Abstract
The roles of CD4 + T cells and CD8 + T cells in hepatitis B virus (HBV) infection have been well documented. However, the role of innate immunity in HBV infection remains obscure. Here we examined the effect of activation of innate immunity by polyinosinic: polycytidylic acid (PolyI:C) on HBV infection. A chronic HBV replication mouse model was established by hydrodynamical injection of pAAV/HBV1.2 plasmid into C57BL/6 mice. We found that HBV did not seem to induce an active NK-cell response in the mouse model. Early PolyI:C treatment markedly decreased serum HBV levels and led to HBV clearance. Following PolyI:C injection, NK cells were activated and accumulated in the liver. Depletion of NK cells markedly attenuated the anti-HBV activity of PolyI:C. Moreover, we found that IFN-γ production from NK cells was essential for the antiviral effect of PolyI:C in the model. Importantly, activation of NK cells by PolyI:C could also lead to HBV suppression in HBV-tolerant mice and HBV-transgenic mice. These results suggest that activated NK cells might suppress HBV and contribute to HBV clearance during natural HBV infection. In addition, therapeutic activation of NK cells may represent a new strategy for the treatment of chronic HBV infection.
Similar content being viewed by others
Introduction
The hepatitis B virus (HBV) is a noncytopathic, hepatotropic DNA virus that causes acute and chronic hepatitis often leading to liver cirrhosis as well as hepatocellular carcinoma1. The chance of clearing HBV infection is dependent on the age of HBV exposure. Ninety-five percent of adult-acquired infections lead to spontaneous clearance, whereas up to 90% of exposed neonates fail to resolve HBV and develop chronic infection2. The outcome of HBV infection in humans (viral clearance or viral persistence) is determined by complicated, as yet not fully understood, interactions between HBV and the immune system3. Both innate and adaptive components of the immune system mediate protective immunity against a viral infection, with innate responses being important for limiting viral replication and spreading very early after infection, as well as for a timely orchestration of virus-specific adaptive responses4. The liver is considered as an innate immune organ as it is highly enriched with innate immune cells that play a critical role in orchestrating the body’s host defense5. Surprisingly, HBV appears to act as a “stealth virus” and does not induce a measurable innate immune response in the infected liver6, 7, it seems to employ active strategies to evade innate immune responses to achieve persistent infection8,9,10. We speculate that boosting of the innate immune response during the early phase of HBV infection may be useful in reducing viral spread and preventing HBV persistence.
Natural killer (NK) cells have been viewed as the most important effectors of the initial antiviral innate immune system11. Compared with their relatively low frequency in the peripheral lymphatic system, NK cells are highly enriched in the liver12, 13, the site of HBV replication, implying that HBV has to evade NK cell-mediated immune responses to establish a persistent viral infection14. Based on the abundance of NK cells in the liver and their ability to produce antiviral cytokines, it is possible that activated NK cells might inhibit viral replication during HBV infection. To test this hypothesis, in the present study, we attempted to elucidate the role of NK cells in HBV infection by monitoring the ability of PolyI:C, a potent stimulator for NK cells15, to control HBV infection.
In the past few years, most in vivo studies on the mechanisms of HBV tolerance have been approached by using HBV transgenic mice which are inherently tolerant to HBV virus and have limitations in addressing what happens at the onset of HBV infection that influences the final outcomes of HBV infection16, 17. Recently, a nontransgenic mouse model of HBV tolerance was established by hydrodynamic injection (HI) of the plasmid pAAV/HBV1.2 into immunocompetent mice18. The characteristics of this mouse model are analogous to those of human chronic HBV infections. In the current study, we used this model to examine the effects of PolyI:C on HBV infection. We found that PolyI:C treatment could control HBV infection in a NK cell and IFN-γ-dependent manner.
Results
Establishment of a HBV-tolerant mouse model
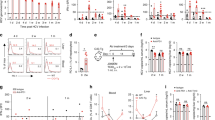
To address whether activation of innate immune system could influence the final outcomes of HBV infection, we utilized a nontransgenic HBV-carrier mouse model developed by hydrodynamic injection of the pAAV/HBV1.2 plasmid into C57BL/6 mice19. Serum HBsAg (Fig. 1A) and HBeAg (Fig. 1B) can be detected 1 day post pAAV/HBV1.2 injection and remained high 6 weeks post injection. There were no detectable serum anti-HBs in these animals (data not shown). Serum alanine aminotransaminase activity (ALT) increased on day 1 due to the hydrodynamic procedure and then returned to baseline levels thereafter (Fig. 1C), suggesting no hepatitis flare following pAAV/HBV1.2 injection. Thus, hydrodynamic injection of pAAV/HBV1.2 leads to a persistent HBV gene expression in mice, which mimics the immune tolerant phase of chronic HBV infection in humans.
Hydrodynamic injection of pAAV/HBV1.2 plasmid leads to the persistence of HBV. (A,B,C) C57BL/6 mice received 6 μg of pAAV/HBV1.2 plasmid through hydrodynamic injection. Serum HBsAg (A), HBeAg (B) and ALT (C) levels were detected at different time points after injection. Data are shown as mean ± SEM, n = 10.
NK cells remain inactivated in the HBV mouse model
We first examined the number and activation status of NK cells in pAAV/HBV1.2-injected mice compared to control mice. Considering that HI of DNA plasmid may induce innate immune responses, mice received HI of pAAV/control plasmid or HI of PBS or intravenous injection (IV) of PBS were used as controls. We found that the percentage of hepatic NK cells did not significantly change after pAAV/HBV1.2 injection (Fig. 2A). Also, the expression of the early activation marker CD69 on hepatic NK cells was not increased in HBV-injected mice compared to all the control groups (Fig. 2B). These data indicated that HBV did not elicit a strong NK-cell response in the HBV mouse model, which may be favourable for HBV replication and persistence.
HBV does not seem to induce an active NK-cell response in the mouse model. C57BL/6 mice received 6 μg of pAAV/HBV1.2 plasmid through hydrodynamic injection. Mice received HI of pAAV/control plasmid or HI of PBS or intravenous injection (IV) of PBS were used as controls. Lymphocytes were isolated from the liver at the indicated time points after injection. (A) The percentages of NK cells (CD3-NK1.1+) were analyzed using anti-NK1.1 and anti-CD3 antibodies. (B) The surface expression of CD69 on gated NK cells (CD3-NK1.1+) was also analyzed. Data are shown as mean ± SEM, n = 4.
Early PolyI:C treatment leads to HBV clearance
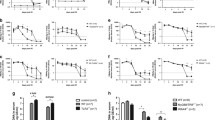
To investigate the effect of NK-cell activation on HBV infection, mice were hydrodynamically injected with pAAV/HBV1.2 and co-treated with PolyI:C. Application of PolyI:C inhibited the initial increase of serum HBsAg and HBeAg levels and eliminated the expression of the two proteins at 5 weeks post injection (Fig. 3A and B). HBV DNA copies in the serum of PolyI:C-treated and mock-treated mice were also assayed. PolyI:C treatment dramatically reduced the level of HBV replication 2 weeks and 5 weeks post pAAV/HBV1.2 injection (Fig. 3C), and HBcAg protein expression in liver tissues of PolyI:C-treated mice could not be detected 5 weeks post injection(Fig. 3D). These data demonstrate that PolyI:C treatment induced clearance of HBV. Additionally, PolyI:C treatment did not increase serum ALT levels (Fig. 3E), indicating that PolyI:C suppressed HBV expression in a noncytolytic process.
Early PolyI:C treatment prevents HBV persistence in the HBV mouse model. C57BL/6 mice were injected with HBV plasmid and chronically treated with Poly I:C as described in Materials and Methods. Titers of serum HBsAg (A), HBeAg (B), HBV DNA (C), and ALT (E) were determined at indicated time points. (D) At 5 weeks post injection, Livers were processed for HBcAg expression (original magnification, x100). Data are shown as mean ± SEM; *p < 0.05, **p < 0.01. n = 6–8 in each group.
PolyI:C induced clearance of HBV via an NK-dependent mechanism
Next, we investigated whether the anti-HBV activity of PolyI:C was due to the effect of PolyI:C on NK cells. We found that NK cells were dramatically increased in the liver but significantly decreased in the spleen following PolyI:C treatment, regardless of whether the treated mice were HBV-bearing or not (Fig. 4A). The expression of activation marker CD69 on hepatic NK cells was also enhanced in PolyI:C-treated mice (Fig. 4B). So, injection with PolyI:C caused recruitment and strong activation of NK cells in the liver. To further elucidate the role of NK cells, anti-ASGM1 Ab was used to deplete NK cells (Fig. 5A). As shown in Fig. 5B, NK cell depletion potently impaired HBV clearance and resulted in persistently maintained serum HBsAg in PolyI:C treated mice. Serum HBV DNA levels also confirmed that NK cell depletion could abrogate the anti-HBV activity of PolyI:C(Fig. 5C). These findings suggest NK cells played a critical role in PolyI:C-induced anti-HBV activity.
PolyI:C treatment activates NK cells and induces accumulation of NK cells in the liver. Mice were injected with HBV plasmid or control plasmid and chronically treated with Poly I:C as described in Materials and Methods. Lymphocytes were isolated from the liver and spleen and analyzed by flow cytometry. (A) The percentages of NK cells (CD3-NK1.1+) were analyzed using anti-NK1.1 and anti-CD3 antibodies. (B) The surface expression of CD69 on gated NK cells (CD3-NK1.1+) was also analyzed. Data are shown as mean ± SEM; **p < 0.01, ***p < 0.001. n = 4–5 in each group.
Depletion of NK cells abrogates the anti-HBV activity of PolyI:C. Mice were injected with HBV plasmid and chronically treated with control Ab or anti–ASGM-1 Ab and Poly I:C, as described in Materials and Methods, for 5weeks. (A) Depletion of NK cells (NK1.1 + CD3−) was confirmed by flow cytometry. (B) Titers of serum HBsAg were determined at the indicated time points post injection. *p < 0.05 vs. PBS + IgG group or PBS + anti–ASGM-1 group or Poly I:C + anti–ASGM-1 group. (C) Titers of serum HBV DNA copies were determined at 2 weeks post injection. Data are shown as mean ± SEM; *p < 0.05, **p < 0.01. n = 4–6 in each group.
Anti-HBV activity of PolyI:C is dependent on NK cell-derived IFN-γ
IFN-γ is known to inhibit HBV gene expression noncytopathically20. As shown in Fig. 6A and B, PolyI:C treatment increased intracellular expression of IFN-γ by NK cells as well as circulating serum IFN-γ levels. Depletion of NK cells significantly decreased serum IFN-γ levels(Fig. 6C), suggesting that IFN-γ was produced by NK cells. To examine the role of IFN-γ in PolyI:C -induced antiviral activity, we monitored the ability of PolyI:C to inhibit HBV gene expression in IFN-γ−/− mice (mice deficient in IFN-γ). As shown in Fig. 6D, IFN-γ deficiency led to a significant increase in expression of HBsAg in PolyI:C-treated mice, suggesting that IFN-γ was required for the anti-HBV effect of PolyI:C.
Anti-HBV activity of PolyI:C is dependent on NK cell-derived IFN-γβ. (A,B) C57BL/6 mice were injected with HBV plasmid and chronically treated with Poly I:C as described in Materials and Methods. Intracellular cytokine staining was performed to examine IFN-γ production from NK cells (CD3-NK1.1+) at 6 days after pAAV/HBV1.2 injection. (C) Mice were injected with HBV plasmid and chronically treated with anti–ASGM-1 Ab and Poly I:C as described in Materials and Methods. Serum levels of IFN-γ at 6 days after pAAV/HBV1.2 injection were determined by ELISA. (D) IFN-γ−/− mice were hydrodynamically injected with HBV plasmid and chronically treated with Poly I:C. Titers of serum HBsAg were determined at the indicated time points post injection. Data are shown as mean ± SEM; *p < 0.05, **p < 0.01. n = 4–6 in each group.
PolyI:C therapy leads to NK cell-dependent HBV suppression in HBV-tolerant mice and HBV-transgenic mice
Next, we examined whether activation of NK cells by PolyI:C could also suppress HBV in HBV-tolerant mice and HBV-transgenic mice. C57BL/6 mice received hydrodynamic injection of pAAV/HBV1.2 and then PolyI:C or PBS 2 weeks later. As shown in Fig. 7A and B, serum levels of HBsAg and HBeAg significantly decreased in HBV-tolerant mice after 3 weeks post PolyI:C therapy, but depletion of NK cells by anti-ASGM1 impaired the anti-HBV activity of PolyI:C, suggesting that NK cells were implicated in PolyI:C therapy for chronic HBV infections. Furthermore, PolyI:C therapy could also suppress HBsAg expression and HBV replication in HBV-transgenic mice, and this is dependent on NK cells (Fig. 7C and D). These data indicate that activation of NK cells by PolyI:C might have therapeutic potential for the treatment of chronic HBV-infected patients.
PolyI:C therapy leads to NK cell-dependent anti-HBV activity in HBV-tolerant mice and HBV-transgenic mice. (A,B) C57BL/6 mice were injected with HBV plasmid, 2 weeks later, HBV-tolerant mice were chronically treated with control Ab or anti–ASGM-1 Ab and Poly I:C, as described in Materials and Methods, for 3 weeks. Titers of serum HBsAg (A) and HBeAg (B) were determined at the indicated time points post injection. *p < 0.05 vs. PBS + IgG group or PBS + anti–ASGM1group or Poly I:C + anti–ASGM-1group. Data are shown as mean ± SEM; n = 5 in each group. (C,D) HBV-transgenic mice were chronically treated with control Ab or anti–ASGM-1 Ab and Poly I:C, as described in Materials and Methods, for 3 weeks. Titers of serum HBsAg (C) and HBV DNA (D) were determined at the indicated time points post injection. *p < 0.05 vs. PBS + IgG group or PBS + anti–ASGM1group or Poly I:C + anti–ASGM-1group. Data are shown as mean ± SEM; n = 3–5 in each group.
Kupffer cells (KCs) are required for the anti-HBV activity of NK cells induced by PolyI:C in the mouse model
KCs, the resident macrophage population of the liver, are poised to initiate innate immune responses by secretion of cytokines and direct cellular contact when primed by pathogen-derived products21. To test whether KCs might be involved in the PolyI:C-induced NK cell response, we depleted KCs by clodronate-liposomes. Successful KC depletion was verified by flow cytometry analysis of reduction in F4/80+ cells as described before (data not shown)22. Depletion of KCs significantly inhibited the accumulation of NK cells in the liver (Fig. 8A), and suppressed the secretion of IFN-γ by NK cells (Fig. 8B), suggesting that KC were involved in the NK cell response. Accordingly, PolyI:C-induced HBV suppression was impaired in KC-depleted mice (Fig. 8C), indicating that KCs were indispensable for HBV suppression in PolyI:C-treated mice. Further in vitro data also showed that PolyI:C could not directly stimulate NK cells to secrete IFN-γ; however, when co-cultured with PolyI:C-stimulated KCs, NK cells were able to produce IFN-γ (Fig. 8D). These results suggested that NK cell anti-HBV activity depends on the presence of KCs in PolyI:C-treated mice.
The presence of KCs is necessary for NK cell anti-HBV activity in PolyI:C-treated mice. (A,B,C) C57BL/6 mice were injected with HBV plasmid, 2 weeks later, HBV-tolerant mice were chronically treated with PBS or PBS-liposomes + PolyI:C or clodronate-liposomes + PolyI:C, as described in Materials and Methods, for 3 weeks. (A) The percentages of NK cells (CD3-NK1.1+) were analyzed using anti-NK1.1 and anti-CD3 antibodies. (B) Intracellular cytokine staining was performed to examine IFN-γ production from NK cells (CD3-NK1.1+). (C) Titers of serum HBsAg were determined at the indicated time points post injection. *p < 0.05 vs. PBS or clodronate-liposomes + PolyI:C. Data are shown as mean ± SEM; n = 5 in each group. (D) Hepatic NK cells were cultured alone or cocultured with KCs at a ratio of 1:1 and stimulated by poly I:C (100 μg/mL). After 48 h, IFN-γ secretion in the supernatant was quantified by ELISA. Data are expressed as mean ± SEM; *p < 0.05. **p < 0.01.
Discussion
In the present study, a mouse model for HBV persistence was generated by hydrodynamically injecting an HBV genome-containing plasmid (pAAV/HBV1.2) into mice. Early application of PolyI:C markedly decreased serum HBV levels and prevented HBV persistence. Also, PolyI:C therapy led to HBV suppression in HBV-tolerant mice and HBV-transgenic mice. We found that PolyI:C -induced anti-HBV activity was dependent on NK cells and IFN-γ, which is an previously undescribed anti-HBV mechanism of PolyI:C.
NK cells are abundant in the normal liver and may play a critical role in immune responses to infections of this organ5. In this study, we did not observe any difference in the abundance or activation of the NK cell populations in the livers of HBV-injected versus control mice, supporting the view that HBV is capable of sneaking through the front line of host defenses.
Toll-like receptors (TLRs) sense pathogen-associated molecule patterns(PAMPs) and activate antiviral mechanisms, including intracellular antiviral pathways and the production of antiviral cytokines, resulting in a suppression of HBV replication and spreading23, 24. Nucleic acids derived from pathogens, including single- or double-stranded RNAs, represent a major class of PAMPs25. The synthetic analogue of double-stranded RNA Poly I:C, which might reflect a natural genetic product from a variety of viruses during replication, can be sensed by TLR3 and melanoma differentiation-associated gene 5 (MDA-5)26. We showed here that early Poly I:C treatment could reduce HBV antigen loads and prevent HBV persistence in mice, indicating that vigorous innate immune responses in the early phase of HBV infection might inhibit initial HBV replication and gene expression and eventually promoted viral clearance.
Apart from its direct antiviral activity, Poly I:C also activated immune cells. It has been shown to enhance the cytotoxicity of NK cells and macrophages and to activate T cells27. In this paper, we presented that NK cells were dramatically recruited into and activated in the liver following PolyI:C injection. Furthermore, depletion of NK cells abrogated the anti-HBV activity of PolyI:C, suggesting that intrahepatic NK cells might control HBV infection if they become activated.
Notably, our results demonstrated that NK cell–mediated anti-HBV activity induced by Poly I:C was dependent on cooperation with KCs. Both in vivo and in vitro data showed that KCs were critical for NK cell–derived IFN-γ production. It was shown that human KCs might activate NK cells through IL-12 and IL-18 and direct cell contact in response to TLR ligands stimulation21. IL-12 has been reported to be involved in promoting NK cell accumulation in the liver28. TNF-α, another cytokine released mainly by activated macrophages, could also recruit NK cells to liver and enhance NK cell IFN-γ production in vitro and in vivo 29, 30. These study may be helpful for further analysis of the mechanisms KCs use to activate NK cells in our model.
As to date, attempts to therapeutically restore HBV–specific T cell responses in chronically HBV-infected patients have not been very successful, probably due to functional exhaustion or physical deletion of virus-specific T cells31. In contrast, since NK cells do not recognize viral peptide antigens, they are less likely to be affected by high antigen loads. Even HBV is poorly sensed by the innate immune system, NK cells remain well-poised to respond to HBV infection given the very low basal expression of MHC class I by liver cells32 and given the high frequency of NK cells in the liver, where NK cells make up approximately 30% of human intrahepatic lymphocytes. Thus, it may be possible to use pharmacological approaches to activate the intrahepatic NK cell population, thereby rapidly and efficiently enhancing the production of antiviral cytokines in the liver of infected patients. Previous studies have found that activation of NKT cells, another major innate immune cell in liver, could inhibit HBV replication in vivo 33, 34. These findings, together with our own, support the view that activating innate immune responses may be a therapeutic approach to compensate for the failure of acquired immune system to treat chronic HBV infection.
Previous studies have found that HBV proteins like HBxAg protein35, 36, polymerase37 can interfere with innate immunity, thereby attenuating the antiviral response of the innate immune system and ultimately contributing to the establishment of persistent infections. The liver receives 80% of its blood supply from the gut through portal vein, as a result, the liver is continuously exposed to gut microbial products which might activate innate immunity during primary HBV infection38. Our results suggest that outcomes of HBV infection might be influenced by the initial activation and strength of the immune response during the early phase of HBV infection in human beings.
In conclusion, this study demonstrates that PolyI:C activates NK cells to secrete antiviral cytokine IFN-γ and induces accumulation of NK cells in the liver. Early PolyI:C treatment leads to NK cell-dependent clearance of HBV in the mouse model; activation of NK cells by PolyI:C therapy could also suppress HBV in HBV-tolerant mice and HBV-transgenic mice. Based on these results, it is conceivable that NK cells are implicated in host defense against HBV during natural infection. In addition, the results suggest that pharmacological activation of NK cells might have a good potential for treating chronic hepatitis B.
Materials and Methods
Animals
Male C57BL/6 mice (5–7 weeks old) were obtained from and raised at Chongqing Medical University. HBV transgenic mice were obtained from Infectious Disease Center of No. 458 Hospital (Guangzhou, China). IFN-γ−/− mice were purchased from Model Animal Research Center (Nanjing, China). All mice were housed under specific pathogen-free conditions and used according to the regulations of animal care of Chongqing Medical University. The study protocol was approved by the ethical committee of the Second Affiliated Hospital of Chongqing Medical University and the methods were carried out in accordance with the approved guidelines.
Plasmids injection and PolyI:C treatment
pAAV/HBV1.2 plasmid was kindly provided by Dr. Pei-Jer Chen18. pAAV/Control plasmid was used as a control plasmid for pAAV/HBV1.2. The plasmids were isolated by using an endotoxin-free kit (Qiagen, Inc.). Hydrodynamic injection of the pAAV/HBV1.2 or pAAV/Control plasmid into mice was performed as described39, 40.
Early PolyI:C treatment. Mice were injected intravenously with 5 μg/g bodyweight poly I:C (Sigma, St. Louis, MO) 24 hours before 6 μg pAAV/HBV1.2 injection and then treated with poly I:C every 48 hours for 3 weeks. Control mice received pAAV/HBV1.2 plasmid plus saline injection.
PolyI:C therapy. C57BL/6 mice were hydrodynamically injected with 6 μg pAAV/HBV1.2 plasmid, 2 weeks later, HBV-tolerant mice were injected intravenously with 5 μg/g bodyweight poly I:C every 48 hours for 3 weeks. Control mice received pAAV/HBV1.2 plasmid plus saline injection.
Detection of HBV antigen and liver transaminase activities
Serum levels of HBsAg, HbeAg were determined with commercially available radioimmunoassay (RIA) kits (Beijing North Institute of Biological Technology, Beijing, China). Serum enzyme activities of alanine aminotransferase (ALT) were measured using commercially available kit (Rong Sheng, Shanghai, China).
HBV DNA detection
Serum HBV DNA copies were assessed by quantitative PCR using a commercial kit for HBV DNA (AMPLLY, Xiamen, China).
Immunohistochemistry
HbcAg+ hepatocytes in liver tissue were examined by immunohistochemistry as previously described40.
Cell preparations and fluorometric analysis
Splenocytes and liver mononuclear cells (MNCs) were isolated essentially as described previously41. Abs against the following proteins were used: anti-NK1.1 (PK136), anti-CD3 (145-2C11), anti-CD69 (H1.2F3), anti-IFN-γ (XMG1.2), anti-F4/80 (BM8) (all of above from eBioscience). Cells in a single-cell suspension were stained with fluorescence-labeled mAbs for surface antigens according to a standard protocol. To detect IFN-γ expression, hepatic MNCs were stimulated with PMA (30 ng/ml; Sigma-Aldrich) and ionomycin (1 µg/ml; Sigma-Aldrich) and monensin (5 µg/ml; Sigma-Aldrich). After 4 h of culture at 37 °C and 5% CO2, the cells were harvested and labeled by anti-NK1.1, anti-CD3. Then the cells were fixed, permeabilized, and stained with anti-IFN-γ. Data were collected on a BD FACSCantoTM II cytometer and analyzed using FlowJo analysis software 7.6.1(Tristar).
Cell depletion
To deplete NK cells, mice were intravenously injected with 30 μg of anti-ASGM1 (Wako, Richmond, VA) or control rabbit IgG per mouse 24 hours before challenge. To chronically deplete NK cells, mice were treated with anti-ASGM1 every 3 days. For KC depletion, mice were injected intravenously with 100 μl of clodronate-liposomes or PBS-liposomes (provided by Dr. N. van Rooijen, Vrije Universiteit, Amsterdam, The Netherlands) 48 hours before challenge. To chronically deplete KCs, mice were treated with clodronate-liposomes every 1week. The elimination of NK cells and KCs was confirmed by flow cytometry.
ELISA
Serum levels of IFN-γ were assessed using a ELISA kit (Dakewei, Beijing, China), according to the manufacturer’s instructions.
Isolation of NK Cells and KCs
Liver NK cells were separated by negative magnetic cell sorting using the NK Cell isolation kit II according to the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA, USA). Approximately 90% of the magnetic cell sorting-purified cells were NK1.1 + CD3−.
Single cell suspensions of hepatic non-parenchymal cells (NPCs) were prepared using two-step collagenase perfusion method as described before42. KCs were enriched by positive magnetic cell sorting using phycoerythrin (PE)-conjugated anti-F4/80 mAb and anti-PE-MicroBeads according to the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA, USA). The separated cells were ≥85% pure.
Culture of hepatic NK cells and KCs
Purified NK cells were cultured alone or co-cultured with KCs at 1:1 ratio in RPMI-1640 complete medium for 48 hours. PolyI:C was used at a final concentration of 100 μg/ml. IFN-γ secretion in the supernatant was quantified by ELISA.
Statistical analysis
All data are expressed as the mean and standard error of the mean (SEM). Results were analyzed by using Student’s t test or ANOVA where appropriate. P < 0.05 was considered statistically significant for all tests.
References
Rehermann, B. & Bertoletti, A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology 61, 712–721, doi:10.1002/hep.27323 (2015).
Frodsham, A. J. Host genetics and the outcome of hepatitis B viral infection. Transplant immunology 14, 183–186, doi:10.1016/j.trim.2005.03.006 (2005).
Bertoletti, A., Maini, M. K. & Ferrari, C. The host-pathogen interaction during HBV infection: immunological controversies. Antiviral therapy 15(Suppl 3), 15–24, doi:10.3851/IMP1620 (2010).
Durantel, D. & Zoulim, F. Innate response to hepatitis B virus infection: observations challenging the concept of a stealth virus. Hepatology 50, 1692–1695, doi:10.1002/hep.23361 (2009).
Gao, B., Jeong, W. I. & Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 47, 729–736, doi:10.1002/hep.22034 (2008).
Wieland, S., Thimme, R., Purcell, R. H. & Chisari, F. V. Genomic analysis of the host response to hepatitis B virus infection. P Natl Acad Sci USA 101, 6669–6674, doi:10.1073/pnas.0401771101 (2004).
Guidotti, L. G., Isogawa, M. & Chisari, F. V. Host-virus interactions in hepatitis B virus infection. Current opinion in immunology 36, 61–66, doi:10.1016/j.coi.2015.06.016 (2015).
Michel, M. L., Deng, Q. & Mancini-Bourgine, M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: perspectives and challenges. Journal of hepatology 54, 1286–1296, doi:10.1016/j.jhep.2010.12.031 (2011).
Rehermann, B. & Nascimbeni, M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature reviews. Immunology 5, 215–229, doi:10.1038/nri1573 (2005).
Wieland, S. F. & Chisari, F. V. Stealth and cunning: hepatitis B and hepatitis C viruses. Journal of virology 79, 9369–9380, doi:10.1128/JVI.79.15.9369-9380.2005 (2005).
Waggoner, S. N. et al. Roles of natural killer cells in antiviral immunity. Current opinion in virology 16, 15–23, doi:10.1016/j.coviro.2015.10.008 (2015).
Tian, Z., Chen, Y. & Gao, B. Natural killer cells in liver disease. Hepatology 57, 1654–1662, doi:10.1002/hep.26115 (2013).
Tjwa, E. T. et al. Intrahepatic natural killer cell activation, but not function, is associated with HbsAg levels in patients with HBeAg-negative chronic hepatitis B. Liver international: official journal of the International Association for the Study of the Liver 34, 396–404, doi:10.1111/liv.12272 (2014).
Sun, C., Sun, H., Zhang, C. & Tian, Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cellular & molecular immunology 12, 292–302, doi:10.1038/cmi.2014.91 (2015).
Schmidt, K. N. et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. Journal of immunology 172, 138–143 (2004).
Guidotti, L. G., Matzke, B., Schaller, H. & Chisari, F. V. High-level hepatitis B virus replication in transgenic mice. Journal of virology 69, 6158–6169 (1995).
Larkin, J. et al. Hepatitis B virus transgenic mouse model of chronic liver disease. Nat Med 5, 907–912, doi:10.1038/11347 (1999).
Huang, L. R., Wu, H. L., Chen, P. J. & Chen, D. S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. P Natl Acad Sci USA 103, 17862–17867, doi:10.1073/pnas.0608578103 (2006).
Huang, L. R., Wu, H. L., Chen, P. J. & Chen, D. S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proceedings of the National Academy of Sciences of the United States of America 103, 17862–17867, doi:10.1073/pnas.0608578103 (2006).
Wieland, S. F. et al. Searching for interferon-induced genes that inhibit hepatitis B virus replication in transgenic mouse hepatocytes. Journal of Virology 77, 1227–1236, doi:10.1128/Jvi.77.2.1227-1236.2003 (2003).
Tu, Z. et al. TLR-dependent cross talk between human Kupffer cells and NK cells. The Journal of experimental medicine 205, 233–244, doi:10.1084/jem.20072195 (2008).
Chou, H. H. et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proceedings of the National Academy of Sciences of the United States of America 112, 2175–2180, doi:10.1073/pnas.1424775112 (2015).
Ma, Z., Zhang, E., Yang, D. & Lu, M. Contribution of Toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses. Cellular & molecular immunology 12, 273–282, doi:10.1038/cmi.2014.112 (2015).
Wu, J. et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology 46, 1769–1778, doi:10.1002/hep.21897 (2007).
Wilkins, C. & Gale, M. Jr. Recognition of viruses by cytoplasmic sensors. Current opinion in immunology 22, 41–47, doi:10.1016/j.coi.2009.12.003 (2010).
Swiecki, M., McCartney, S. A., Wang, Y. & Colonna, M. TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes. Journal of leukocyte biology 90, 691–701, doi:10.1189/jlb.0311166 (2011).
Pfeffer, L. M. et al. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer research 58, 2489–2499 (1998).
Fogler, W. E. et al. Recruitment of hepatic NK cells by IL-12 is dependent on IFN-gamma and VCAM-1 and is rapidly down-regulated by a mechanism involving T cells and expression of Fas. Journal of immunology 161, 6014–6021 (1998).
Pilaro, A. M. et al. TNF-alpha is a principal cytokine involved in the recruitment of NK cells to liver parenchyma. Journal of immunology 153, 333–342 (1994).
Almishri, W. et al. TNFalpha Augments Cytokine-Induced NK Cell IFNgamma Production through TNFR2. Journal of innate immunity 8, 617–629, doi:10.1159/000448077 (2016).
Ferrari, C. HBV and the immune response. Liver international: official journal of the International Association for the Study of the Liver 35(Suppl 1), 121–128, doi:10.1111/liv.12749 (2015).
Gehring, A. J. et al. The level of viral antigen presented by hepatocytes influences CD8 T-cell function. Journal of virology 81, 2940–2949, doi:10.1128/JVI.02415-06 (2007).
Kakimi, K., Guidotti, L. G., Koezuka, Y. & Chisari, F. V. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. The Journal of experimental medicine 192, 921–930 (2000).
Kakimi, K., Lane, T. E., Chisari, F. V. & Guidotti, L. G. Cutting edge: Inhibition of hepatitis B virus replication by activated NK T cells does not require inflammatory cell recruitment to the liver. Journal of immunology 167, 6701–6705 (2001).
Wei, C. et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol 185, 1158–1168, doi:10.4049/jimmunol.0903874 (2010).
Kumar, M. et al. Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. Journal of virology 85, 987–995, doi:10.1128/JVI.01825-10 (2011).
Wang, H. & Ryu, W. S. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog 6, e1000986, doi:10.1371/journal.ppat.1000986 (2010).
Kesar, V. & Odin, J. A. Toll-like receptors and liver disease. Liver international: official journal of the International Association for the Study of the Liver 34, 184–196, doi:10.1111/liv.12315 (2014).
Liu, F., Song, Y. & Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene therapy 6, 1258–1266, doi:10.1038/sj.gt.3300947 (1999).
Yin, W., Xu, L., Sun, R., Wei, H. & Tian, Z. Interleukin-15 suppresses hepatitis B virus replication via IFN-beta production in a C57BL/6 mouse model. Liver international: official journal of the International Association for the Study of the Liver 32, 1306–1314, doi:10.1111/j.1478-3231.2012.02773.x (2012).
Wang, J. et al. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. Journal of hepatology 44, 446–454, doi:10.1016/j.jhep.2005.08.015 (2006).
Nakashima, H. et al. Activation and increase of radio-sensitive CD11b+ recruited Kupffer cells/macrophages in diet-induced steatohepatitis in FGF5 deficient mice. Scientific reports 6, 34466, doi:10.1038/srep34466 (2016).
Acknowledgements
We thank Dr. Pei-Jer Chen (National Taiwan University, Taipei, Taiwan) for providing us the plasmid pAAV/HBV1.2. This work was supported by National Natural Science Foundation of China (81401664, 81301423); Program for Outstanding Young Talent of Chongqing Kuanren Hospital; China Postdoctoral Science Foundation funded project(2014M552539XB); Chongqing Research Program of Basic Research and Frontier Technology(cstc2015jcyjA10063); Chongqing Postdoctoral Science Foundation funded project(Xm2014132); and Science and Technology Research Project of Chongqing Municipal Education Commission (KJ1500214).
Author information
Authors and Affiliations
Contributions
S.T. performed and designed (with W.Y.) most of the included studies. G.L. and X.L. provided the HBV-transgenic mice and designed experiments related to HBV-transgenic mice. M.L. and Q.L. provided support for flow cytometry. H.P. and S.L. contributed to the Kupffer cell isolation and culture experiments. H.R. provided comments on the manuscript. W.Y. supervised the research project, assisted in the experimental design, provided support on the hydrodynamic injection mouse model and co-wrote the paper with S.T. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tong, S., Liu, G., Li, M. et al. Natural killer cell activation contributes to hepatitis B viral control in a mouse model. Sci Rep 7, 314 (2017). https://doi.org/10.1038/s41598-017-00387-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00387-2
- Springer Nature Limited
This article is cited by
-
Perforin inhibition protects from lethal endothelial damage during fulminant viral hepatitis
Nature Communications (2018)
-
NK cells in liver homeostasis and viral hepatitis
Science China Life Sciences (2018)
-
Interleukin-28B dampens airway inflammation through up-regulation of natural killer cell-derived IFN-γ
Scientific Reports (2017)