Abstract
Dicalcium phosphate cement preparation requires the addition of setting retarders to meet clinical requirements regarding handling time and processability. Previous studies have focused on the influence of different setting modifiers on material properties such as mechanical performance or injectability, while ignoring their influence on biological cement properties as they are used in low concentrations in the cement pastes and the occurrence of most compounds in human tissues. Here, analyses of both material and biological behavior were carried out on samples with common setting retardants (citric acid, sodium pyrophosphate, sulfuric acid) and novel (phytic acid). Cytocompatibility was evaluated by in vitro tests with osteoblastic (hFOB 1.19) and osteoclastic (RAW 264.7) cells. We found cytocompatibility was better for sodium pyrophosphate and phytic acid with a three-fold cell metabolic activity by WST-1 test, whereas samples set with citric acid showed reduced cell number as well as cell activity. The compressive strength (CS) of cements formed with phytic acid (CS = 13 MPa) were nearly equal to those formed with citric acid (CS = 15 MPa) and approximately threefold higher than for other setting retardants. Due to a proven cytocompatibility and high mechanical strength, phytic acid seems to be a candidate replacement setting retardant for dicalcium phosphate cements.
Similar content being viewed by others
Introduction
Trauma or disease can cause bone defects exceeding a critical size such that bone grafts have to be implanted into the defect to fill space and to prevent fibrous tissue ingrowth during healing. The development of synthetic bone graft substitutes provides a wide range of possibilities to treat these defects. Major advantages of such synthetic materials compared to autologous transplants are their unlimited availability, constant quality and the absence of donor related pathogen transfer compared with natural resources1, 2. These synthetic materials can be either of an organic nature such as polylactid-co-glycolic acid3, 4 or they can be based on inorganic calcium phosphate chemistry to mimic the natural chemical composition of the bone mineral phase. Calcium phosphates are applied as either pre-formed monoliths or granules composed of sintered hydroxyapatite or tricalcium phosphate (or mixtures of both) or they can be used as self-setting cement pastes which can be molded or even injected5, 6 into a defect following the formation of a mechanically stable implant directly at the application site. Currently, calcium phosphate cements (CPC) are clinically used to fill and restore cancellous bone, to fix implants and to reinforce osteoporotic bone7. CPC inhibit fibrous tissue ingrowth8 and may have the potential to increase the mechanical strength of bone enabling treatments with screws for fixation9,10,11.
The setting mechanism of CPC occurs by a continuous dissolution/precipitation reaction based on the differential of solubilities of cement raw materials and their setting product. Mineral phases formed during cement setting are either stoichiometric or calcium deficient hydroxyapatite at neutral or basic pH conditions12, while secondary protonated dicalcium phosphates such as brushite (CaHPO4·2H2O)13, 14 or monetite (CaHPO4)15, 16 will be formed at an acidic pH. The latter have the advantage of a higher solubility under in vivo conditions compared with apatitic cements17, 18 (solubility of brushite (monetite) in water: 85–88 (41–48) mg/L, HA: 0.2 mg/L)19. A couple of studies have demonstrated the bone remodelling capacity of brushite forming cements in various animal models within a time period of 16–18 weeks20, 21, whereas other studies have indicated a phase conversion of brushite into lower soluble octacalcium phosphate or hydroxyapatite in vivo 22, which slows down degradation compared to monetite.
Since the crystallization rate of brushite crystals is approximately three orders of magnitude higher than those for hydroxyapatite23, setting retarders have to be used in these cements to adjust clinically appropriate setting times of 3–5 min. Most commonly applied setting retarders for brushite cements are citric acid24, 25, pyrophosphate ions24, 26, sulfate ions27 or magnesium ions28, 29. Among those, citric acid was shown to result in superior physical properties such as mechanical strength, setting time and injectability30, 31. Although all of these retarders were compounds naturally occurring in tissues32, the biological properties of brushite cements were recently demonstrated to be influenced by these additives. Jamshidi et al. showed that the cell attachment to brushite cements is mostly inhibited by using citric acid as retarder33. This was attributed to the formation of an amorphous dicalcium citrate phase during setting, which slowly dissolved afterwards in the culture medium during cell experiments. Another study by Kanter et al.17 revealed that citric acid may also influence the degradation rate of brushite cement in vivo. Even those cement samples, which were not converted into lower solubility octacalcium phosphate remained mostly stable during 10 months of orthotopic implantation, either because the calcium citrate complexes formed in the cement decreased the chemical solubility of the cement matrix or they decreased the metabolic activity of bone resorbing osteoclasts near the implant.
This study aimed to investigate this observation in more detail to identify the effect of different setting retarders on both basic properties of cements as well as on their influence on the cytocompatibility against bone forming and degrading cells. Several possible alternatives to citric acid as setting retarder in brushite cements such as sodium pyrophosphate, sulfuric acid and phytic acid were investigated and compared with citric acid and cements without any setting retardant. Apart from analyzing the influence of these additives on the physico-chemical cement properties such as initial setting time, compressive strength and the exothermic setting reaction, biological investigations concerning cytocompatibility were carried out with a human osteoblastic cell line (hFOB 1.19) and a murine macrophage cell line (RAW 264.7) differentiating into osteoclasts.
Results
Cell proliferation of the osteoblastic cell line hFOB1.19 over a period of 10 days on the surface of set cements revealed cell numbers in the range of the control (cement with no retarding additive, set as 100% at day 4) or above and only those made with citric acid and sulfuric acid as retarder had a large decrease to approx. 27–45% in cell number after 10 days (Fig. 1A). Additionally, cell proliferation assayed by WST-test (Fig. 1B) showed lower cell activity for samples containing citric acid or sulfuric acid compared to the reference without setting retardant. However, more than three-times higher metabolic activity per cell could be reached by the use of sodium pyrophosphate or phytic acid (Fig. 1C).
Percent cell number with regard to cement without setting retardant (solid line at 100%) (A), cell activity (B) and cell activity per cell (C) of hFOB. Samples without setting retardant (wsr) and retardants citric acid (0.5 M ca), sodium pyrophosphate (5.4 wt% sp), sulfuric acid (0.1 M sa) and phytic acid (0.1 M pa) were used on day 4, 6 and 10.
Additionally, cements were seeded with the macrophage cell line RAW 264.7, which were differentiated into osteoclasts after addition of 50 ng/mL RANKL. Surprisingly, cement surfaces prepared with citric acid as setting retardant fully inhibited osteoclast growth during the whole course of the experiment (Fig. 2A). The cell number of the other retarders was comparable to the reference and increased during culture of 21 days. Cell activity measured by WST-1 test is shown in Fig. 2B. Due to the lack of cells, samples with citric acid had no cell activity. Sulfuric acid had very low activity, which increased during culture period. Cements formed with sodium pyrophosphate and phytic acid were overwhelmingly comparable to the reference, even though the highest activity of the reference on day 21 could not be reached by any other composition (Fig. 2C).
Cell number of RAW 264.7 osteoclasts with regard to cement without setting retardant (solid line at 100%) (A), Cell activity (B) and cell activity per cell (C). Samples without setting retardant (wsr) and retardants citric acid (0.5 M ca), sodium pyrophosphate (5.4 wt% sp), sulfuric acid (0.1 M sa) and phytic acid (0.1 M pa) were cultured over 21 days.
Differentiation of RAW macrophages into multinucleated osteoclasts by addition of RANKL was verified by TRAP staining (Fig. 3). Samples without RANKL addition served as control and were cultured over 21 days resulting in no staining for any cement composition (data not shown). Cement surfaces prepared with citric acid or sulfuric acid as retarder showed hardly any staining and only at day 21, on both surfaces little TRAP staining could be detected. In contrast, cement surfaces prepared with sodium pyrophosphate or phytic acid retarder exhibited continuously increasing TRAP staining during the culture period which was at least comparable or even more than the reference. After 15–21 days, these surfaces were confluently grown with cells.
TRAP staining of RANKL treated RAW 264.7 cells. Differentiation can be seen for different setting retardants citric acid (0.5 M ca), sodium pyrophosphate (5.4 wt% sp), sulfuric acid (0.1 M sa) and phytic acid (0.1 M pa) as well as cement without setting retardant (wsr) over 21 days. Scale bar corresponds to 1.5 mm.
The solubility of the preset cements was tested using DMEM cell culture medium (Fig. 4) for up to 24 h. All cements showed an adsorption of magnesium ions (2.3–7.3 mg/l) from the medium and a high release of phosphate in the range of 71–89 mg/l. Strong differences were observed regarding calcium ions, where only cements formed with sodium pyrophosphate and phytic acid increased the Ca2+ concentration of the medium (21–47 mg/l), whereas all other cements adsorbed calcium ions.
Ion concentrations after immersion of different cement samples in DMEM cell culture medium for 24 h at 37 °C in relation to fresh medium. Samples were prepared without setting retardant (wsr) and retardants citric acid (0.5 M ca), sodium pyrophosphate (5.4 wt% sp), sulfuric acid (0.1 M sa) and phytic acid (0.1 M pa).
Regarding the influence of setting retardants on the mechanical cement properties (Fig. 5A), the highest compressive strength was found for samples set with citric acid (15 MPa) and phytic acid (13 MPa), with both being significantly higher than the reference. In contrast, the use of sodium pyrophosphate and sulfuric acid weakened the cement matrix and compressive strength was significantly lower compared to the reference without setting retardant. Initial setting time was evaluated by the Gillmore needle test (Fig. 5B) indicating a setting time of less than one minute in the absence of any retarder. While sodium pyrophosphate, sulfuric acid and phytic acid increased the setting time to approx. 3–4 min, citric acid had a much stronger influence as retarder and prolonged cement solidification to 5.5 min. Porosity of set cements (Fig. 5C) was found to be in the range 21–23% for the reference as well as citric acid/phytic acid retarder, whereas higher values (29–31%) were obtained for sodium pyrophosphate and sulfuric acid.
(A) Compressive strength of brushite with four different setting retardants. Cement without setting retardant served as reference. Samples marked with ** were highly significant (p < 0.001) with regard to the reference, (B) initial setting times according to Gillmore needle test (boundary for the initial setting time of 3 min is marked) and (C) porosity of set cements. Setting retardants citric acid (0.5 M ca), sodium pyrophosphate (5.4 wt% sp), sulfuric acid (0.1 M sa) and phytic acid (0.1 M pa) were compared to the reference without setting retardant (wsr).
Figure 6 shows the temperature evolution during cement setting over a period of 60 min and a dot marks the initial setting time measured with Gillmore needle test. As could be already seen at the Gillmore needle test, cement samples set without retardant had the earliest exothermic reaction and highest maximum temperature (45 °C). The maximum peak during setting reaction is shifted to later time points when retarders were employed and also the temperatures achieved during setting decreased by the addition of retarders below 37 °C. Morphological analysis using SEM revealed three types of crystals: plates (Fig. 7A), needles (Fig. 7C) and paving stone like structures (Fig. 7B), differing in terms of aspect ratio (two-dimensional) and three-dimensional expansion. While cements formed with sodium pyrophosphate and citric acid showed a predominantly needle like structure with a crystal size of 5–10 µm, sulfuric acid as well as phytic acid as retarders led to the formation of plate-like crystals of <5 µm size. A more compact morphology was observed in the absence of any retarder (Fig. 7B). The size distribution of the cement raw materials (Fig. 7D) was monomodal with a medium size of ß-TCP (9.4 µm) and MCPA (14.5 µm).
Temperature profile between 30 s and 60 min (left) and maximum temperature reached during setting (right). Samples with setting retardants citric acid (0.5 M ca), sodium pyrophosphate (5.4 wt% sp), sulfuric acid (0.1 M sa) and phytic acid (0.1 M pa) as well as a reference without setting retardant (wsr) ware examined. Dots mark the initial setting time determined by Gillmore needle test.
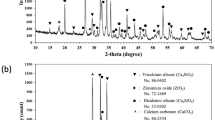
SEM images of three different types of morphology taken at 3 kV and 2000-fold magnification. Images show plates (A), plate-like crystals (B) and needles (C). The cements with 0.1 M sulfuric acid (A), no setting retarder (B) and 0.5 M citric acid (C) served as examples. The particle size distribution of the reactant powders β-TCP and MCPA are shown (D). Both had a monomodal particle size distribution between 1 µm and 100 µm. Analysis of phase composition via XRD (E) exhibited crystalline structures for brushite (b), monetite (m) and the reactant powder β-TCP (β).
Phase analysis performed with XRD (Fig. 7E) exhibited three different phases: brushite (b), the anhydride monetite (m) and the cement raw powder β-TCP (β). The second cement component MCPA was not found in crystalline form after setting. When phytic acid or sulfuric acid were used as retarders, monetite was the predominant phase formed during setting, whereas for all other retardants the formation of mixtures of both brushite and monetite was observed, even though there were residues of β-TCP in all compositions.
Discussion
Dicalcium phosphate crystals are formed during cement setting by a comparatively fast dissolution/precipitation process5. The reactants β-TCP and MCPA dissolve in the liquid phase, whereas brushite or monetite precipitates at a pH-value less than 4.25. However, the setting time of dicalcium phosphate cements without any retardant of less than one minute is very short. An extension of the setting time to a clinically applicable time frame requires the use of setting retarders, such as pyrophosphates, sulfates or citrates24. These compounds delay the crystal growth rate of the setting product by adsorbing at the active growth sites of dicalcium phosphate crystal faces34, leading to both a longer setting time (Fig. 5B) and a decrease in the maximum setting temperature at later time points (Fig. 6B). Among these retarders, citric acid has been proven to simultaneously retard setting and attain excellent material properties such as high compressive strength31 and good injectability30. This was confirmed in the present study demonstrating the highest cement strength and longest setting times compared with all other additives (Fig. 5). The only other compound leading to nearly similar cement characteristics was phytic acid, which is known to form Ca2+/Mg2+ chelates with compounds such as α-tricalcium phosphate, ß-tricalcium phosphate or trimagnesium phosphate35,36,37 and which acts as a regulator of brushite and hydroxyapatite crystal growth38.
Although for all cements a constant powder to liquid ratio was used, porosity as a strength determining parameter39, 40 was found to vary in the range from 21–31% (Fig. 5C). These porosity differences likely correlate with strength variation as those cements with the lowest porosity (citric acid, phytic acid, no retarder) had the highest strength, whereas the Ca2+ chelating capacity of citric acid and phytic acid may have further contributed to the mechanical performance. In addition, the observed variation in brushite crystal morphology may be a regulating parameter for cement strength as it is also known for hydroxyapatite forming cements41. Examination of surface morphology of the different setting retardants via SEM revealed three types of crystal shapes. Plates and needles could be detected due to a non-centrosymmetric monoclinic crystallization structure as reported elsewhere42, 43. Furthermore, paving stone like structures were found having a more compact structure with a lower aspect ratio compared to plates and needles. In most cases, the morphology of cement samples was a mixture of these three types since the complex kinetics influenced by numerous factors may play a decisive role in crystallization leading to a variety of crystal morphologies. In terms of the mechanical cement properties, the predominant needle like character of brushite crystals formed by the addition of citric acid are thought to result in a better entanglement of crystals compared to the compact morphology of brushite crystals without setting retarder (Fig. 7).
Despite of the excellent cement material properties, recent studies indicated a reduced cell attachment on cement surfaces33 and a strongly delayed degradation in vivo 17 for citric acid modified brushite cements. Especially the latter is surprising, since the authors did not observe any phase changes during 10 months of orthotopic implantation for low porosity brushite cements. This anomalous behavior may have two reasons: firstly, the calcium citrate complexes formed in the cement may generally decrease the solubility of the cement and/or secondly, the calcium citrate complexes are not only affecting the proliferation/activity of osteoblasts but also those of bone resorbing osteoclasts. Regarding the cement solubility, cements formed with citric acid showed a similar dissolution behavior like cements with sulfuric acid or without setting retarder (Fig. 4). The release of phosphate in conjunction with the adsorption of Ca2+ and Mg2+ is related to the well know tendency of brushite cements to convert into calcium phosphate phases with a higher Ca: P ratio44,45,46,47. Surprisingly, sodium pyrophosphate and phytic acid behaved differently and expressed both Ca2+ and phosphate ions. However, since the release was measured only at an early time point, an absence or a retarded phase transformation of brushite into lower soluble phases in a long term implantation regime is speculative.
The biological part of our study confirmed recent results of Jamshidi et al.33 regarding the low cytocompatibility of brushite cements formed with citric acid retarder. The human fetal osteoblast cell line hFOB used here is likely more suitable for bone models than tumor derived MG63 exhibiting numerous karyotypic alterations48. Cell number of hFOB was measured via cell counting on day 4, 6 and 10 (Fig. 1) showing that most cement samples were in the range of the retardant free reference with a slight tendency to higher cell numbers for sodium pyrophosphate modification. Citric acid, in contrast, had decreasing cell numbers, especially on day 10. Cell activity measurements confirmed the tendency suggested for cell number and activity on citric acid and sulfuric acid modified cements was even lower than for the reference tracing back to a reduced cell metabolism. In contrast, the addition of sodium pyrophosphate or phytic acid enhanced cell activity up to three times indicating a metabolism activating influence on osteoblasts.
Complementary to osteoblast cell tests, osteoclast precursor cells RAW264.7 were cultured on cement surfaces over 21 days (Fig. 2). This cell line is an established model to study bioceramic dissolution for both sintered hydroxyapatite or tricalcium phosphate ceramics49 as well as for low temperature cements50. The effect of citric acid on osteoclasts was even more striking than for osteoblastic cells showing practically no cell growth or cell activity during the whole culture period. Sulfuric acid also showed very low cell number and cell activity in the first eleven days but increased until day 21. Sodium pyrophosphate and phytic acid were almost comparable to the reference and showed no inhibiting effect on osteoclast proliferation and activity. To prove differentiation of precursor cells into multinucleated osteoclasts by supplementation of RANKL to the medium, staining with TRAP was performed (Fig. 3). On surfaces with citric acid and sulfuric acid, no staining could be found which is in accordance with cell number and cell activity. In contrast, on samples with sodium pyrophosphate and phytic acid all cells differentiated within 15 days and stayed vital until day 21. The results clearly support the hypothesis that citric acid used as a retarder in brushite cements alters the degradation ability of the cement in vivo, most likely because of a cytotoxic effect on osteoclastic cells. However, brushite cements in the current study contained a relatively high concentration of citric acid (0.5 M), since this concentration provided the maximum effect on cement material properties. Previous experiments with other citrate modified cements were performed with 10 times lower concentrations of 0.05 M and showed medium activity of osteoclasts. This demonstrates that citrate ions are not generally cytotoxic for osteoclasts and hence this may open the possibility for further cement optimization regarding cytocompatibility and material properties. The mechanism by which citrate ions inhibit osteoclast formation and activity is however still unclear. A recent study by Drager et al.51 has shown a strong effect of iron chelators on osteoclast function, as iron is needed for mitochondrial biogenesis during osteoclastic differentiation. Since citrate has also the capacity to form iron-chelates52, the formation of such complexes from dissolved calcium citrate from the cement might have provoked iron chelation with the observed inhibition of osteoclast formation. Although also phytic acid is a strong chelating agent for iron53, we assume, that the calcium-phytate complexes formed during cement setting are stable under in vitro neutral pH conditions as demonstrated by Gosselin et al.54 and Grynspan et al.55 in contrast to calcium citrate which dissolves in this environment56. It is then likely that for phytic acid as setting retarder, the free iron concentration is maintained within the cell culture (and hence osteoclast activity), which is not the case for citric acid retarder due to the solubility of calcium citrate.
Conclusion
An in vitro cytocompatibility test with both osteoblastic (hFOB1.19) and osteoclastic (RAW264.7) cell lines revealed a very low cell number and activity for citric acid as a common dicalcium phosphate cement setting retardant. To overcome this drawback, we analyzed the retarders sodium pyrophosphate, sulfuric acid and phytic acid, whereas the best in vitro performance was achieved with phytic acid with a high cell proliferation and cell activity. The mechanical properties were investigated with a compression test, showing that cements with sodium pyrophosphate (CS = 3.2 ± 0.7 MPa) and sulfuric acid (CS = 4.7 ± 1.2 MPa) were mechanically much weaker than cements formed with phytic acid (CS = 13.2 ± 1.9 MPa) or citric acid (CS = 15.0 ± 1.6 MPa). Altogether, phytic acid as a novel setting retardant for dicalcium phosphate cements seemed to be promising in terms of material properties as well as biological behavior. Nevertheless, further knowledge has to be gained by in vivo tests, since complex organisms always have a different behavior compared to mono-cultures.
Materials and Methods
Sample preparation
β-TCP was made of an equimolar mixture of CaHPO4 and CaCO3 (both Merck, Darmstadt, Germany) by sintering for 14 h at 1400 °C followed by 6 h at 1000 °C. Crushing with mortar and pestle followed by grinding for 10 min in a planetary ball mill (PM400, Retsch, Germany) resulted in a medium particle size of 9.4 µm. Monocalcium phosphate anhydrate (MCPA) was used as phosphate source. MCPA (LOT: MV3272, Budenheim) was mixed in a stoichiometric ratio of 0.755:1 with β-TCP within a coffee mill (Privileg, type: PCML2012, Quelle GmbH, Fürth, Germany). The setting retardants citric acid monohydrate (Sigma-Aldrich, LOT: SZBB2010V), sulfuric acid (Merck, LOT: 318 K19691813) and phytic acid (Sigma-Aldrich, LOT: BCBH8997V) were added to the liquid phase, whereas tetra-sodium pyrophosphate decahydrate (Merck, LOT: F1061091 139) was added to the powder. The concentrations were 0.5 mol/L citric acid (0.5 M ca), 5.4 wt% sodium pyrophosphate (5.4 wt% sp), 0.1 mol/L sulfuric acid (0.1 M sa) and 0.1 mol/L phytic acid (0.1 M pa). All samples had a powder to liquid ratio of 3 g/mL and were produced in silicon molds. Samples were set for 1 h at 37 °C and 100% humidity prior to testing.
Characterization
Particle sizes of raw powders were measured with a laser diffractometer (Horiba LA 300 Wet, Horiba, Japan) in ethanol. Characterization of the cement morphology was performed by cross beam scanning electron microscopy (SEM; CB 340, Zeiss, Germany) where the samples were sputtered with 4 nm platinum prior to imaging. The SEM operated at 3 kV with a 2000-fold magnification. For mechanical testing, cuboid samples of 6 mm × 6 mm × 12 mm (n = 12) were prepared and stored in water at 37 °C for 24 h. The mechanical testing device (Zwick GmbH & Co.KG, Ulm, Germany) was operated in compression mode with a 10 kN load cell at a testing speed of 1 mm/min. The solubility of preset cement samples was tested by immersion in 10 ml DMEM cell culture medium for 24 h at 37 °C. Ion concentrations in the immersion liquids were quantified by inductively coupled plasma mass spectrometry (ICP-MS; Varian, Darmstadt, Germany) against standard solutions of 5 and 10 mg/mL (Merck, Darmstadt, Germany). To measure the initial setting time, a Gillmore needle test was performed within a chamber at 37 °C and 100% humidity. Exothermic behavior of isolated samples of 1.5 g cement (n = 5) within 60 min was recorded by a thermometer (Data-Logger Thermometer 306, Conrad Electronic GmbH, Hirschau, Germany). Statistical analysis was calculated with ANOVA using the program SigmaPlot 12.5 (Systat Software, Inc., 2011). The porosity of the cement samples (6 mm × 6 mm × 12 mm) was measured according to Unosson et al.57, by solvent resaturation (n = 12). Therefore, the weight of water saturated samples was recorded followed by drying under vacuum at room temperature. The porosity Φ in percent was calculated according to Eq. (1), where m sat and m dry are the weight of saturated and dry samples, respectively, ρ w is the density of water and V is the apparent volume of the sample.
Phase analysis was performed with x-ray diffraction (XRD; Siemens D5005, Bruker AXS, Karlsruhe, Germany) operating with Cu-Kα radiation (λ = 0.15418 nm) at a voltage of 40 kV and a current of 40 mA. Samples were scanned in a range of 2Θ = 20°–40° with a scan rate of 1.5 s/step and a step size of 0.02°. Phases were identified with the help of ICDD (International Centre for Diffraction Data) database for brushite (PDF-No. 09–0077), β-TCP (PDF-No. 09–0169), MCPA (PDF-No. 09–0390) and monetite (PDF-No. 09–0080).
Biological testing
To exclude any influence of different cement pHs on the biological results, sample discs with a diameter of 15 mm and a height of 2 mm were washed with autoclaved ultrapure water and phosphate buffered saline (PBS) until a physiological pH-value of the washing solution was achieved, followed by twice an immersion in 70% ethanol for 30 min. Cells of the human fetal osteoblast cell line hFOB 1.19 were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM/F12, Gibco, Cat. No.: 313311–028) supplemented with 1% Penicillin-Streptomycin (Gibco, Cat. No.: 15140–122), 0.3 g/L Geneticin G-418 Sulphate (Gibco, Cat. No.: 11811–064) and 10% fetal calf serum (FCS, Gibco, Cat. No.: 10270–106). Cement samples (n = 4) and polystyrene plates serving as reference were placed in 24-well plates and seeded with a cell density on cement surfaces of 105 cells/well and 5 · 104 cells/well on polystyrene. The seeded samples were incubated at 34 °C and 5% CO2 and cell activity and cell number were evaluated at day 4, 6 and 10. Samples were incubated for 30 min with WST-1 reagent (dilution of 1:10) and the cell activity was measured twice with a spectrometer (Tecan SpectraFluor Plus, Tecan, Maennedorf, Switzerland). Cell number was evaluated by a cell counter (Casy, Roche innovates AG, Bielefeld, Germany) after detaching cells from the surface by 12 min incubation with Accutase (PAA, Cat. No.: L11–007). Cell counting was performed in isotonic liquid with a dilution of 1:100. Furthermore, cell tests with the murine macrophage cell line RAW 264.7 were performed to investigate the influence of retardant addition on activity of bone degrading cells. RAW cells were used up to passage 18 and were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; ATCC), supplemented with 1% Penicillin-Streptomycin (Gibco, Cat. No.: 15140–122) and 10% FCS (Gibco, Cat. No.: 10270–106). Therefore, samples (n = 4) with a diameter of 5 mm and a height of 2 mm were placed in 96-well plates, seeded with 6.4 · 103 cells/well and incubated at 37 °C and 5% CO2. After 24 h of culture medium was supplemented with 50 ng/mL receptor activator of the nuclear factor κB ligand (RANKL) to induce differentiation to multinucleated osteoclasts and culture medium was changed every 2–3 days. Osteoclast differentiation was evaluated by tartrate resistant acid phosphatase (TRAP) staining after 5, 11, 15 and 21 days by using a commercial TRAP-staining kit (Sigma, Cat. No. 387). Moreover, cell number of osteoclasts was determined using Auto T4 cell counter (Nexcellom Bioscience, Lawrence, Massachusetts, USA) after detaching cells from cement surface by incubation with Accutase (PAA, Cat. No.: L11–007) for 45 min. Similar to hFOB cell tests, activity of osteoclasts was measured by WST-1 reagent also over 21 days.
References
Tamimi, F. et al. Brushite-collagen composites for bone regeneration. Acta Biomater 4, 1315–1321 (2008).
Ewald, A. et al. Effect of cold-setting calcium- and magnesium phosphate matrices on protein expression in osteoblastic cells. J. Biomed. Mater. Res. B 96B, 326–332 (2011).
Karp, J. M., Shoichet, M. S. & Davies, J. E. Bone formation on two-dimensional (DL-lactide-co-glycolide) (PLGA) films and three-dimensional PLGA tissue engineering scaffolds in vitro. J. Biomed. Mater. Res. A 64A, 388–396 (2003).
Salgado, A. J., Coutinho, O. P. & Reis, R. L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 4, 743–765 (2004).
Tamimi, F., Sheikh, Z. & Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater 8, 474–487 (2012).
Bohner, M., Gbureck, U. & Barralet, J. E. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials 26, 6423–6429 (2005).
Dorozhkin, S. V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 43, 3028–3057 (2008).
Grossardt, C., Ewald, A., Grover, L. M., Barralet, J. E. & Gbureck, U. Passive and Active In Vitro Resorption of Calcium and Magnesium Phosphate Cements by Osteoclastic Cells. Tissue Eng. Part A 16, 3687–3695 (2010).
Stadelmann, V. A., Bretton, E., Terrier, A., Procter, P. & Pioletti, D. P. Calcium phosphate cement augmentation of cancellous bone screws can compensate for the absence of cortical fixation. J. Biomech. 43, 2869–2874 (2010).
Barralet, J. E. et al. Cortical bone screw fixation in lonically modified apatite cements. J. Biomed. Mater. Res. B 73B, 238–243 (2005).
Mermelstein, L. E., Chow, L. C., Friedman, C. & Crisco, J. J. The reinforcement of cancellous bone screws with calcium phosphate cement. J. Orthop. Trauma 10, 15–20 (1996).
Carrodeguas, R. G. & De Aza, S. alpha-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 7, 3536–3546 (2011).
Mirtchi, A. A., Lemaitre, J. & Munting, E. Calcium-phosphate cements - action of setting regulators on the properties of the beta-tricalcium phosphate monocalcium phosphate cements. Biomaterials 10, 634–638 (1989).
Mirtchi, A. A., Lemaitre, J. & Terao, N. Calcium-phosphate cements - study of the beta-tricalcium phosphate - monocalcium phosphate system. Biomaterials 10, 475–480 (1989).
Cama, G. et al. A novel method of forming micro- and macroporous monetite cements. J. Mater. Chem. B 1, 958–969 (2013).
Sahin, E. & Ciftcioglu, M. Monetite promoting effect of NaCl on brushite cement setting kinetics. J. Mater. Chem. B 1, 2943–2950 (2013).
Kanter, B., Geffers, M., Ignatius, A. & Gbureck, U. Control of in vivo mineral bone cement degradation. Acta Biomater 10, 3279–3287 (2014).
Klammert, U., Ignatius, A., Wolfram, U., Reuther, T. & Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 7, 3469–3475 (2011).
Holzapfel, B. M. et al. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliver. Rev 65, 581–603 (2013).
Theiss, F. et al. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 26, 4383–4394 (2005).
Xia, Z. D. et al. In vitro biodegradation of three brushite calcium phosphate cements by a macrophage cell-line. Biomaterials 27, 4557–4565 (2006).
Sheikh, Z. et al. In vitro degradation and in vivo resorption of dicalcium phosphate cement based grafts. Acta Biomater. 26, 338–346 (2015).
Oliveira, C., Ferreira, A. & Rocha, F. Dicalcium phosphate dihydrate precipitation: Characterization and crystal growth. Chem. Eng. Res. Des. 85, 1655–1661 (2007).
Bohner, M., Lemaitre, J. & Ring, T. A. Effects of sulfate, pyrophosphate, and citrate ions on the physicochemical properties of cements made of beta-tricalcium phosphate-phosphoric acid-water mixtures. J. Am. Ceram. Soc. 79, 1427–1434 (1996).
Sahin, E. & Ciftcioglu, M. Monetite promoting effect of citric acid on brushite cement setting kinetics. Mater. Res. Innovat 18, 138–145 (2014).
Grover, L. M., Gbureck, U., Young, A. M., Wright, A. J. & Barralet, J. E. Temperature dependent setting kinetics and mechanical properties of beta-TCP-pyrophosphoric acid bone cement. J. Mater. Chem. 15, 4955–4962 (2005).
Bohner, M., Merkle, H. P., Landuyt, P. V., Trophardy, G. & Lemaitre, J. Effect of several additives and their admixtures on the physico-chemical properties of a calcium phosphate cement. J. Mater. Sci.: Mater. Med 11, 111–116 (2000).
Bohner, M. et al. Compositional changes of a dicalcium phosphate dihydrate cement after implantation in sheep. Biomaterials 24, 3463–3474 (2003).
Kuemmerle, J. M. et al. Assessment of the suitability of a new brushite calcium phosphate cement for cranioplasty - an experimental study in sheep. J. Cranio Maxill. Surg. 33, 37–44 (2005).
Barralet, J. E., Grover, L. M. & Gbureck, U. Ionic modification of calcium phosphate cement viscosity. Part II: hypodermic injection and strength improvement of brushite cement. Biomaterials 25, 2197–2203 (2004).
Hofmann, M. P., Mohammed, A. R., Perrie, Y., Gbureck, U. & Barralet, J. E. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 5, 43–49 (2009).
Fleisch, H. & Bisaz, S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am. J. Physiol. 203, 671 (1962).
Jamshidi, P., Bridson, R. H., Wright, A. J. & Grover, L. M. Brushite cement additives inhibit attachment to cell culture beads. Biotechnol. Bioeng 110, 1487–1494 (2013).
Marshall, R. W. & Nancollas, G. H. Kinetics of crystal growth of dicalcium phosphate dihydrate. J. Phys. Chem. 73, 3838 (1969).
Konishi, T. et al. Fabrication of Novel Biodegradable alpha-Tricalcium Phosphate Cement Set by Chelating Capability of Inositol Phosphate and Its Biocompatibility. J. Nanomater. 864374 (2013).
Takahashi, S. et al. Fabrication of novel bioresorbable beta-tricalcium phosphate cement on the basis of chelate-setting mechanism of inositol phosphate and its evaluation. J. Ceram. Soc. Jpn 119, 35–42 (2011).
Christel, T., Christ, S., Barralet, J. E., Groll, J. & Gbureck, U. Chelate Bonding Mechanism in a Novel Magnesium Phosphate Bone Cement. J. Am. Ceram. Soc. 98, 694–697 (2015).
Grases, F., Ramis, M. & Costa-Bauza, A. Effects of phytate and pyrophosphate on brushite and hydroxyapatite crystallization - Comparison with the action of other polyphosphates. Urol. Res 28, 136–140 (2000).
Barralet, J. E., Gaunt, T., Wright, A. J., Gibson, I. R. & Knowles, J. C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J. Biomed. Mater. Res. 63, 1–9 (2002).
Geffers, M., Groll, J. & Gbureck, U. Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements. Materials 8, 2700–2717 (2015).
Lopez-Heredia, M. A. et al. The effect of ball milling grinding pathways on the bulk and reactivity properties of calcium phosphate cements. J. Biomed. Mater. Res. B 98B, 68–79 (2011).
Giocondi, J. L., El-Dasher, B. S., Nancollas, G. H. & Orme, C. A. Molecular mechanisms of crystallization impacting calcium phosphate cements. Philos. T. R. Soc. A 368, 1937–1961 (2010).
Qiu, S. R. & Orme, C. A. Dynamics of Biomineral Formation at the Near-Molecular Level. Chem. Rev. 108, 4784–4822 (2008).
Constantz, B. R. et al. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J. Biomed. Mater. Res. 43, 451–461 (1998).
Penel, G. et al. Raman microspectrometry studies of brushite cement: In vivo evolution in a sheep model. Bone 25, 81S–84S (1999).
Bannerman, A., Williams, R. L., Cox, S. C. & Grover, L. M. Visualising phase change in a brushite-based calcium phosphate ceramic. Sci. Rep. 6 (2016).
Tas, A. C. Transformation of Brushite (CaHPO4·2H2O) to Whitlockite (Ca9Mg(HPO4)(PO4)(6)) or other CaPs in physiologically relevant solutions. J. Am. Ceram. Soc. 99, 1200–1206 (2016).
Subramaniam, M. et al. Further characterization of human fetal osteoblastic hFOB 1.19 and hFOB/ER alpha cells: bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J. Cell. Biochem. 87, 9–15 (2002).
Detsch, R., Mayr, H. & Ziegler, G. Formation of osteoclast-like cells on HA and TCP ceramics. Acta Biomater. 4, 139–148 (2008).
Le Nihouannen, D., Hacking, S. A., Gbureck, U., Komarova, S. V. & Barralet, J. E. The use of RANKL-coated brushite cement to stimulate bone remodelling. Biomaterials 29, 3253–3259 (2008).
Drager, J., Sheikh, Z., Zhang, Y. L., Harvey, E. J. & Barralet, J. E. Local delivery of iron chelators reduces in vivo remodeling of a calcium phosphate bone graft substitute. Acta Biomater. 42, 411–419 (2016).
Gomez, M. A. et al. Protein Tyrosine Phosphatases Are Regulated by Mononuclear Iron Dicitrate. J. Biol. Chem. 285, 24620–24628 (2010).
Minihane, A. M. & Rimbach, G. Iron absorption and the iron binding and anti-oxidant properties of phytic acid. Int. J. Food Sci. Tech 37, 741–748 (2002).
Gosselin, R. E. & Coghlan, E. R. The stability of complexes between calcium and orthophosphate, polymeric phosphate, and phytate. Arch. Biochem. Biophys. 45, 301–311 (1953).
Grynspan, F. & Cheryan, M. Calcium phytate - effect of ph and molar ratio on in vitro solubility. J. Am. Oil Chem. Soc 60, 1761–1764 (1983).
Goss, S. L., Lemons, K. A., Kerstetter, J. E. & Bogner, R. H. Determination of calcium salt solubility with changes in pH and P-CO2, simulating varying gastrointestinal environments. J. Pharm. Pharmacol 59, 1485–1492 (2007).
Unosson, J. E., Persson, C. & Engqvist, H. An evaluation of methods to determine the porosity of calcium phosphate cements. J. Biomed. Mater. Res. B 103, 62–71 (2015).
Acknowledgements
The authors would like to acknowledge financial support from the Deutsche Forschungsgemeinschaft (DFG GB1/15-1 and DFG IG18/11-1) and the possibility of measuring porosity at the Chair of Pharmaceutics and Biopharmacy (Prof. Dr. Dr. Lorenz Meinel) at the University of Würzburg. Additionally, we would like to acknowledge financial support by DFG State Major Instrumentation Programme, funding the crossbeam scanning electron microscope Zeiss CB 340 (INST 105022/58-1 FUGG).
Author information
Authors and Affiliations
Contributions
S.M. undertook the majority of the experimental work and wrote the initial manuscript. C.B. and M.S. helped in analysing the samples. J.E.B. and A.I. advised on the experimental design and modified the manuscript. U.G. planned and supervised the work and helped writing and modifying the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meininger, S., Blum, C., Schamel, M. et al. Phytic acid as alternative setting retarder enhanced biological performance of dicalcium phosphate cement in vitro . Sci Rep 7, 558 (2017). https://doi.org/10.1038/s41598-017-00731-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00731-6
- Springer Nature Limited
This article is cited by
-
Biological and mechanical performance of calcium phosphate cements modified with phytic acid
Journal of Materials Science: Materials in Medicine (2024)
-
Fabrication of a novel bone adhesive (crosslinked phytic acid-gelatin coordinated with magnesium phosphate and calcined dolomite, and montmorillonite) for enhancing adhesion strength and biocompatibility
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2024)
-
Osteogenic effect of magnesium oxychloride cement modified with phytic acid and loaded with strontium ranelate
Biomaterials Research (2023)
-
Enzymatically hardened calcium phosphate biocement with phytic acid addition
Journal of Materials Science: Materials in Medicine (2020)