Abstract
Cholesterol efflux capacity (CEC) from macrophages, the first step in the reverse cholesterol transport pathway, is inversely associated with residual risk for atherosclerotic cardiovascular disease. Fatty acid-binding protein 4 (FABP4) and FABP5 are expressed in both adipocytes and macrophages and play significant roles in the development of insulin resistance and atherosclerosis. Both FABP4 and FABP5 are secreted from cells, and their circulating levels are associated with insulin resistance and atherosclerosis. We investigated the association between CEC and levels of FABP4 and FABP5 in 250 subjects without any medications. CEC was positively correlated with HDL cholesterol level and negatively correlated with concentrations of high-sensitivity C-reactive protein (hsCRP) and FABP5, but not FABP4. Multiple regression analysis demonstrated that FABP5 concentration was an independent predictor of CEC after adjustment of age, gender and levels of HDL cholesterol and hsCRP. In 129 of the 250 subjects who underwent carotid ultrasonography, mean intima-media thickness was negatively correlated with CEC and was positively correlated with concentrations of FABP4 and FABP5. In conclusion, in contrast to FABP4, circulating FABP5 is associated with decreased CEC and carotid atherosclerosis, suggesting that FABP5 level is a regulatory factor of CEC and a potential biomarker for residual risk of atherosclerosis.
Similar content being viewed by others
Introduction
Several risk factors for atherosclerotic cardiovascular disease, including dyslipidemia, diabetes mellitus, hypertension and smoking, have been reported1. Regarding dyslipidemia, the level of high-density lipoprotein (HDL) cholesterol is a potential candidate for reduction of residual risk after treatment with a statin for patients with a high level of high low-density lipoprotein (LDL) cholesterol2, 3. However, it has been shown that an increase in HDL level induced by cholesteryl ester transfer protein inhibitors failed to reduce the risk of recurrent cardiovascular events4,5,6. Quantitative measurement of HDL cholesterol is inadequate to assess the impact of HDL cholesterol on cardiovascular events, and improving the quality of HDL would be a better therapeutic target than simply raising HDL cholesterol level. We and others previously reported that cholesterol efflux capacity (CEC) from macrophages as an HDL function, which represents the first step of the reverse cholesterol transport pathway, is inversely associated with residual risk for atherosclerotic cardiovascular disease even after adjustment of HDL cholesterol level7,8,9,10, providing supportive evidence for the significance of HDL functionality over simple measurement of HDL cholesterol level.
Fatty acid-binding proteins (FABPs) are a family of intracellular lipid chaperones and they are approximately 14–15-kDa predominantly cytosolic proteins that regulate lipid trafficking and responses in cells11,12,13. Among FABPs, fatty acid-binding protein 4 (FABP4) and fatty acid-binding protein 5 (FABP5) are expressed in both adipocytes and macrophages. Previous studies using FABP4- and FABP5-deficient mice demonstrated that both FABP4 and FABP5 play significant roles in the development of insulin resistance, diabetes mellitus and atherosclerosis14,15,16,17,18,19. We previously demonstrated that inhibition of FABP4 in cells would be a novel therapeutic strategy against insulin resistance, diabetes mellitus and atherosclerosis20.
FABP4 is secreted from adipocytes in association with lipolysis via a non-classical secretion pathway21, 22, though there are no typical secretory signal peptides in the sequence of FABP411. It has recently been shown that FABP4 is also secreted from macrophages23. Elevated circulating FABP4 level is associated with obesity, insulin resistance, type 2 diabetes mellitus, dyslipidemia, hypertension, renal dysfunction, cardiac dysfunction, atherosclerosis and cardiovascular events23,24,25,26,27,28,29,30,31,32,33,34. Circulating FABP4 has recently been reported to act as an adipokine for the development of insulin resistance21, and neutralization of FABP4 with an antibody to FABP4 could be a feasible approach for the treatment of diabetes mellitus35. On the other hand, secretome analyses showed that FABP5 is secreted from cells36,37,38,39, though the mechanism remains unclear. It has been shown that circulating FABP5 level is associated with several components of metabolic syndrome, including atherosclerosis of carotid and coronary arteries26, 40, 41.
However, little is known about the link between CEC and levels of FABP4 and FABP5 regarding the development of atherosclerosis. In the present study, we investigated the cross-sectional association between CEC, circulating levels of FABP4 and FABP5 and intima-media thickness (IMT), a marker of carotid atherosclerosis assessed by using carotid ultrasonography, in a general population who had not regularly taken any medications.
Results
Basal characteristics of the studied subjects with no medication
Characteristics of the 250 recruited subjects with no medication (male/female: 88/162) are shown in Table 1. Mean age, BMI and waist circumference of the recruited subjects were 61 ± 14 years, 22.7 ± 3.5 kg/m2 and 82.6 ± 10.3 cm, respectively. Male subjects had significantly larger BMI and waist circumference and had higher levels of diastolic blood pressure, triglycerides, fasting glucose, insulin, HOMA-R, HbA1c, BUN, creatinine, uric acid, AST, ALT, γGTP and hsCRP and lower levels of pulse rate, total cholesterol, LDL cholesterol, HDL cholesterol and FABP4 than did female subjects. No significant difference in age, systolic blood pressure, eGFR, BNP, FABP5 or CEC was found between the male and female subjects.
Correlations of cholesterol efflux capacity and FABP5 level with clinical parameters
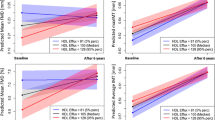
As shown in Table 2, CEC was positively correlated with levels of total cholesterol, HDL cholesterol (r = 0.580, P < 0.001) (Fig. 1A) and AST and was negatively correlated with age, systolic blood pressure and levels of triglycerides, fasting glucose, insulin, HOMA-R, HbA1c, BNP and hsCRP. A significantly negative correlation was found between CEC and concentration of FABP5 (r = −0.216, P < 0.001) (Fig. 1B) but not between CEC and concentration of FABP4 (r = −0.004, P = 0.945).
Correlations between cholesterol efflux capacity, levels of HDL cholesterol and FABP5 and carotid atherosclerosis. (A,B) HDL cholesterol level (A) and log FABP5 (B) were plotted against cholesterol efflux capacity in each subject (n = 250). Open circle and broken regression line: males (n = 88), closed circle and solid regression line: females (n = 162). (C,D) Mean intima-media thickness (IMT) was plotted against cholesterol efflux capacity (C) and log FABP5 (D) in each subject who underwent carotid ultrasonography (n = 129). Open circle: males (n = 44), closed circle: females (n = 85).
Serum FABP5 level was positively correlated with age, systolic blood pressure and levels of fasting glucose, HOMA-R, HbA1c, BUN, creatinine, uric acid, AST, ALT, BNP, hsCRP and FABP4 (r = 0.174, P = 0.006) and was negatively correlated with level of eGFR (r = −0.307, P < 0.001) and CEC (r = −0.216, P < 0.001) (Table 3).
Multiple regression analysis showed that CEC was independently associated with gender and levels of HDL cholesterol, hsCRP and FABP5, explaining a total of 37.8% of the variance in this measure (R2 = 0.378, AIC = 420.8) (Table 4). On the other hand, CEC was an independent predictor of FABP5 concentration after adjustment of age, gender and eGFR, explaining a total of 15.7% of the variance in this measure (R2 = 0.157, AIC = 293.9) (Table 4).
Associations of carotid atherosclerosis with cholesterol efflux capacity and FABP5 level
Among the recruited subjects, a total of 129 applicants (male/female: 44/85) underwent carotid ultrasonography, and basal characteristics are shown in Table S1. Clinical characteristics of the applicants were similar to those of the 250 enrolled subjects (Table 1, S1). No significant difference in mean IMT or mean stiffness parameter β was found between male and female subjects. Similar results were obtained for correlations of CEC or FABP5 level with clinical parameters (Table S2).
As shown in Table 5, mean IMT was negatively correlated with levels of HDL cholesterol and eGFR and with CEC (r = −0.248, P = 0.005) (Fig. 1C) and was positively correlated with age (r = 0.707, P < 0.001), systolic blood pressure and levels of HbA1c, BUN, creatinine, BNP, hsCRP, FABP4 (r = 0.178, P = 0.043) and FABP5 (r = 0.194, P = 0.028) (Fig. 1D). After adjustment of age and gender, mean IMT was independently associated with systolic blood pressure and CEC but not with levels of FABP4 and FABP5 (Table 5).
Mean stiffness parameter β, a marker of vascular stiffness, was negatively correlated with eGFR and positively correlated with mean IMT (r = 0.427, P < 0.001), age (r = 0.507, P < 0.001) and levels of systolic and diastolic blood pressures, fasting glucose, uric acid, AST, ALT and FABP4 (r = 0.211, P = 0.017) (Table 5). However, there was no significant correlation of mean stiffness β with CEC (r = −0.109, P = 0.220) or FABP5 level (r = 0.054, P = 0.543). After adjustment of age and gender, mean stiffness β was independently assocaited with systolic blood pressure and levels of uric acid, AST and ALT (Table 5).
Discussion
The present study showed for the first time that serum FABP5 concentration was an independent negative predictor of CEC in connection with mean IMT in a general population who had not taken any medication, suggesting a link between circulating FABP5 and carotid atherosclerosis via reduction of cholesterol efflux in macrophages. Circulating FABP5 may directly regulate HDL function independently of HDL cholesterol level and decrease cholesterol efflux in macrophages. It has recently been shown that CEC was inversely associated with the incidence of cardiovascular events in a population-based cohort study42. Therapies targeting quality of HDL rather than quantity of HDL might be effective for prevention of atherosclerotic cardiovascular disease and prevention of recurrence, though it is necessary to determine whether CEC is associated with progression of atherosclerosis in subjects with no medication. Reduction of FABP5 level would be a novel therapeutic strategy for increasing CEC and preventing atherosclerotic cardiovascular disease.
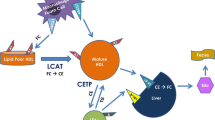
The two proteins FABP4 and FABP5 have 52% amino acid similarity and bind to various long-chain fatty acids with similar selectivity and affinity11. The expression of FABP5 is only about one-hundredth of that of FABP4 in adipose tissue11. Furthermore, circulating FABP5 level is detected at levels of about one tenth or less of FABP4 concentrations26, 40, 41, which was confirmed in the present study. However, it is notable that stoichiometry of FABP4 and that of FABP5 in macrophages are almost identical15. Previous studies using in vitro and in vivo experiments showed that FABP4 acts as an adipokine for the development of hepatic insulin resistance through increased hepatic glucose production21 and for the development of coronary atherosclerosis via induction of proinflammatory responses in macrophages, vascular smooth muscle cells and vascular endothelial cells23. Serum FABP4 level has also been reported to predict long-term cardiovascular events32,33,34. On the other hand, it has been reported that macrophage FABP5 deficiency suppresses atherosclerosis17. Interestingly, the impact of FABP5 on reduction of atherosclerosis was even greater than that of FABP4. In the present study, whereas mean IMT was negatively correlated with concentrations of FABP4 (r = 0.178, P = 0.043) and FABP5 (r = 0.194, P = 0.028), the level of FABP5, but not that of FABP4, was an independent predictor of CEC. A relatively high circulating FABP5 level derived from macrophages might be a reason for the difference in the impact of FABP4 and FABP5 levels on CEC. The putative mechanism of circulating FABP4 and FABP5 underlying the development of atherosclerosis is shown in Fig. 2.
Putative mechanism of the development of atherosclerosis by secreted FABP4 and FABP5. The expression of FABP5 is about one-hundredth of that of FABP4 in adipose tissue, and the amount of FABP4 in adipocytes is about 10,000-fold larger than that in macrophages (ref. 11). The stoichiometry of FABP4 and that of FABP5 in macrophages are almost identical (ref. 15). Both FABP4 and FABP5 are secreted from adipocytes and macrophages (refs 21,22,23 and 39). Direct effects of exogenous FABP4 in various types of cells have been demonstrated. Treatment with recombinant FABP4 inhibited activation of endothelial nitric oxide synthase in vascular endothelial cells, increased proliferation/migration of vascular smooth muscle cells (VSMC) and induced inflammatory responses in macrophages, vascular endothelial cells and VSMC (ref. 23), leading to the development of atherosclerosis. In the present study, the level of FABP5, but not that of FABP4, was an independent negative predictor of cholesterol efflux capacity (CEC) as an HDL function, indicating that circulating FABP5 contributes to the development of atherosclerosis via reduction of CEC in macrophages. The mechanism of direct association between CEC and FABP5 level needs to be addressed in experimental models.
FABP4 is secreted from adipocytes in association with lipolysis21, 22, and several drugs, including a statin43, omega-3 fatty acid ethyl esters44, a dipeptidyl peptidase-4 inhibitor45, a sodium glucose cotransporter 2 inhibitor46, a thiazolidinedione47 and angiotensin II receptor blockers48, 49, have been reported to modulate circulating FABP4 level. On the other hand, secretion of FABP5 remains to be elucidated. The mechanism of FABP5 secretion as well as direct association between CEC and FABP5 level needs to be addressed in experimental models. It is unclear whether FABP5 is loaded on HDL particles and directly regulates cholesterol efflux in macrophages. Identification of FABP5 using mass spectrometry analysis, such as in-depth proteomic analysis of purified HDL50, 51, would provide important information for predicting risks of atherosclerotic cardiovascular disease. Furthermore, the receptor for FABP5 remains unknown. It is unclear whether extracellular FABP5 is internalized into the cell or whether it acts by an intracellular signaling mechanism. A further understanding of the mechanism of FABP5 action may enable the development of new therapeutic strategies for atherosclerotic cardiovascular disease, such as neutralization of FABP5 and/or blockade of the FABP5 receptor, if any.
The present study has several limitations. First, the study was a cross-sectional design, which does not prove causal relations between serum level of FABP5, CEC and correlated biomarkers. A longitudinal study and interventional study are needed to clarify what underlies the relationship between FABP5 and CEC. Second, because the recruited subjects were only Japanese people, it is unclear whether the present findings can be generalized to other ethnicities. Third, the CEC assay quantifies not only one component of the reverse cholesterol transport pathway but also one property among several atheroprotective functions of HDL, including anti-inflammatory and anti-oxidative effects3. Lastly, CEC measurement is not standardized across laboratories. Therefore, values of CEC measured in the present study are not directly comparable to those measured in another laboratory using a different method.
In conclusion, in contrast to FABP4, circulating FABP5 is independently associated with both decreased CEC and carotid atherosclerosis, suggesting that FABP5 level is a regulatory factor of CEC and a potential biomarker for residual risk of atherosclerosis in relation to cholesterol efflux from macrophages. A further understanding of the mechanism underlying the link between FABP5 level and CEC may enable development of new therapeutic strategies for atherosclerotic cardiovascular diseases.
Methods
Study population
In the Tanno-Sobetsu Study, a study with a population-based cohort design in two rural towns, Tanno and Sobetsu, in Hokkaido, the northernmost island of Japan, a total of 617 Japanese subjects (male/female: 260/357, mean age: 66 ± 13 years) were recruited from residents of Sobetsu-town in 2011. Subjects who were being treated with any medications were excluded, and subjects who were not on any medication (n = 250, male/female: 88/162) were enrolled in the present study. This study conformed to the principles outlined in the Declaration of Helsinki and was performed with the approval of the Ethical Committee of Sapporo Medical University. Written informed consent was received from all of the study subjects.
Medical check-ups were performed between 06:00 h and 09:00 h after an overnight fast. After measuring anthropometric parameters, blood pressure was measured twice consecutively on the upper arm using an automated sphygmomanometer (HEM-907, Omron Co., Kyoto, Japan) with subjects in a seated resting position, and average blood pressure was used for analysis. Body mass index (BMI) was calculated as body weight (in kilograms) divided by the square of body height (in meters). Peripheral venous blood samples were obtained from study subjects after physical examination for complete blood count and biochemical analyses. Samples of the serum and plasma were analyzed immediately or stored at −80 °C until biochemical analyses.
Measurements
Concentrations of FABP4 and FABP5 were measured using commercially available enzyme-linked immunosorbent assay kits for FABP4 (Biovendor R&D, Modrice, Czech Republic) and FABP5 (USCN Life Science, Houston, USA), respectively. The intra- and inter-assay coefficients of variation in the kits were <5%. According to the manufacturer’s protocol, no cross-reactivity of FABP4 or FABP5 with other FABP types was observed. Plasma glucose was determined by the glucose oxidase method. Fasting plasma insulin was measured by a chemiluminescent enzyme immunoassay method. Hemoglobin A1c (HbA1c) was determined by a latex coagulation method and was expressed in National Glycohemoglobin Standardization Program (NGSP) scale. Creatinine, blood urea nitrogen (BUN), uric acid, aspartate transaminase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP) and lipid profiles, including total cholesterol, HDL cholesterol and triglycerides, were determined by enzymatic methods. LDL cholesterol level was calculated by the Friedewald equation. Brain natriuretic peptide (BNP) was measured using an assay kit (Shionogi & Co., Osaka, Japan). High-sensitivity C-reactive protein (hsCRP) was measured by a nephelometry method. Homeostasis model assessment of insulin resistance (HOMA-R), an index of insulin resistance, was calculated by the previously reported formula: HOMA-R = insulin (μU/ml) × glucose (mg/dl)/405. As an index of renal function, estimated glomerular filtration rate (eGFR) was calculated by an equation for Japanese52: eGFR (ml/min/1.73 m2) = 194 × creatinine(−1.094) × age(−0.287) × 0.739 (if female).
Cholesterol efflux capacity
CEC was measured as previously reported10. In brief, apolipoprotein B (apoB)-depleted serum was obtained from whole serum by precipitating apoB-containing lipoproteins with a polyethylene glycol solution as described previously53. J774.1 cells, a murine macrophage cell line, were obtained from National Institute of Biomedical Innovation (Osaka, Japan) and were incubated with 0.33 μCi of 3H-cholesterol (Perkin-Elmer Analytical Sciences, MA, US) per milliliter for 24 h. Then the cells were washed with phosphate buffered saline, and an efflux medium containing 2.8% apolipoprotein B–depleted serum was added and left for 4 h. All steps were performed in the presence of 2 μg/ml Sandoz 58-035 (Santa Cruz Biotech), an acyl-coenzyme A:cholesterol acyltransferase inhibitor. Liquid scintillation counting (Perkin-Elmer Analytical Sciences, MA, US) was used to quantify the efflux of radioactive cholesterol from the cells. The quantity of radioactive cholesterol incorporated into cellular lipids was calculated by means of hexane and isopropanol extraction of control wells, which had not been exposed to the apoB-depleted serum. Percent efflux was calculated by the following formula: [(μCi of 3H-cholesterol in the medium containing 2.8% apoB-depleted serum - μCi of 3H-cholesterol in serum-free medium)/μCi of 3H-cholesterol in cells extracted before the efflux step] × 100. All assays were performed in duplicate. To correct for inter-assay variation across plates, a pooled serum control from eleven healthy volunteers was included on each plate, and values for serum samples from patients were normalized to the value of the pooled sample in subsequent analyses.
Carotid ultrasonography
After medical check-ups and collection of urine and blood samples, carotid ultrasonographic examinations were performed in applicants by three well-experienced examiners certified by the Japan Society of Ultrasonics in Medicine, who were blinded to clinical data, using Vivid 9 (GE Health Care, Tokyo, Japan) equipped with a multifrequency 4- to 10-MHz linear-array transducer. Mean IMT of the far wall of the bilateral common carotid arteries was measured using commercially available semiautomated edge-detection software (IMT Option, General Electric Medical System, Milwaukee, WI, USA). The region of interest was placed from the beginning of carotid bulbs to a 2-cm proximal site in each common carotid artery. Stiffness parameter β of bilateral common carotid arteries was calculated from blood pressure and the dimension of common carotid arteries assessed by B-mode ultrasonography as stiffness parameter β = log (systolic blood pressure/diastolic blood pressure) × Dd/(Ds − Dd), where Dd and Ds are dimensions of the common carotid artery at end-diastole and end-systole, respectively. Mean values of IMT and stiffness parameter β in the bilateral common carotid arteries were averaged.
Statistical analysis
Numeric variables are expressed as means ± SD for normal distributions or medians (interquartile ranges) for skewed variables. The distribution of each parameter was tested for its normality using the Shapiro-Wilk W test, and non-normally distributed parameters were logarithmically transformed for regression analyses. Comparison between two groups was done with the Mann-Whitney U test. The correlation between two variables was evaluated using Pearson’s correlation coefficient. Multivariate regression analysis was performed to identify independent determinants of CEC and FABP5 level using the variables with a significant and non-confounding correlation as independent predictors, showing the t-ratio calculated as the ratio of regression coefficient and standard error of regression coefficient and the percentage of variance in the object variables that the selected independent predictors explained (R2). Several models for independent determinants of CEC and FABP5 level were prepared by using all or different combinations of parameters as independent variables for calculation of both regression coefficients and Akaike’s Information Criterion (AIC). Among the candidate models, the best-fit model using AIC for each dependent variable was selected. Multiple regression analysis was also performed to identify the correlation of mean IMT or mean stiffness parameter β after adjustment of age and gender. A p value of less than 0.05 was considered statistically significant. All data were analyzed by using JMP 9 for Macintosh (SAS Institute, Cary, NC).
References
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Reith, C. & Armitage, J. Management of residual risk after statin therapy. Atherosclerosis 245, 161–170 (2016).
Kosmas, C. E., Christodoulidis, G., Cheng, J. W., Vittorio, T. J. & Lerakis, S. High-density lipoprotein functionality in coronary artery disease. Am J Med Sci 347, 504–508 (2014).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357, 2109–2122 (2007).
Schwartz, G. G. et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367, 2089–2099 (2012).
Keene, D., Price, C., Shun-Shin, M. J. & Francis, D. P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ 349, g4379 (2014).
de la Llera-Moya, M. et al. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 30, 796–801 (2010).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364, 127–135 (2011).
Saleheen, D. et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 3, 507–513 (2015).
Ogura, M., Hori, M. & Harada-Shiba, M. Association Between Cholesterol Efflux Capacity and Atherosclerotic Cardiovascular Disease in Patients With Familial Hypercholesterolemia. Arterioscler Thromb Vasc Biol 36, 181–188 (2016).
Furuhashi, M. & Hotamisligil, G. S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7, 489–503 (2008).
Furuhashi, M., Ishimura, S., Ota, H. & Miura, T. Lipid chaperones and metabolic inflammation. Int J Inflam 2011, 642612 (2011).
Furuhashi, M., Saitoh, S., Shimamoto, K. & Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin Med Insights Cardiol 8, 23–33 (2014).
Hotamisligil, G. S. et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 274, 1377–1379 (1996).
Makowski, L. et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 7, 699–705 (2001).
Maeda, K. et al. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes 52, 300–307 (2003).
Babaev, V. R. et al. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-gamma-regulated genes. Arterioscler Thromb Vasc Biol 31, 1283–1290 (2011).
Maeda, K. et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 1, 107–119 (2005).
Furuhashi, M. et al. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest 118, 2640–2650 (2008).
Furuhashi, M. et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 447, 959–965 (2007).
Cao, H. et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab 17, 768–778 (2013).
Mita, T. et al. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring) 23, 359–367 (2015).
Furuhashi, M. et al. Local Production of Fatty Acid-Binding Protein 4 in Epicardial/Perivascular Fat and Macrophages Is Linked to Coronary Atherosclerosis. Arterioscler Thromb Vasc Biol 36, 825–834 (2016).
Xu, A. et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 52, 405–413 (2006).
Xu, A. et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation 115, 1537–1543 (2007).
Ishimura, S. et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One 8, e81318 (2013).
Ota, H. et al. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am J Hypertens 25, 1124–1130 (2012).
Fuseya, T. et al. Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc Diabetol 13, 126 (2014).
Cabre, A. et al. Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J Lipid Res 49, 1746–1751 (2008).
Furuhashi, M. et al. Independent Link Between Levels of Proprotein Convertase Subtilisin/Kexin Type 9 and FABP4 in a General Population Without Medication. Am J Cardiol 118, 198–203 (2016).
Yeung, D. C. et al. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol 27, 1796–1802 (2007).
Furuhashi, M. et al. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One 6, e27356 (2011).
von Eynatten, M. et al. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol 32, 2327–2335 (2012).
Chow, W. S. et al. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc 2, e004176 (2013).
Burak, M. F. et al. Development of a therapeutic monoclonal antibody that targets secreted fatty acid-binding protein aP2 to treat type 2 diabetes. Sci Transl Med 7, 319ra205 (2015).
Hwang, H. H. et al. Identification of the target proteins of rosiglitazone in 3T3-L1 adipocytes through proteomic analysis of cytosolic and secreted proteins. Mol Cells 31, 239–246 (2011).
Wasinger, C. et al. Autocrine secretion of 15d-PGJ2 mediates simvastatin-induced apoptotic burst in human metastatic melanoma cells. Br J Pharmacol 171, 5708–5727 (2014).
Pepaj, M., Bredahl, M. K., Gjerlaugsen, N. & Thorsby, P. M. Proteomic analysis of the INS-1E secretome identify novel vitamin D-regulated proteins. Diabetes Metab Res Rev (2016).
Yamamoto, T. et al. Transcriptome and Metabolome Analyses in Exogenous FABP4- and FABP5-Treated Adipose-Derived Stem Cells. PLoS One 11, e0167825 (2016).
Yeung, D. C. et al. Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur Heart J 29, 2156–2163 (2008).
Bagheri, R. et al. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. Am J Cardiol 106, 1118–1123 (2010).
Rohatgi, A. et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371, 2383–2393 (2014).
Karpisek, M. et al. Treatment with atorvastatin reduces serum adipocyte-fatty acid binding protein value in patients with hyperlipidaemia. Eur J Clin Invest 37, 637–642 (2007).
Furuhashi, M. et al. Reduction of circulating FABP4 level by treatment with omega-3 fatty acid ethyl esters. Lipids Health Dis 15, 5 (2016).
Furuhashi, M. et al. Reduction of serum FABP4 level by sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes mellitus. J Lipid Res 56, 2372–2380 (2015).
Furuhashi, M. et al. Possible Increase in Serum FABP4 Level Despite Adiposity Reduction by Canagliflozin, an SGLT2 Inhibitor. PLoS One 11, e0154482 (2016).
Cabre, A. et al. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis 195, e150–158 (2007).
Miyoshi, T. et al. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart Vessels 26, 408–413 (2011).
Furuhashi, M. et al. Angiotensin II receptor blockers decrease serum concentration of fatty acid-binding protein 4 in patients with hypertension. Hypertens Res 38, 252–259 (2015).
Rezaee, F., Casetta, B., Levels, J. H., Speijer, D. & Meijers, J. C. Proteomic analysis of high-density lipoprotein. Proteomics 6, 721–730 (2006).
Dashty, M. et al. Proteome of human plasma very low-density lipoprotein and low-density lipoprotein exhibits a link with coagulation and lipid metabolism. Thromb Haemost 111, 518–530 (2014).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53, 982–992 (2009).
Asztalos, B. F. et al. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res 46, 2246–2253 (2005).
Acknowledgements
We are grateful to Megumu Morimoto for excellent technical assistance in measurement of cholesterol efflux capacity. M.F. has been supported by grants from JSPS KAKENHI, MEXT Translational Research Network Program, Uehara Memorial Foundation, SENSHIN Medical Research Foundation, Japan Diabetes Foundation, Takeda Medical Research Foundation, Ono Medical Research Foundation, Takeda Science Foundation, Akiyama Life Science Foundation, Yamaguchi Endocrine Research Foundation, Naito Foundation Natural Science Scholarship, Suhara Memorial Foundation, Terumo Foundation for Life Science and Arts, and Kondou Kinen Medical Foundation. M.O. has been supported by grants from JSPS KAKENHI and Daiwa Securities Health Foundation.
Author information
Authors and Affiliations
Contributions
M.F. and M.O. designed the project; M.F., T.M., N.M., H.O., and S.S. collected samples; S.Y., A.M. and M.K. performed echocardiography. M.F., M.O., M.M., A.O., and M.H.-S. performed experiments; M.F., M.O., M.H.-S., K.S. and T.M. analyzed and interpreted data; M.F. prepared the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Furuhashi, M., Ogura, M., Matsumoto, M. et al. Serum FABP5 concentration is a potential biomarker for residual risk of atherosclerosis in relation to cholesterol efflux from macrophages. Sci Rep 7, 217 (2017). https://doi.org/10.1038/s41598-017-00177-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00177-w
- Springer Nature Limited
This article is cited by
-
Identification of key genes for atherosclerosis in different arterial beds
Scientific Reports (2024)
-
Fatty acid-binding protein 5 is a functional biomarker and indicator of ferroptosis in cerebral hypoxia
Cell Death & Disease (2024)
-
Transcriptome-wide N6-methyladenosine methylation profile of atherosclerosis in mice
BMC Genomics (2023)
-
FABP5 Deficiency Impairs Mitochondrial Function and Aggravates Pathological Cardiac Remodeling and Dysfunction
Cardiovascular Toxicology (2021)
-
New Classification of Macrophages in Plaques: a Revolution
Current Atherosclerosis Reports (2020)