance were selected for qRT-PCR confirmation. For a quick reference, the fold change based on microarray results and functions of these genes are mentioned in Table 1. TCH4, a touch-inducible gene, was induced in the SV-treated plants compared to control plants at 0 and 24 hpi. Three genes (ARD3, AIG1, and GASA6) were strongly up-regulated at 24 hpi, although they were down-regulated at 0 hpi; while seven genes (WRKY51, DMR6, MYB29, LECTIN, RLP53, WRKY38 and NUDX6) were up-regulated at 12 and 24 hpi in the SV-treated plants. AIG1 is involved in recognition of bacterial pathogens carrying avrRpt2, the avirulence gene18. Expression of DMR6 can be induced by SAR and benzothiadiazole (BTH)19. LECTIN has vital role in plant defense against pathogens and predators20. Moreover, lectin-like protein (SAI-LLP1) in Arabidopsis is involved in SA-mediated immunity against Pseudomonas syringae 21. RLP53 encodes a member of receptor-like proteins (RLPs) which are involved in early pathogen recognition and activation of protective immune signaling in plants22. NUDX6 has significant impact on plant immune response as a positive regulator of NPR1-dependent SA signaling pathways23. Both WRKY51 and WRKY38 seem to have a potential role in SA-mediated downstream signaling24. MYB29 is involved in the biosynthesis of aliphatic glucosinolate, a sulphur containing defense molecules25. ARR6, an important member of cytokinin (CK) signaling network, showed higher level in the SV-treated plants than control at all-time points after fungal inoculation. MDAR3, LTP, and TPS4, were up-regulated in the SV-treated plants at 0 and 24 hpi. LTP is a bacterial flagellin (flg22)-induced gene26. CYP71A13, GRX480, FMO1, and PBS3 were up-regulated at 12 hpi, while PME41 was strongly up-regulated at 24 hpi in the SV-treated plants compared to control. PME41 synthesizes a member of pectin methylesterases that has an important role in active immune response27. PBS3 (also known as GH3.12) is important for pathogen-induced SA accumulation, and directly regulates the synthesis of important upstream molecule of SA28. FMO1, has a crucial role in basal resistance to invasive virulent pathogens and functions as a positive regulator of EDS1, an important player in SA signaling29. GRX480 is SA-inducible and requires NPR1 (also known as NIM1 or SAI1)30. On the basis of our microarray and qRT-PCR data, the overall defense mechanism in SV-treated Arabidopsis appears to be effectively triggered at early stage (12 and 24 hpi) of infection and was mediated by SA signaling.
Validation of microarray data using qRT-PCR. Microarray results of twenty representative genes are confirmed by qRT-PCR. Expression of each gene in the A. thaliana exposed to a 1000 Hz sound vibration (gray) was compared with that of control plants (black). AtGAPDH was used for normalization. Bars represent mean and standard error of three biological replications. The time (0, 12, 24 and 48 h) indicates h post inoculation (hpi). Details of genes and their fold changes in microarray experiment are available in Table 1. P-value ranges are marked by asterisks: *** P < 0.01, ** 0.01 < P < 0.05, * P < 0.1.
SV treatment with 1000 Hz affects B. cinerea pathogenicity and growth in A. thaliana
SV treatment increases plant disease resistance, which might be due to the alteration of plant defense mechanism, reduced pathogenicity of B. cinerea, or combination of both. To test this hypothesis, we analyzed gene expression patterns of B. cinerea using qRT-PCR in detached leaves inoculated with fungal spore (Fig. 5). The transcript level of B. cinerea PME1 (BcPME1), encoding pectin methyltransferase that is a crucial determinant of B. cinerea virulence, was down-regulated in SV-treated plant compared to control. The transcript level for BcBOT1, encoding cytochrome P450 monooxygenase involved in B. cinerea phytotoxin (botrydial) synthesis, was significantly lower in SV-treated plants than control at 12, 24 and 48 hpi. Three genes encoding chitin synthases (BcCHSI, BcCHSIIIa and BcCHSIV) showed similar expression patterns, where they were significantly up-regulated at early time points (12 and 24 hpi) and down-regulated at later time points (48 and 72 hpi) in SV-treated plants. B. cinerea ACTIN transcript (BcACT) level relative to A. thaliana ACTIN (AtACT) was calculated to estimate the fungal growth in planta. BcACT was less in the SV-treated plants than control from 24 to 48 hpi, suggesting that the induced plant defense by SV treatment reduced fungal growth inside the host cell.
Expression patterns of fungal virulence genes after infection with B. cinerea in A. thaliana exposed to 1000 Hz sound vibration. Five representative genes for fungal growth and virulence were tested at four time points (12, 24, 48, 72 h post inoculation). Expression of each fungal gene in the Arabidopsis exposed to a 1000 Hz sound vibration (gray) was compared with control (black) after normalization with B. cinerea ACTIN (BcACT). Accumulation of BcACT transcript in the inoculated leaves was calculated relative to A. thaliana ACTIN (AtACT) transcript. Bars represent mean and standard error of three biological replications. BcPME1, encoding pectin methyltransferase; BcBOT1, encoding cytochrome P450 monooxygenase; BcCHSI, BcCHSIIIa and BcCHSIV, encoding chitin synthases. P-value ranges are marked by asterisks: *** P < 0.01, ** 0.01 < P < 0.05, * P < 0.1.
SA-mediated response is primarily induced in 1000 Hz-treated A. thaliana during B. cinerea infection
Transcriptomic analyses indicate the involvement of SA –mediated signaling and subsequent resistance in the SV-treated plants. In addition, JA also plays a critical role in plant immunity and generally shows antagonism to the SA-mediated defense pathway31. Hence, the accumulation of SA and JA was estimated in order to ascertain their role in SV-induced fungal resistance (Fig. 6). Simultaneously expression of genes involved in SA- and JA-defense signaling was quantified (Fig. 7 and Supplementary Fig. S3). SA level in the SV-treated plants was increased compared to control plants during fungal infection from 12 to 72 hpi (Fig. 6). Moreover, increased SA concentration was observed after SV treatment relative to the untreated control at 0 h. Similarly, transcript levels of genes involved in SA-defense signaling (PAD4, EDS1 and EDS5)32 were more abundant in the SV-treated plants related to control at 12 and 24 hpi (Fig. 7). In addition, NPR1, which is primarily involved in downstream of SA signaling, was also induced at 0 hpi in the SV-treated plants. In contrast, JA level in the SV-treated plants remained stable during the infection, while it was gradually induced in control plants (Fig. 6). Transcript levels of PDF1.2, a JA-regulated gene, and its upstream regulator ERF1 31 were reduced in the SV-treated plants relative to control at 24 to 72 hpi (Fig. 7). Unlike PDF1.2, VSP1 was more abundant in the SV-treated plants at 24 and 48 hpi, which is one of the JA-inducible markers.
Salicylic acid (SA)- and jasmonic acid (JA)- contents after infection with B. cinerea in A. thaliana exposed to 1000 Hz sound vibration. Analysis of SA and JA contents was performed by liquid chromatography-mass spectrometry (LC-MS) and shown in a line graph, where red and blue colors indicate SV-treated and control plants respectively. Analysis was performed with three biological replications. P-value ranges are marked by asterisks: *** P < 0.01, ** 0.01 < P < 0.05, * P < 0.1.
Expression pattern of the genes involved in SA- and JA-defense signaling pathways. Expression patterns are shown in colored panel where red, green, and black colors indicate transcript levels with induction, reduction and no-difference, respectively based on qRT-PCR results (Supplementary Fig. S3). The boxes within a panel indicate the expression levels of each gene in SV-treated plants compared to control during 0 to 72 h post infection (hpi). The genes marked in red color are based on the microarray results (Table 1). Pointed arrows indicate activation and blunt arrows indicate repression. All experiments were performed with three biological replications. P-value ranges are marked by asterisks: *** P < 0.01, ** 0.01 < P < 0.05, * P < 0.1.
Discussion
In this study, we demonstrated that the application of single frequency SV (1000 Hz) in Arabidopsis could induce resistance upon infection of necrotrophic fungal pathogen, B. cinerea (Fig. 1). Stronger defense response and up-regulation of defense genes in the SV-treated plants compared to that of control plants can be explained by priming13. In addition to the well-known chemical agents (like- SA, azelaic acid, proline, β-aminobutyric acid)33, mechanical stimuli has been reported to prime plants and improve the tolerance against stresses in various vegetables like bean, maize, tomato, and cucumber15. It has been demonstrated that SV-treatment can alter the gene expressions, hormonal levels and enzyme activities in higher plants6, 9, which eventually may result in increased defense metabolites and subsequent priming. The present study indicates that SV treatment could induce gene expression (TCH4, LTP, MDAR3, and GRX480) and prime Arabidopsis for potentiated gene expression (AIG1, WRKY51, DMR6, MYB29, LECTIN, RLP53, WRKY38, NUDX6, FMO1, PBS3, and PME41) in response to subsequent infection by B. cinerea (Fig. 4). Apparently, most of the potentiated defense genes (NUDX6, WRKY51, WRKY38, PBS3, GRX480, LECTIN, DMR6, PAD4, EDS1, and EDS5) are associated with SAR and/or SA-mediated response and significantly up-regulated at 12 and 24 hpi in the SV-treated plants compared to control plants (Figs 4 and 7). Furthermore, microarray analysis indicates that the up-regulated genes are significantly enriched with SA responsive/signaling GO term (Fig. 2a). Microarray data suggests the induction of several well-known defense-related genes (like-AZI1, NHL25, PR1 and NIMIN1) in the SV-treated plants during the infection (Table 1 and Fig. 7). AZI1, encoding a lipid transfer protein (LTP), has a prominent role in systemic immunity priming in distal tissues34. NHL25, a SA-induced gene, is assumed to be involved in pathogenesis-related (PR) gene expression35. Up-regulation of PR1 and NIMIN1 was observed in the SV-treated plants which are SA-induced genes and can be controlled by NPR123. NPR1was up-regulated by SV at 0 h (Fig. 7), which is involved in priming13. Overall, the transcript analyses indicate that SV might prime plants by SA-mediated response accompanied with SAR.
Recently, we have demonstrated that short-term exposure of SV treatment induces SA accumulation in Arabidopsis9. Increased level of SA in the SV-treated plants compared to the control plants as observed in this study further strengthens the likeliness of SV providing an impetus to the SA-mediated priming (Fig. 6). Up-regulation of MDAR3, a SA-induced gene36 and down-regulation of GASA6, a SA-repressed gene37 also corroborates with the higher concentration of SA in the SV-treated plants compared to control plants at 0 h (Figs 4 and 6). Increased concentration of SA along with stable production of JA in the SV-treated plants during infection process (Fig. 6), exhibits a non-antagonistic interaction between these two hormones. Although, the antagonistic relationship between SA and JA is often reported, synergistic interactions between these two hormones during immune response are also well-documented31, 38, 39. Furthermore, the genes involved in SA or JA defense signaling were co-upregulated in control as well as SV-treated plants after Botrytis challenge (Supplementary Fig. S3). However, the transcript levels of SA-signaling genes (PAD4, EDS1, and EDS5)32 were more abundant and those of JA- signaling genes (ERF1 and PDF1.2)31 was less abundant in the SV-treated plants compared to the control plants (Fig. 7 and Supplementary Fig. S3). NPR1 has an important role in SA/JA crosstalk, such as suppression of PDF1.2 by GRX480 depending on the presence of TGA factors in planta 30. High abundance of NPR1 and GRX480 transcripts at 0 and/or 12 hpi might be responsible for reduced accumulation of PDF1.2 in the SV-treated plants, related to control plants. Similarly, lesser amount of JA in SV-treated plants caused lower abundance of ERF1, compared to control plants. Although high accumulation of VSP1 in SV-treated plants at 24 and 48 hpi, it was down-regulated at other time points (Fig. 7). VSP1 is well-known as a JA-inducible marker, which may have a role in defense against herbivores40. Thus, VSP1 could be independently induced for priming against B. cinerea. Accumulation of both SA and JA was co-upregulated after Botrytis infection in control plants (Fig. 6). The concentration of JA was almost stable in SV-treated plants after Botrytis challenge. Interestingly, SA and/or its derivatives shows fungistatic effect on Botrytis 41. Therefore, elevated SA levels at 0 hpi in the SV-treated plants may hinder the successful Botrytis infection compared to control plants. Seemingly, the already triggered effective SA defense metabolism is enough to impede the disease progression in SV-primed plants, compared to the surging of two defense hormones together. After all, production of defense hormone requires huge energy42. On the other hand, co-upregulation of JA- and SA- defense signaling genes in the SV-treated plants after Botrytis infection might be the result of an unavoidable synergistic effect at upstream immune response. It is well-known that exogenous application of SA or its chemical analog BTH can induce resistance against B. cinerea in several plant species43. SA and BTH are considered as SAR inducers, which potentiate plants for priming13. However, exogenous SA treatment could increase resistance to B. cinerea through an apparently different mechanism than that involved in establishing the localized resistance observed in untreated plants. Overall, plants with SA- and SAR-mediated defense mechanisms can build a resistance against necrotrophic pathogens. Therefore, enhanced SA level by SV treatment might be able to protect plants from pathogens temporarily. Nonetheless, further efforts are required to establish how SA predominantly controls the defense mechanism in SV-treated plants.
Arabidopsis mutants with constitutive production of SA or defense gene expression displayed reduced growth and reproduction, while knock-out mutants blocked in induced resistance increased their growth under pathogen-free conditions44. However, primed plants have advantages over plants with direct induction of defense response in cost of fitness such as growth and yield45. In this study, we did not find any significant morphological changes after SV treatment, which might be advantageous.
It is believed that cytokinin (CK) has synergistic effect on SA signaling network and plant defense46. There are two types of ARRs: type A is a negative regulator of cytokinin signaling and type B is a positive regulator46. ARR6, a type A ARR, is an important member of CK signaling network. In our experiment, continuous up-regulation of ARR6 (Fig. 4), indicates the possibility of CK-independent immune response, instead of SA/CK-mediated synergistic defense or balancing between downstream CK signaling and other defense hormone crosstalk.
In general, defense responses against phytopathogens are energy-consuming processes45. Therefore, energy metabolism needs to be reconfigured to support the increased demands for plant defense process. Reduced expression of abiotic stress related genes in the SV-treated plants at 24 hpi (Fig. 2a), might be one of the strategies to increase energy efficiency for defense metabolism. Simultaneously, the up-regulation of CYP71A13 and MYB29 was noted in the SV-treated plants at early stage of infection (12 and/or 24 hpi) which are involved in camalexin47 and glucosinolate25 biosynthesis, respectively (Fig. 4). In addition, ARD3, which is involved in methionine biosynthesis (according to gene ontology), a precursor of aliphatic glucosinolate25, was up-regulated in the SV-treated plants at 24 hpi. Hence, increase in defense molecules like camalexin and glucosinolates could be one of the reasons behind induced resistance in the SV-treated plants. Corroboratively, Arabidopsis rosette pre-exposed to SV caused by feeding of caterpillar exhibited effective accumulation of glucosinolate and anthocyanin which increased the defense response against herbivore4.
Fungal spores require sufficient time for adhesion, germination, penetration and hyphal propagation into the leaf which leads to the successful infection. Botrytis conidiospores require ~24 h for germination and subsequent penetration into the plant tissue48, 49 which causes more differential expression of genes at 24 hpi compared to 12 hpi as observed through microarray analysis (Fig. 2b). Moreover, the up-regulation of several genes, involved in cell wall biogenesis, organization, biosynthesis of wall components (like- pectin and xyloglucan) and cutin, was observed in the SV-treated plants at 24 hpi (Figs 2a and 3). This finding indicates sturdy reinforcement of the cell wall in the SV-treated plants as one of the defense strategies to prevent pathogen invasion50. Simultaneously, B. cinerea virulence genes were broadly down-regulated in the SV-treated plants compared to control plants after the fungal inoculation (Fig. 5). B. cinerea mutants at BcPME1, BcBOT1, BcCHSIIIa and BcCHSI loci showed reduced colonization phenotype in planta 48 which suggests their importance for successful infection to the host. Taken together, it can be stated that SV-treatment make plants more efficient to combat early infection events and reduces Botrytis growth temporally.
Certain perturbations with mechano-stimulation are preceded by the perception of a stimulus and signal transduction cascade. Changes in gene expression are one of the indicators of signal transduction cascade. Previously touch–induced genes (TCH) were identified upon exposure of various mechanical stimulation like touch and wind51. TCH1, TCH2 and TCH3 encode for calmodulin or calmodulin-like proteins, and TCH4 encodes XTH51. Even SV treatment with audible frequencies also induced the expression of TCH genes in Arabidopsis9, 52. Thus, induction of the TCHs can be considered as a signature response to mechano-stimuli. It was hypothesized that soft mechanical stress (SMS) like touch could be perceived by membrane-bound mechanosensors with simultaneous triggering of damage-associated molecular patterns (DAMPs) mediated signaling, which lead to TCH expression and innate immune response17. We checked the expression level of three DAMP-marker genes (PROPEP2, PROPEP3, and prePIP1)53 to know whether SV can induce the DAMP-mediated signaling (Supplementary Fig. S4). SV-mediated up-regulation of PROPEP2 and PROPEP3 was observed at 0 h compared to control. Simultaneously, the up-regulation of TCH4 was noted in the SV-treated plants compared to control at 0 h (Fig. 4). It is known that XTHs encode cell-wall modifying enzymes which catalyze the cleavage of xyloglucan polymers54. Previous studies showed that SV treatment could change cell wall fluidity, secondary structure of cell wall proteins, and ultrastructure of the membrane6. In response to SV, XTH could function as breaking the xyloglucan chains and making the cell wall to have more elasticity possible, which later might turn on downstream signaling cascade. However, in-depth study regarding interaction between cytoskeleton-plasma membrane-cell wall interface and SV stimulation is still required. SV causes plant leaves to vibrate which may mimic wound-mediated downstream signaling and JA accumulation55. Nevertheless, reduced concentration of JA in the SV-treated plants compared to control plants (Fig. 6), suggests that SV-mediated priming is distinct from wound-mediated priming at molecular level.
In summary, SV-primed Arabidopsis plants delayed the infection process against B. cinerea through SA- and SAR-mediated pathways. Previously, it has been noted that pretreatment with vibrations caused by chewing sound of caterpillar can induce Arabidopsis chemical defense against herbivore4. Interestingly, complex frequency profile for insect chewing and leafhopper song identifies the highest amplitude occurring between 0 - 1000 Hz4. Another study indicated that exposure to natural SV (like-green music) elevates the polyamines content, a chemical priming agent33, in Chinese cabbage5. Therefore, future experiments with natural SV are required to establish the ecological relevance of plant-acoustic research. Simultaneously, these findings can further apply to biotrophic pathogens where resistance is almost SA-dependent. Choosing biotrophic fungus instead of necrotrophic B. cinerea, could show stronger resistance in SV-primed plants. Comprehensive studies on SV-treated plants are warranted in future, that could open avenues in green technology for plant resistance against biotic and abiotic stresses.
Methods
Plant materials and SV treatment
Arabidopsis thaliana (Columbia-0) seeds were sown in soil mixture (Punong, Korea) and kept at 4 °C for 2 days in the dark, then transferred to growth room and grown under continuous light (~150 μmol m−2 s−1) at 23 ± 1 °C. The 14-day-old plants were exposed to SV with three separate frequencies (500, 1000 and 3000 Hz) at constant amplitude (100 dB) for daily 3 h up to 10 days in a specialized sound-proof chamber without light. The control 14-day-old plants were kept in a similar sound-proof chamber without SV exposure (daily 3 h) up to 10 days. Control and SV-treated plants were transferred back to the growth room after the daily 3 h treatment. Sound-proof chamber was customized by Korea Scientific Technique Industry as mentioned previously56 and single frequency SV was generated by Adobe Audition version 3.0 software. After 10 days, rosette leaves from SV-treated and control plants were harvested in liquid nitrogen, stored at −80 °C, and marked as 0 h sample. The rest of the SV-treated and control plants were used to inoculate with Botrytis cinerea. Inoculated plants were transferred to growth room for harvesting infected samples at various time points. A schematic diagram shown in Supplementary Fig. S1 represents the SV treatment methodology, pathogen inoculation and sample harvesting time.
Fungal culture and disease resistance assay
B. cinerea obtained from Korea Agricultural Culture Collection (KACC) 40573 was grown on potato dextrose agar (PDA, Difco) at 23 ± 1 °C. Conidiospores were isolated according to the previous method57. For whole plant experiment, conidiospore suspension (3 ml) in potato dextrose broth (PDB, 5 × 105 spores ml−1) was sprayed to both SV-treated and control plants. After spraying, the plants were grown in the growth room with transparent lid to maintain humidity. At 72 hpi, disease level was calculated in whole plant as follows: percentage of disease level (%) = (number of fully senescent leaves / total number of leaves) × 100. Rosette leaves were harvested 12, 24, 48, and 72 hpi. These samples were used for microarray, qRT-PCR, and hormone analysis.
For the experiment with detached leaves, the leaves in similar developmental stage from SV-treated and control plants were used and placed on two layers of wet Whatman filter paper in Petri dish for disease resistance assay. One drop (5 µl) of conidiospores (5 × 105 spores ml−1 in PDB) was applied to the middle of adaxial side of leaves. The Petri dish was covered with transparent lid and kept in the growth room.
Microarray and qRT-PCR
Total RNA was isolated from Arabidopsis rosette leaves at 0, 12 and 24 hpi using Qiagen RNeasy Plant Mini Kit according to the manufacturers’ instructions. RNA quality was examined by Agilent 2100 Bioanalyzer and quantity was determined by ND-1000 spectrophotometer (NanoDrop Technologies Inc.). RNA was labeled and hybridized to Arabidopsis Gene 1.1 ST Array according to the manufacturer’s instructions (Affymetrix). Array was scanned at GeneAtlas station and normalized based on standard Robust Multi-Average (RMA) algorithm. All microarray data have been deposited in the ArrayExpress database (https://www.ebi.ac.uk/arrayexpress/; accession no. E-MTAB-4077). For hierarchical clustering, fold change data of selected genes were processed using a commercial software package (MATLAB 7.14, The MathWorks Inc., Natick, MA, 2000). Z-score transformation, the classical method of microarray data normalization, was employed to normalize the variance in the gene expression. The hierarchical clustering of the variance in gene expression over the time was performed to reveal the co-regulated and functionally related genes by using cluster algorithms developed by Eisen et al.58. The gene expression level presented as grid of colored points are shown as red-green heatmap (Fig. 3). GO enrichment analysis of selected genes was performed through DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/).
Microarray results were confirmed using qRT-PCR analysis. Total RNA (1 µg) was used to generate cDNA using cDNA synthesis kit (Promega). The qRT-PCR analysis was performed as described previously59 using Mx3000P QPCR system (Agilent) with SYBR Green QPCR Master Mix (LPS solution). The expression patterns of twenty Arabidopsis genes, which were selected from microarray results and additional seven genes that are involved in JA- or SA- defense signaling, were reconfirmed using qRT-PCR. The expression levels of transcripts were normalized to Arabidopsis GAPDH (At1g13440) encoding glyceraldehyde 3-phosphate dehydrogenase as follows: ∆CT = (CT.GOI)- (CT. GAPDH); where (CT.GOI) and (CT. GAPDH) indicate threshold cycles for genes of interest (GOI) and GAPDH, respectively, for each replication60. Relative transcript levels of each gene were calculated with respect to GAPDH transcript levels (% relative expression to GAPDH) using 2−∆CT value [2−∆CT × 100] and plotted in graph60. Transcript levels of six B. cinerea genes (PME1, BOT1, CHSI, CHSIIIa, CHSIV and ACT) were also tested. Arabidopsis ACTIN (At3g18780) and B. cinerea ACTIN (AJ000335) were used to assess B. cinerea mycelial growth in planta. Expression level of fungal growth and virulence genes were normalized to B. cinerea ACTIN (BcACT) and transcript level of BcACT was calculated relative to A. thaliana ACTIN (AtACT). Primer 3 software from Biology Workbench (http://workbench.sdsc.edu/) was used to design primers. Primer details are available in Supplementary Table S3. Three independent biological replicates were used for all experiments.
Hormonal profiling using HPLC-MS and ESI-MS/MS
Analysis of hormonal changes was performed as described previously61. JA (Sigma-Aldrich) and SA (Tokyo Chemical Industry Co.) were used as internal standards (IS). The stock solutions of JA and SA were prepared in 100% methanol at a concentration of 1 mg ml−1. The experiments were conducted using Agilent 1100 series liquid chromatography (LC) system equipped with a degasser, pump, auto sampler, and column oven. SunFire C18 column (2.1 × 10 mm, Waters) was used for chromatographic separations. The mobile phase consisted of isocratic mobile phase of 15:85 v/v 0.1% formic acid in water, 0.1% formic acid in methanol, at a flow rate of 300 µl min−1, column temperature of 30 °C. Sample injection was 10 µl for all experiments. MS condition was as follows: 500 °C source temperature, 5.5 kV (positive), −4.5 kV (negative) ion spray voltages, 3 collision gas (CAD), 15 curtain gas (CUR), 45 ion source gas 1 and 2, and 150 ms dwell time. Unit resolution was used for Q1 and Q3. For linearity, JA and SA standard solutions were prepared at a concentration of 0.25, 0.5, 1.0, 2.5, 5.0, 50, and 500 ng ml−1. Calibration curves were measured using the ratio of JA/IS and SA/IS area versus the ratio of JA/IS and SA/IS concentration. Each calibration curve was studied separately by using 1/× weighted linear regression, constructed by determining each hormone/IS peak area ratio versus each hormone/IS concentration ratio. The regression equations for the analysis were as follows: Y = 0.242X + 0.0171 (r = 0.9994) for JA, and Y = 0.704X + 0.115 (r = 0.9927) for SA using a weight factor of 1/×. The limit of detection (LOD) was determined using a signal-to-noise ratio of 3, and the limit of quantification (LOQ) was calculated using a signal-to-noise of 10. The LODs of JA and SA were 0.15 ng ml−1. The LOQs of JA and SA were 0.5 ng ml−1. Three independent biological replicates were used for hormone analysis.
Statistical analysis
Statistical analyses with 3 independent biological replicates were performed using Student’s t test at P < 0.05. P-value ranges are marked by asterisks: *** P < 0.01, ** 0.01 < P < 0.05, * P < 0.1.
References
Chehab, E. W., Eich, E. & Braam, J. Thigmomorphogenesis: a complex plant response to mechano-stimulation. Journal of experimental botany 60, 43–56, doi:10.1093/jxb/ern315 (2009).
Mishra, R. C., Ghosh, R. & Bae, H. Plant acoustics: in the search of a sound mechanism for sound signaling in plants. Journal of experimental botany, doi:10.1093/jxb/erw235 (2016).
De Luca, P. A. & Vallejo-Marin, M. What’s the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Current opinion in plant biology 16, 429–435, doi:10.1016/j.pbi.2013.05.002 (2013).
Appel, H. M. & Cocroft, R. B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175, 1257–1266, doi:10.1007/s00442-014-2995-6 (2014).
Qin, Y. C., Lee, W. C., Choi, Y. C. & Kim, T. W. Biochemical and physiological changes in plants as a result of different sonic exposures. Ultrasonics 41, 407–411, doi:10.1016/S0041-624X(03)00103-3 (2003).
Hassanien, R. H. E., Hou, T. Z., Li, Y. F. & Li, B. M. Advances in Effects of Sound Waves on Plants. J Integr Agr 13, 335–348, doi:10.1016/S2095-3119(13)60492-X (2014).
Bochu, W., Jiping, S., Biao, L., Jie, L. & Chuanren, D. Soundwave stimulation triggers the content change of the endogenous hormone of the Chrysanthemum mature callus. Colloids and surfaces. B, Biointerfaces 37, 107–112, doi:10.1016/j.colsurfb.2004.03.004 (2004).
Gagliano, M., Mancuso, S. & Robert, D. Towards understanding plant bioacoustics. Trends in plant science 17, 323–325, doi:10.1016/j.tplants.2012.03.002 (2012).
Ghosh, R. et al. Exposure to Sound Vibrations Lead to Transcriptomic, Proteomic and Hormonal Changes in Arabidopsis. Scientific reports 6, 33370, doi:10.1038/srep33370 (2016).
Ghosh, R. et al. Expression Analysis of Sound Vibration-Regulated Genes by Touch Treatment in Arabidopsis. Frontiers in plant science 8, 100, doi:10.3389/fpls.2017.00100 (2017).
Qi, L., Teng, G., Hou, T., Zhu, B. & Liu, X. in Computer and Computing Technologies in Agriculture (III) Vol. 317 449–454 (Springer Berlin Heidelberg, 2010).
Jeong, M. J. et al. Sound Frequencies Induce Drought Tolerance in Rice Plant. Pak J Bot 46, 2015–2020 (2014).
Conrath, U., Pieterse, C. M. & Mauch-Mani, B. Priming in plant-pathogen interactions. Trends in plant science 7, 210–216, doi:10.1016/S1360-1385(02)02244-6 (2002).
Pastor, V., Luna, E., Mauch-Mani, B., Ton, J. & Flors, V. Primed plants do not forget. Environmental and Experimental Botany 94, 46–56, doi:10.1016/j.envexpbot.2012.02.013 (2013).
Li, Z. G. & Gong, M. Mechanical Stimulation-Induced Cross-Adaptation in Plants: An Overview. J Plant Biol 54, 358–364, doi:10.1007/s12374-011-9178-3 (2011).
Chehab, E. W., Yao, C., Henderson, Z., Kim, S. & Braam, J. Arabidopsis Touch-Induced Morphogenesis Is Jasmonate Mediated and Protects against Pests. Curr Biol 22, 701–706, doi:10.1016/j.cub.2012.02.061 (2012).
Benikhlef, L. et al. Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC plant biology 13, 133, doi:10.1186/1471-2229-13-133 (2013).
Reuber, T. L. & Ausubel, F. M. Isolation of Arabidopsis genes that differentiate between resistance responses mediated by the RPS2 and RPM1 disease resistance genes. Plant Cell 8, 241–249, doi:10.1105/tpc.8.2.241 (1996).
van Damme, M., Huibers, R. P., Elberse, J. & Van den Ackerveken, G. Arabidopsis DMR6 encodes a putative 2OG-Fe(II) oxygenase that is defense-associated but required for susceptibility to downy mildew. Plant J 54, 785–793, doi:10.1111/j.1365-313X.2008.03427.x (2008).
Peumans, W. J. & van Damme, E. J. The role of lectins in plant defence. The Histochemical J 27, 253–271, doi:10.1007/BF00398968 (1995).
Armijo, G. et al. A salicylic acid-induced lectin-like protein plays a positive role in the effector-triggered immunity response of Arabidopsis thaliana to Pseudomonas syringae Avr-Rpm1. Molecular Plant-Microbe Interactions 26, 1395–1406, doi:10.1094/MPMI-02-13-0044-R (2013).
Tor, M., Lotze, M. T. & Holton, N. Receptor-mediated signalling in plants: molecular patterns and programmes. J Exp Bot 60, 3645–3654, doi:10.1093/jxb/erp233 (2009).
Ishikawa, K. et al. AtNUDX6, an ADP-ribose/NADH pyrophosphohydrolase in Arabidopsis, positively regulates NPR1-dependent salicylic acid signaling. Plant physiology 152, 2000–2012, doi:10.1104/pp.110.153569 (2010).
Eulgem, T. & Somssich, I. E. Networks of WRKY transcription factors in defense signaling. Current opinion in plant biology 10, 366–371, doi:10.1016/j.pbi.2007.04.020 (2007).
Yatusevich, R. et al. Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J 62, 1–11, doi:10.1111/j.1365-313X.2009.04118.x (2010).
Qutob, D. et al. Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18, 3721–3744, doi:10.1105/tpc.106.044180 (2006).
Bethke, G. et al. Arabidopsis PECTIN METHYLESTERASEs Contribute to Immunity against Pseudomonas syringae. Plant Physiol 164, 1093–1107, doi:10.1104/pp.113.227637 (2014).
Chen, Z., Zheng, Z., Huang, J., Lai, Z. & Fan, B. Biosynthesis of salicylic acid in plants. Plant signaling & behavior 4, 493–496 (2009).
Bartsch, M. et al. Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the nudix hydrolase NUDT7. Plant Cell 18, 1038–1051, doi:10.1105/tpc.105.039982 (2006).
Ndamukong, I. et al. SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. The Plant journal: for cell and molecular biology 50, 128–139, doi:10.1111/j.1365-313X.2007.03039.x (2007).
Pieterse, C. M., Van der Does, D., Zamioudis, C., Leon-Reyes, A. & Van Wees, S. C. Hormonal modulation of plant immunity. Annual Review of Cell and Developmental Biology 28, 489–521, doi:10.1146/annurev-cellbio-092910-154055 (2012).
Nawrath, C., Heck, S., Parinthawong, N. & Metraux, J. P. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14, 275–286, doi:10.1105/tpc.010376 (2002).
Savvides, A., Ali, S., Tester, M. & Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends in Plant Science 21, 329–340, doi:10.1016/j.tplants.2015.11.003 (2016).
Parker, J. E. The Quest for Long-Distance Signals in Plant Systemic Immunity. Sci Signal 2, pe31–pe31, doi:ARTN pe31 10.1126/scisignal.270pe31 (2009).
Varet, A. et al. NHL25 and NHL3, two NDR1/HIN1-1ike genes in Arabidopsis thaliana with potential role(s) in plant defense. Molecular plant-microbe interactions 15, 608–616, doi:10.1094/MPMI.2002.15.6.608 (2002).
Sewelam, N. et al. Ethylene Response Factor 6 Is a Regulator of Reactive Oxygen Species Signaling in Arabidopsis. PloS One 8, doi:ARTN e70289 10.1371/journal.pone.0070289 (2013).
Qu, J., Kang, S. G., Hah, C. & Jang, J. C. Molecular and cellular characterization of GA-Stimulated Transcripts GASA4 and GASA6 in Arabidopsis thaliana. Plant Sci 246, 1–10, doi:10.1016/j.plantsci.2016.01.009 (2016).
Liu, L. J. et al. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat Commun 7, doi:Artn 13099 10.1038/Ncomms13099 (2016).
Mur, L. A., Kenton, P., Atzorn, R., Miersch, O. & Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant physiology 140, 249–262, doi:10.1104/pp.105.072348 (2006).
Liu, Y. L. et al. Arabidopsis vegetative storage protein is an anti-insect acid phosphatase. Plant Physiol 139, 1545–1556, doi:10.1104/pp.105.066837 (2005).
Dieryckx, C. et al. Beyond plant defense: insights on the potential of salicylic and methylsalicylic acid to contain growth of the phytopathogen Botrytis cinerea. Frontiers in Plant Science 6, 859, doi:10.3389/fpls.2015.00859 (2015).
Vos, I. A., Pieterse, C. M. J. & van Wees, S. C. M. Costs and benefits of hormone-regulated plant defences. Plant Pathol 62, 43–55, doi:10.1111/ppa.12105 (2013).
Ferrari, S., Plotnikova, J. M., De Lorenzo, G. & Ausubel, F. M. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35, 193–205, doi:10.1046/j.1365-313X.2003.01794.x (2003).
Heil, M. & Baldwin, I. T. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends Plant Sci 7, 61–67, doi:10.1016/S1360-1385(01)02186-0 (2002).
Bolton, M. D. Primary Metabolism and Plant Defense-Fuel for the Fire. Mol Plant Microbe In 22, 487–497, doi:10.1094/Mpmi-22-5-0487 (2009).
Hwang, I., Sheen, J. & Muller, B. Cytokinin signaling networks. Annual Review of Plant Biology 63, 353–380, doi:10.1146/annurev-arplant-042811-105503 (2012).
Nafisi, M. et al. Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis. Plant Cell 19, 2039–2052, doi:10.1105/tpc.107.051383 (2007).
Choquer, M. et al. Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. Fems Microbiol Lett 277, 1–10, doi:10.1111/j.1574-6968.2007.00930.x (2007).
Shlezinger, N. et al. Anti-apoptotic machinery protects the necrotrophic fungus Botrytis cinerea from host-induced apoptotic-like cell death during plant infection. PLoS Pathogens 7, e1002185, doi:10.1371/journal.ppat.1002185 (2011).
Bellincampi, D., Cervone, F. & Lionetti, V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Frontiers in Plant Science 5, 228, doi:10.3389/fpls.2014.00228 (2014).
Braam, J. In touch: plant responses to mechanical stimuli. The New Phytologist 165, 373–389, doi:10.1111/nph.2005.165.issue-2 (2005).
Johnson, K. A., Sistrunk, M. L., Polisensky, D. H. & Braam, J. Arabidopsis thaliana responses to mechanical stimulation do not require ETR1 or EIN2. Plant Physiol 116, 643–649, doi:10.1104/pp.116.2.643 (1998).
Li, B., Meng, X., Shan, L. & He, P. Transcriptional Regulation of Pattern-Triggered Immunity in Plants. Cell host & microbe1 9, 641–650, doi:10.1016/j.chom.2016.04.011 (2016).
Campbell, P. & Braam, J. Co- and/or post-translational modifications are critical for TCH4 XET activity. Plant J 15, 553–561, doi:10.1046/j.1365-313X.1998.00239.x (1998).
Savatin, D. V., Gramegna, G., Modesti, V. & Cervone, F. Wounding in the plant tissue: the defense of a dangerous passage. Frontiers in plant science 5, 470, doi:10.3389/fpls.2014.00470 (2014).
Jeong, M. J. et al. Plant gene responses to frequency-specific sound signals. Mol Breeding 21, 217–226, doi:10.1007/s11032-007-9122-x (2008).
Mengiste, T., Chen, X., Salmeron, J. & Dietrich, R. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15, 2551–2565, doi:10.1105/tpc.014167 (2003).
Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95, 14863–14868, doi:10.1073/pnas.95.25.14863 (1998).
Bae, H. et al. The drought response of Theobroma cacao (cacao) and the regulation of genes involved in polyamine biosynthesis by drought and other stresses. Plant Physiol Bioch 46, 174–188, doi:10.1016/j.plaphy.2007.10.014 (2008).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols 3, 1101–1108, doi:10.1038/nprot.2008.73 (2008).
Pan, X. Q., Welti, R. & Wang, X. M. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5, 986–992, doi:10.1093/mp/ssr118 (2010).
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (PJ010497)”.
Author information
Authors and Affiliations
Contributions
B.C. performed the experiments shown in Figs 1, 5 and 7; R.G. performed experiment shown in Fig. 4; R.G., G.S. and J.J. analysed microarray data; D.W.B. performed the hormone analysis; H.B. supervised the experiments; S.-C.P., M.-J.J., D.W.B., and H.B. conceived the project and designed the experiments; B.C., R.G., M.A.G., K-H. H., J.K., and H.B. wrote the article with contributions of all the authors. All authors discussed the results.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, B., Ghosh, R., Gururani, M.A. et al. Positive regulatory role of sound vibration treatment in Arabidopsis thaliana against Botrytis cinerea infection. Sci Rep 7, 2527 (2017). https://doi.org/10.1038/s41598-017-02556-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02556-9
- Springer Nature Limited
This article is cited by
-
Mass spectrometry-based metabolomics study for delay tomato fruit ripening by sound waves
Journal of Analytical Science and Technology (2023)
-
Assessing the Effect of Sound Vibrations on Plant Neurotransmitters in Arabidopsis
Journal of Plant Growth Regulation (2023)
-
The role of sound stimulation in production of plant secondary metabolites
Natural Products and Bioprospecting (2023)
-
Characterization of a hypovirulent strain of Botrytis cinerea from apple and quantification of the ICs related gene expression
Mycological Progress (2021)