Abstract
Cigarette smoke is a complex chemical mixture, involving health-damaging components such as carbon monoxide, ammonia, pyridine, toluene and nicotine. While cognitive functions have been well documented in heavy smokers, spatial vision has been less characterized. In the article, we investigated smoking effects through contrast sensitivity function (CSF), a rigorous procedure that measures the spatial vision. Data were recorded from 48 participants, a group of non-smokers (n = 16), a group of chronic and heavy cigarette smokers (n = 16) and deprived smokers (n = 16); age range 20–45 years. Sinewave gratings with spatial frequencies ranging from 0.25 to 20 cycles per degree were used. All subjects were free from any neurological disorder, identifiable ocular disease and had normal acuity. No abnormalities were detected in the fundoscopic examination and in the optical coherence tomography exam. Contrary to expectations, performance on CSF differed between groups. Both smokers and deprived smokers presented a loss of contrast sensitivity compared to non-smokers. Post-hoc analyses suggest that deprived smokers were less sensitive at all spatial frequencies. These results suggest that not only chronic exposure to cigarette compounds but also withdrawal from nicotine affected spatial vision. This highlights the importance of understanding diffuse effects of smoking compounds on visual spatial processing.
Similar content being viewed by others
Introduction
Cigarette smoking consists of numerous compounds harmful to health1. Data from the World Health Organization (WHO) hypothesize that by 2030, cigarettes could kill nearly 9 million people a year around the world2, 3.
Nicotine, one psychoactive compound of cigarette smoke, is an alkaloid that binds and activates nicotinic acetylcholine receptors (nAChRs)4. There are nAChRs receptors in functional units of visual processing such as retinal cells, lateral geniculate nucleus, and primary visual cortex of numerous species5, 6.
The imbalance in the neurotransmission of acetylcholine, dopamine and glutamate as well as impairments in the receptors functioning or conformation alter visual information processing7, 8. Studies have shown that nicotinic agonists (nicotine, for example) affect the release of these neurotransmitters4, 9.
Whereas there is a lack of studies about smoking effects on spatial vision, the existing data are a little controversial and highlight the need of a rigorous testing procedure that measures spatial vision10,11,12. In addition, there is a need to understand which (if any) mechanisms involve cigarette toxicity in sensory integration. Therefore, we first need to understand how smoking can change early visual processing.
Contrast sensitivity function (CSF), an important measure of visual function and the first analysis performed by the visual cortex13, 14, may be used to assess the performance of the visual system. Luminance contrast differences, which are perceived as lightness differences, can be identified and quantified through spatial frequency responses13, 14.
Some benefits from the use of acute nicotine were reported in some studies that investigated cognitive functions such as memory and attention reported. However, it is interesting to point out that these same cognitive functions are affected due to the nicotine chronic intake in the form of tobacco addiction. Since vision may be a gateway to cognition15, understanding changes in primary visual processing may help promote smoke cessation strategies.
Based on this information, the purpose of the present study was to investigate the influence of chronic heavy smoking and smoking abstinence on spatial vision.
Results
In this study, CSF is expressed as the reciprocal logarithm of the threshold contrast. There was a statistically significant difference in CSF among all groups, χ²(2) = 14.66, p < 0.001. Contrast sensitivity peak values occurred at spatial frequencies of 2.5 cpd and 5 cpd with decays at low (0.2 cpd) and high (10 and 20 cpd) spatial frequencies.
Psychophysical measurements
The results of the psychophysical contrast sensitivity measurements are shown in Fig. 1. The non-smokers group had significantly higher sensitivity than deprived smokers at all spatial frequencies, 0.2 cpd (U = 91, p = 0.001, r = −0.70), 2.5 cpd (U = 92, p = 0.001, r = −0.69), 5.0 cpd (U = 94, p = 0.003, r = −0.66), 10.0 cpd (U = 81, p < 0.001, r = −0.82) and 20.0 cpd (U = 96, p = 0.002, r = −0.64). Thus, non-smokers needed 0.37, 0.35, 0.34, 0.43 and 0.32 log units less contrast for low, mid and high spatial frequencies, respectively.
Contrast sensitivity curves as a function of spatial frequency (cpd) for non-smokers, smokers and deprived smokers. Each data point represents the sensitivity (reciprocal of contrast threshold) and error bars represents the standard deviation (SD) of the median sensitivity based on 1000 bootstrap resamplings. CSF is plotted in logarithmic units.
The CSF of deprived smokers was significantly lower than smokers were at spatial frequencies of 0.2 cpd (U = 110, p = 0.019, r = −0.48), 2.5 cpd (U = 109, p = 0.018, r = −0.48), 5.0 cpd (U = 107, p = 0.013, r = −0.51), and 10.0 cpd (U = 110, p = 0.021, r = −0.47) except for 20.0 cpd (U = 124, p = 0.133). In general, deprived smokers needed 0.24, 0.24, 0.25 and 0.23 log units more contrast than smokers did for 0.2 cpd, 2.5 cpd, 5.0 cpd and 10.0 cpd, respectively.
Briefly, post-hoc test showed statistically significant differences between non-smokers and smokers at spatial frequencies of 5.0 cpd (U = 116, p = 0.025, r = −0.46), 10.0 cpd (U = 117, p = 0.030, r = −0.44) and 20.0 cpd (U = 97, p = 0.002, r = −63). There were no differences between 0.2 cpd (U = 126, p = 0.145) and 2.5 cpd (U = 131, p = 0.455).
Correlation analysis of demographics data
There is no relationship between CSF and gender (chi-square = 36, df = 33, p > 0.05) and education years [rho = 0.080, p = 0.578]. On the other hand, there was a strong, negative correlation between age and CSF data (p < 0.05) at low (0.2 and 2.5 cpd) and high spatial frequencies (20 cpd). Middle-aged adults had poorer CSF at 0.2 cpd (rho = −0.597, p < 0.001), 2.5 cpd (rho = −0.621, p < 0.001), 10.0 cpd (rho = −0.343, p = 0.045) and 20 cpd (rho = −0.434, p = 0.008), except for 5.0 cpd (rho = −0.222, p = 0.193) (Fig. 2). A spearman correlation showed no correlation between FTND and CSF (p > 0.050).
Contrast sensitivity bars as a function of age groups for non-smokers, smokers and deprived smokers at each spatial frequency. Color bars represents the sensitivity (reciprocal of contrast threshold) for each spatial frequency. Error bars represents the standard deviation (SD) of the mean sensitivity. ***p < 0.001, **p < 0.01, *p < 0.05.
Discussion
The data indicated that smokers, as a whole, demonstrated reduced CSF when compared to non-smokers. High spatial frequencies deficits were the most frequent type of divergences observed among smokers group, at about 0.25 log units of differences between non-smokers and deprived smokers groups. Despite of a lower sensitivity, the relative difference between smokers and non-smokers was minimal if we take as a parameter the CSF of deprived smokers group (Fig. 1).
It is known that there are numerous compounds in the cigarette that, per se, are harmful such as carbon monoxide, toluene, tar, nickel and many others. Thus, the intent of the present study was not to specifically evaluate the effect of nicotine, one of the components of the cigarette, but to observe and investigate the effects of cigarette smoking in visual spatial processing. In general, the results indicated that smokers presented less sensitivity when compared to the non-smokers group. In agreement with some studies16,17,18 and in disagreement with other studies12, 19, 20 that showed effects of cigarette and nicotine on visual processing, these data point to a new investigation direction.
The results do not suggest the existence of selective changes in spatial channels of visual processing12, 20, but support the idea that tobacco constituents play a role as addiction chemical modulators and visual sensitivity through dopamine (DA), GABA and glutamate neurotransmission deficits21,22,23,24,25,26.
Nicotine, for example, a tobacco psychoactive component, enhances DA release through a dynamic balance of activation and desensitization of nAChRs located mainly in the ventral tegmental area (VTA) and in the striatum9, 27. Since there are also nAChRs and DA receptors on the retina, it is not hard to understand that acute use of nicotine would enhance cognition at some points28,29,30, since nicotine’s effects on multiple neurotransmitters are thought to contribute to cognitive performance27.
In this case, we did not observe a large reduction of CSF among smokers, but also did not observe improvements. So, what would be the relation between chronic smoking and visual processing? The answer may lie in desensitization, which is one of many changes caused my addiction31. Besides, chronic nicotine exposure leads to nAChRs desensitization through brain upregulation4, 32. Another property of tobacco, specifically from nicotine, is that the more exposure, the greater the need for it activate the receptors, which changes affinity and response properties of the nAChRs33, 34. Considering that nicotine-enhancing effects decrease over time and remain unchanged after chronic exposure, this may explain not only the lower CSF but also the similarity between smokers and non-smokers in our data (Fig. 1).
On the one hand, smokers group needed nearly two times more contrast to detect high frequencies when compared to non-smokers (p < 0.001, r = −0.60). When we compare with deprived smokers, they were less sensitive in order of 0.5 log units at low (p < 0.005) and mid spatial frequencies (p < 0.007) (Fig. 1). Thus, the high spatial frequencies were the most affected comparing smokers and deprived smokers to non-smokers, and the least affected when comparing smokers and non-smokers. As stated, these results do not support the idea of channel selectivity deficits, despite the existence of nAChRs in the primary visual cortex35. However, we hypothesize the existence of a diffuse deficit in visual processing, which may include magno- and parvocelular pathways, since we used a photopic condition and observed significant changes at high spatial frequencies8, 11.
On the other hand, deprived smokers showed a large reduction in the CSF (Fig. 1). As expected, the reduction of CSF occurred at all frequencies, when comparing with non-smokers (p < 0.001). However, we expected that differences between smokers and deprived smokers would be minimal, without statistically significant differences. Our data showed that deprived smokers were more sensitive for almost all spatial frequencies (p < 0.05), except 20.0 cpd. This can be explained by withdrawal effect (≤24 h) which induces a hypofunctional effect of dopamine release36, 37, reflecting at both visual processing38,39,40 and brain reward function41. Tobacco deprivation, even in a short period, play a role affecting motivation and attention in responding to the test stimuli42, 43. Therefore, we suggest the existence of another factor within tobacco deprivation: attentional bias.
In order to detect environmental stimuli, visual attention is a key mechanism the brain uses. Some psychophysical studies indicated that directing attention to a certain location has an influence on spatiotemporal aspects of visual processing44,45,46. Considering attention and motivation as functions affected with tobacco deprivation, they would then play an important role in the CSF decrease, even comparing against smokers group. Besides, this may explain the larger decrease at low spatial frequencies (p < 0.001), once that deprivation stimulates the amygdala and activates the magnocellular system which, in turn is specialized in providing low spatial frequency information47.
Although smoking behavior is slightly different between men and women48, there were no gender differences in our data. We observed a strong negative correlation between age and CSF, being that the middle-aged adults had a worse CSF than young adults (Fig. 2). This same pattern was found in other studies using psychophysics49,50,51. It is hypothesized that the normal aging affects both magno- and parvocelular pathways in humans and the parvocellular is the most largely affected. Our data pointed out that high frequencies were the most impaired between groups (Fig. 2). Thus, it is consistent with Flaubert’s theory of visual perception and aging52.
Even though there are a limited number of studies about smoking and visual processing, specifically about spatial vision, our limitations should be considered. First, this was an observational study, and such a design does not permit evaluation of the physiological, mechanistic, or genetic associations between smoking and spatial vision per se. Second, even the use of a classic, reliable and rigorous testing procedure such as CSF may be unable to assess all aspects of visual function important during smoking abstinence. Therefore, we recommend the combined use of multiple methods (e.g., physiological, behavioral and imaging) with psychophysics to facilitate the understanding of the mechanisms involved. Third, we evaluated tobacco as a whole, not only the nicotine effects19, 20, which brings us the idea of further studies, using nicotine gum and the same paradigm used here.
Clearly, further work is needed but this study highlights the relation between smoking and spatial visual processing. We suggest that cigarette components may affect vision more than nicotine separately - which even helps in disorders such as Schizophrenia and Parkinson’s Disease53. Indeed, tobacco components may increase free radical that would cause macular degeneration along with the action of ischemia. Besides, these mechanisms affect color vision19, 21, 54, which is our future research target.
Methods
Participants
This research followed the ethical principles from the Declaration of Helsinki and was approved by the Committee of Ethics in Research of the Health Sciences Center of Federal University of Paraiba (CAAE: 60944816.3.0000.5188). Written informed consent was obtained from all participants.
In this study, 16 non-smokers (mean age = 34.1 years; SD = 7.9; 8 male), 16 heavy cigarette smokers (mean age = 34.4 years; SD = 7.3; 8 male) and 16 deprived smokers (mean age = 30.3 years; SD = 5.1; 8 male), from 25 to 45 years, who were working as staff or were students at Federal University of Paraiba, were recruited through newspaper advertisements. Exclusion criteria were: (I) older than 45 years (since the effects of the human visual system maturation or aging could superestimate the results51), (II) current history of neurological disorder, (III) a history of head trauma, (IV) history of contact with substances such as solvents, (V) met criteria for a lifetime diagnosis of substance abuse or dependence other than nicotine dependence (for smokers groups), (VI) drinking more than 10 alcoholic drinks per week, or (VII) current use of medications that may affect visual processing and cognition. In addition, subjects were required to have a good ocular health: no abnormalities were detected in the fundoscopic examination and in the optical coherence tomography exam. All observers were screened for color blindness using Ishihara’s55 tests for color deficiency and had normal or corrected-to-normal vision as determined by a visual acuity of at least 20/20.
Smokers reported a smoking history of at least 8 years, currently smoked more than 20 cigarettes/day and had a score >5 on the Fagerström Test for Nicotine Dependence (FTND)56. Smokers and deprived smokers began smoking on average at the age of 16.5 years (SD = 1.05) and had been smoking on average for 17 years (SD = 4.05). Smokers were allowed to smoke until the beginning of the experiment. An abstinence period of 12 hours was chosen based on previous studies. Non-smokers had never smoked a cigarette. To assess subjective craving for a cigarette (a form of evaluation of abstinence), participants provided self-reporting and completed psychometric measures such as Program to Aid Smokers (PAS)28 comfort scale and the brief version of the Questionnaire of Smoking Urges (QSU-B)57. There were no statistical differences between depression and anxiety symptoms pre- and post-experiment, as measured by Hamilton Scale for Depression and Hamilton Anxiety Rating Scale.
The groups did not differ in age, F (2, 47) = 0.17, p = 0.98, or ratio of males to females (Pearson’s Chi Square Value = 1.9, ns). There was also no difference in FTND scores (t(1) = 2.35, p = 0.98), age at first cigarette use (t(1) = 5.71, p = 0.99), and years of cigarette use (t(1) = 1.63, p = 0.93) between the two groups of smokers who took part in this experiment (Table 1).
Stimuli and Apparatus
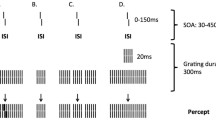
Stimuli were presented on a 19-inch LG CRT monitor with 1024 × 786 resolution and a rate of 100 Hz. Stimuli were generated using a VSG 2/5 video card (Cambridge Research Systems Ltd., Rochester, Kent, UK), which was run on a microcomputer Precision T3500 with W3530 graphics card. The average luminance was 50 cd/m². All procedures were performed in a room at 26 ± 1 °C, with the walls covered in grey for better control of luminance during the experiments. Measurements were performed with binocular vision at a distance of 150 cm from the monitor screen. Monitor luminance and chromatic calibrations were performed with a ColorCAL MKII photometer (Cambridge Research Systems).
The visual contrast sensitivity measurements were taken through Metropsis software (Cambridge Research Systems Ltd., Rochester, Kent, UK). This software provides a CSF clinical evaluation. The Metropsis vision-testing suite provides precise, repeatable, psychophysical threshold measurements for general research applications.
Similar to Andrade et al.58, we used the same procedure where Metropsis was the software responsible for generating vertical sinewave gratings. Spatial frequencies of 0.2, 2.5, 5.0, 10.0 and 20.0 cpd, with an initial phase of 180°, were responsible for the luminance contrast sensitivity measurements (for details, see ref. 11). Stimuli consisted in equiluminant gratings with dimensions of 5 degrees of visual angle, being presented in the monitor at 2.5 degrees from the cross-shaped fixation point.
Procedure
Before scheduling the experiments, participants were advised about the research procedures and perspectives. The division of smokers and deprived smokers groups occurred randomly. Along with the recommendations (e.g., abstinence from caffeine or psychoactive substances), information on cigarette deprivation was given instantaneously. To schedule participation in the study, deprived smokers were asked to avoid the use of cigarettes 12 hours before the beginning of the experiments.
Contrast sensitivity measurements were performed using the two-alternative forced choice (2-AFC) method, and the subjects’ task was to identify, using a remote control response box, whether the stimulus was presented at the left or right side of the screen. Subjects were instructed to maintain fixation on a small black fixation cross in the center of the display monitor. The order of frequencies tested was randomized within a session.
A three-down one-up logarithmic staircase with dynamic steps was used, tracking performance at 75% accuracy. Initially, the contrast values appeared in suprathreshold level (which was expected that participants had a series of correct answers) to reach the staircase criteria of three consecutive correct responses and an error. Thus, after three consecutive correct responses contrast decreased 0.7 dB and increased by 1.0 dB after every incorrect response. Stimuli had exposure time of 600 ms with between-intervals of 300 ms. This process took place throughout the experiment.
To obtain the reversals for each grating, an average of 150–250 trials (depending on the participant) were presented. The session ended after eight response reversals that were recorded for each grating tested. The thresholds were calculated averaging the contrast values of the response reversals for each grating, and contrast sensitivity was estimated as the reciprocal of the threshold values. Higher contrast sensitivity values mean that the participant presents higher sensitivity to the spatial frequency evaluated in the test (for procedure details, refer to refs 58 and 59).
Statistics
The distributions for each group were compared with Shapiro-Wilk. Both groups showed non-normal distribution, thus non-parametric statistical methods were used to analyze the data. For group comparisons, the non-parametric univariate analysis was used, with pairwise comparisons by Mann-Whitney U test. Spearman’s rank correlation coefficients (rho) were conducted to assess the relation between outcomes of color discrimination data and biosociodemographic variables such as age, gender and education level.
The effect size (r) estimation was used from the conversion of z-score60. Each data point represents the medians and errors bars represent standard deviations (SD) of the median based on 1000 bootstrap resamplings. Bonferroni correction was the method of adjusting p-value that was used.
References
Mathers, C. D. & Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLOS Med 3, e442 (2006).
WHO. Who Report On The Global Tobacco Epidemic 2013 (2013).
WHO, W. H. WHO report on the global tobacco epidemic, 2015: Raising taxes on tobacco (2015).
Balfour, D. J. & Munafò, M. R. The Neuropharmacology of Nicotine Dependence. (Springer, 2015).
Metherate, R. Nicotinic Acetylcholine Receptors in Sensory Cortex. Learn. Mem. 11, 50–59 (2004).
Paterson, D. & Nordberg, A. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 61, 75–111 (2000).
Brandies, R. & Yehuda, S. The possible role of retinal dopaminergic system in visual performance. Neurosci. Biobehav. Rev. 32, 611–656 (2008).
DeValois, R. L. & DeValois, K. K. Spatial Vision. (Oxford University Press, 1990).
D’Souza, M. S. & Markou, A. Neuronal Mechanisms Underlying Development of Nicotine Dependence: Implications for Novel Smoking-Cessation Treatments. Addict. Sci. Clin. Pract. 6, 4–16 (2011).
Cornsweet, T. Visual Perception. (Academic Press, 2012).
Santos, N. Ados & Simas, M. LdeB. Contrast Sensitivity Function: Indicator of the Visual Perception of Form and of the Spatial Resolution. Psicol. Reflex. E Crítica 14, 589–597 (2001).
Kunchulia, M., Pilz, K. S. & Herzog, M. H. Small effects of smoking on visual spatiotemporal processing. Sci. Rep. 4, 7316 (2014).
Pelli, D. G. & Bex, P. Measuring contrast sensitivity. Vision Res. 90, 10–14 (2013).
Santos, N. A. dos & Simas, M. L. deB. Perception and visual processing of form: discussing contemporary models. Psicol. Reflex. E Crítica 14, 157–166 (2001).
Pylyshyn, Z. Is vision continuous with cognition? The case for cognitive impenetrability of visual perception. Behav. Brain Sci. 22, 341–365–423 (1999).
Bell, S. L., Taylor, R. C., Singleton, E. G., Henningfield, J. E. & Heishman, S. J. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob. Res. 1, 45–52 (1999).
Zhang, X. et al. Smoking and visual impairment among older adults with age-related eye diseases. Prev. Chronic. Dis. 8, A84 (2011).
Ga, W., Ap, F. & N, W. Smoke gets in your eyes: smoking and visual impairment in New Zealand. N. Z. Med. J. 114, 471–474 (2001).
Naser, N. T., Loop, M., Than, T., Varghese, S. & Hartmann, E. E. Color Vision: Effects of Nicotine Gum in Non-Smokers. Invest. Ophthalmol. Vis. Sci. 52, 4902–4902 (2011).
Varghese, S. B., Reid, J. C., Hartmann, E. E. & Keyser, K. T. The Effects of Nicotine on the Human Electroretinogram. Invest. Ophthalmol. Vis. Sci. 52, 9445–9451 (2011).
Fernandes T. M. d. P., Almeida, N. L. & dos Santos N. A. Comparison of color discrimination in chronic heavy smokers and healthy subjects [version 2; referees: 1 approved, 1 approved with reservations]. F1000Research 2017, 6, 85, doi:10.12688/f1000research.10714.2
Melis, M., Diana, M., Enrico, P., Marinelli, M. & Brodie, M. S. Ethanol and acetaldehyde action on central dopamine systems: mechanisms, modulation, and relationship to stress. Alcohol Fayettev. N 43, 531–539 (2009).
Shiraishi-Yokoyama, H., Yokoyama, H., Matsumoto, M., Imaeda, H. & Hibi, T. Acetaldehyde inhibits the formation of retinoic acid from retinal in the rat esophagus. Scand. J. Gastroenterol. 41, 80–86 (2006).
Ward, R. J., Colantuoni, C., Dahchour, A., Quertemont, E. & De Witte, P. Acetaldehyde-induced changes in monoamine and amino acid extracellular microdialysate content of the nucleus accumbens. Neuropharmacology 36, 225–232 (1997).
Radcliffe, K. A., Fisher, J. L., Gray, R. & Dani, J. A. Nicotinic Modulation of Glutamate and GABA Synaptic Transmission in Hippocampal Neurons. Ann. N. Y. Acad. Sci. 868, 591–610 (1999).
Timmerman, W. & Westerink, B. H. C. Brain microdialysis of GABA and glutamate: What does it signify? Synapse 27, 242–261 (1997).
Levin, E. D. Nicotinic Receptors in the Nervous System (CRC Press, 2001).
Potter, A. S. & Newhouse, P. A. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol. Biochem. Behav. 88, 407–417 (2008).
Quisenaerts, C. et al. Acute nicotine improves social decision-making in non-smoking but not in smoking schizophrenia patients. Front. Neurosci 7, 197 (2013).
Quisenaerts, C. et al. The nicotinergic receptor as a target for cognitive enhancement in schizophrenia: barking up the wrong tree? Psychopharmacology (Berl.) 231, 543–550 (2014).
Koob, G. F., Sanna, P. P. & Bloom, F. E. Neuroscience of Addiction. Neuron 21, 467–476 (1998).
Govind, A. P., Vezina, P. & Green, W. N. Nicotine-induced Upregulation of Nicotinic Receptors: Underlying Mechanisms and Relevance to Nicotine Addiction. Biochem. Pharmacol. 78, 756–765 (2009).
Besson, M. et al. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc. Natl. Acad. Sci. 104, 8155–8160 (2007).
Vallejo, Y. F., Buisson, B., Bertrand, D. & Green, W. N. Chronic Nicotine Exposure Upregulates Nicotinic Receptors by a Novel Mechanism. J. Neurosci. Off. J. Soc. Neurosci. 25, 5563–5572 (2005).
Groleau, M., Kang, J. I., Huppé-Gourgues, F. & Vaucher, E. Distribution and effects of the muscarinic receptor subtypes in the primary visual cortex. Front. Synaptic Neurosci. 7 (2015).
De Biasi, M. & Dani, J. A. Reward, Addiction, Withdrawal to Nicotine. Annu. Rev. Neurosci. 34, 105–130 (2011).
Zhang, L., Dong, Y., Doyon, W. M. & Dani, J. A. Withdrawal from Chronic Nicotine Exposure Alters Dopamine Signaling Dynamics in the Nucleus Accumbens. Biol. Psychiatry 71, 184–191 (2012).
Bodis-Wollner, I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci. 13, 296–302 (1990).
Jackson, C. R. et al. Retinal Dopamine Mediates Multiple Dimensions of Light-Adapted Vision. J. Neurosci. 32, 9359–9368 (2012).
Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. Adv. Ophthalmol. 108, 17–40 (2004).
Wise, R. A. & Rompre, P. P. Brain Dopamine and Reward. Annu. Rev. Psychol. 40, 191–225 (1989).
Aguirre, C. G., Madrid, J. & Leventhal, A. M. Tobacco withdrawal symptoms mediate motivation to reinstate smoking during abstinence. J. Abnorm. Psychol. 124, 623–634 (2015).
Figueiró, L. R. et al. Assessment of changes in nicotine dependence, motivation, and symptoms of anxiety and depression among smokers in the initial process of smoking reduction or cessation: a short-term follow-up study. Trends Psychiatry Psychother. 35, 212–220 (2013).
Lee, T.-H., Baek, J., Lu, Z. & Mather, M. How Arousal Modulates the Visual Contrast Sensitivity Function. Emot. Wash. DC 14, 978–984 (2014).
Pestilli, F., Viera, G. & Carrasco, M. How do attention and adaptation affect contrast sensitivity? J. Vis. 7, 9.1–912 (2007).
Pollux, P. M. J., Hall, S., Roebuck, H. & Guo, K. Event-related potential correlates of the interaction between attention and spatiotemporal context regularity in vision. Neuroscience 190, 258–269 (2011).
Bocanegra, B. R. & Zeelenberg, R. Emotional cues enhance the attentional effects on spatial and temporal resolution. Psychon. Bull. Rev. 18, 1071–1076 (2011).
Muir, A. D., McKeown, P. P. & Bayraktutan, U. Role of gender, smoking profile, hypertension, and diabetes on saphenous vein and internal mammary artery endothelial relaxation in patients with coronary artery bypass grafting. Oxid. Med. Cell. Longev. 3, 199–205 (2010).
Derefeldt, G., Lennerstrand, G. & Lundh, B. Age Variations in Normal Human Contrast Sensitivity. Acta Ophthalmol. (Copenh.) 57, 679–690 (1979).
Sieiro, R. & de, O. et al. Contrast sensitivity assessment in different age group in medium and high spatial frequency. Rev. Bras. Oftalmol. 75, 296–299 (2016).
Santos, N. A., dos Simas, M. L., de, B. & Nogueira, R. M. T. B. L. Visual processing of form in the aged: threshold contrast curves to angular and spatial frequency. Psicol. Reflex. E Crítica 16, 271–277 (2003).
Faubert, J. Visual perception and aging. Can. J. Exp. Psychol. Rev. Can. Psychol. Exp. 56, 164–176 (2002).
Barreto, G. E., Iarkov, A. & Moran, V. E. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Front. Aging Neurosci. 6 (2015).
Arda, H. et al. Effects of chronic smoking on color vision in young subjects. Int. J. Ophthalmol. 8, 77–80 (2015).
Ishihara, S. The Series of Plates Designed as a Test for Colour-Blindness (1972).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerström, K. O. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 86, 1119–1127 (1991).
Cox, L. S., Tiffany, S. T. & Christen, A. G. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 3, 7–16 (2001).
Andrade, M. J. O., Silva, J. A. & Santos, N. A. Influência do Cronotipo e do Horário da Medida na Sensibilidade ao Contraste Visual. 28, 522–531 (2015).
Santos, D., Antonio, N., Andrade, S. M. & Calvo, B. F. Detection of spatial frequency in brain-damaged patients: influence of hemispheric asymmetries and hemineglect. Front. Hum. Neurosci. 7 (2013).
Field, A. Discovering Statistics Using IBM SPSS Statistics (SAGE, 2013).
Acknowledgements
We appreciate the participation of all who have provided time for this research. National Brazilian National Council of Technological and Scientific Development (CNPq), Brazil (303822/2010-4), sponsored this paper.
Author information
Authors and Affiliations
Contributions
T.M.: work design, data collection and interpretation, and article drafting. N.L. performed the experiments, participated in the study design and performed the statistical analysis. N.A.: work design, data analysis and interpretation, and critical revision of the article. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernandes, T.M.d.P., Almeida, N.L.d. & Santos, N.A.d. Effects of smoking and smoking abstinence on spatial vision in chronic heavy smokers. Sci Rep 7, 1690 (2017). https://doi.org/10.1038/s41598-017-01877-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01877-z
- Springer Nature Limited
This article is cited by
-
Health behavior and psychological treatment utilization in adults with avoidant/restrictive food intake disorder symptoms
Journal of Eating Disorders (2024)
-
Smoking, urinary cotinine levels and incidence of visual impairment
Scientific Reports (2021)
-
Longevity pathways in stress resistance: targeting NAD and sirtuins to treat the pathophysiology of hemorrhagic shock
GeroScience (2021)
-
Colour discrimination thresholds in type 1 Bipolar Disorder: a pilot study
Scientific Reports (2017)