Abstract
Our study presents the assembly of a high-quality Taihu goose genome at the Telomere-to-Telomere (T2T) level. By employing advanced sequencing technologies, including Pacific Biosciences HiFi reads, Oxford Nanopore long reads, Illumina short reads, and chromatin conformation capture (Hi-C), we achieved an exceptional assembly. The T2T assembly encompasses a total length of 1,197,991,206 bp, with contigs N50 reaching 33,928,929 bp and scaffold N50 attaining 81,007,908 bp. It consists of 73 scaffolds, including 38 autosomes and one pair of Z/W sex chromosomes. Importantly, 33 autosomes were assembled without any gap, resulting in a contiguous representation. Furthermore, gene annotation efforts identified 34,898 genes, including 436,162 RNA transcripts, encompassing 806,158 exons, 743,910 introns, 651,148 coding sequences (CDS), and 135,622 untranslated regions (UTR). The T2T-level chromosome-scale goose genome assembly provides a vital foundation for future genetic improvement and understanding the genetic mechanisms underlying important traits in geese.
Similar content being viewed by others
Background & Summary
The domestic goose (Anser cygnoides domesticus) holds significant economic value as a poultry species that is extensively raised for its meat, eggs, and ornamental purposes. It boasts a rich history of domestication that dates back over 6000 years, establishing geese as one of the earliest domesticated poultry species. Geese possess distinctive biological characteristics, such as rapid growth, robust disease resistance, and livers that exhibit exception tolerance to fat accumulation, enabling them to adapt to coarse feed materials. Additionally, geese manifest unique genomic traits, including a reduced susceptibility to certain avian viruses and a remarkable capacity for lipid accumulation in the liver. These distinctive traits offer valuable insights into the study of human lipid metabolism disorders. Genomic research has emerged as a pivotal tool in unravelling the genetic characteristics and biological functions of geese. Recent advancements in genome sequencing techniques have paved the way for an in-depth exploration of the genomic structure and functional attributes of geese.
Over the years, scientists have been diligently working towards the assembly of an ideal, high-quality genome for geese. In 2015, Lu et al. conducted the sequencing and assembly of the goose genome using second-generation sequencing data1. For assembly, they employed the SOAPdenovo software2, resulting in a draft genome size of 1.12 Gb. The draft genome consisted of 1,049 scaffolds, with a scaffold N50 of 5.2 Mb. Subsequently, in 2016, Gao published the genome sequence map of a female Sichuan White Goose. Through re-sequencing the ancestor species, the Swan Goose (Anser cygnoides), they unveiled that the divergence between these two species occurred approximately 3.4–6.3 million years ago3. Furthermore, in 2020, a chromosome-scale genome of the goose was released. Researchers unveiled a 1.11 Gb Tianfu Goose genome with a Contig N50 of 1.85 Mb and a Scaffold N50 of 33.12 Mb4. In recent years, high-quality chromosome-scale reference genomes of other goose breeds, such as Xingguo gray goose5 and Shitou goose6, have been successively published. These publications have provided genetic resources and established a robust foundation for breeding and biological research in geese.
However, previous genome assemblies for geese have been hindered by the presence of numerous gaps, particularly in regions rich in repetitive sequence such as telomeres and centromeres. These regions contain crucial genetic information but are challenging to accurately sequence and assemble due to their highly repetitive nature. Telomeres and centromeric DNA sequences predominantly consist of satellite DNA and are known to evolve rapidly in eukaryotic genomes7,8,9. Advancements in sequencing technologies have paved the way for the utilization of long-read technologies such as Oxford Nanopore (ONT) and Pacific Biosciences’ high-fidelity (HiFi) sequencing to address these challenges and fill gaps in animal and plant genomes. Notably, a recent breakthrough in genomics research led to the development of a Telomere-to-Telomere (T2T) assembly of human10 and the domestic chicken11 genome, successfully bridging most of the gaps in the previous assembly and providing valuable insights into the structural characteristics of telomeres and centromeres. However, a comprehensive, gapless reference genome for geese is currently unavailable. In this study, we present the first gapless assembly of the goose genome, employing various assembly strategies and leveraging high coverage and accurate long-read sequencing data. This assembly significantly enhance our understanding of the structural features of highly repetitive regions, including telomeres and centromeres, in geese. Consequently, it established a solid foundation for further investigations into the genomic structure and functionality of geese.
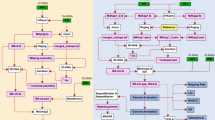
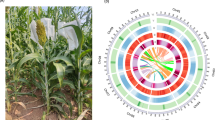
In this study, we selected the Taihu goose, an exemplary local breed in China, as our research subject (Fig. 1A). By utilizing advanced techniques such as Pacific Biosciences HiFi reads, nanopore long reads, Illumina short reads, and chromatin conformation capture (Hi-C), we successfully achieved a Telomere-to-Telomere (T2T) level assembly of the goose genome (Fig. 1B). This newly assembled T2T goose genome significantly bridges the gaps present in the existing reference genome for geese. The assembled genome has a total length of 1,197,991,206 bp, with a scaffold N50 value of 81,007,908 bp. Our assembly consists of 73 scaffolds, including 38 autosomes and one pair of Z/W sex chromosomes. Importantly, 33 autosomes have reached a completely gapless state. The completion of the T2T-level goose genome provides a robust foundation for future investigations in goose functional genomics and facilitates breeding enhancements targeting economically important traits.
Methods
Sample collection and sequencing
Collection and extraction of DNA and RNA samples
The research samples were obtained from an adult female Taihu goose belonging to the conservation population of the National Waterfowl Gene Bank in Jiangsu, China. A comprehensive sequencing strategy involving multiple techniques was employed for the assembly of the goose genome. Prior to the slaughter of the goose, blood samples were collected from the wing vein using a 5 ml anticoagulant blood collection tube (BD Vacutainer® EDTA). DNA extraction was subsequently performed on the blood samples to facilitate further sequencing analysis. To obtain complete genome fragments, a combination of third-generation long-read sequencing and second-generation sequencing methods was utilized. Additionally, pectoral muscle tissue samples were dissected into small pieces and subjected to a formaldehyde cross-linking reaction for Hi-C library preparation and subsequent sequencing. Moreover, various tissues including brain, heart, liver, spleen, lung, and kidney were collected. Each tissue sample was cut into small pieces and placed in 1.8 ml cryotubes (Nunc Cryo Tube). The samples were rapidly frozen in liquid nitrogen and temporarily stored at −80 °C in an ultra-low temperature freezer (Hair DW-86L728J) for second-generation transcriptome sequencing. Furthermore, to enhance the accuracy of gene annotation, equal amounts of the six tissue samples were mixed and subjected to third-generation full-length transcriptome sequencing. All sampling procedures described above strictly adhered to the regulations set by the Animal Welfare Committee at Jiangsu College of Agriculture and Animal Husbandry Technology (22110313195050999).
The DNA extraction process from the samples strictly followed the protocols outlined in the Tiangen Blood/Cell/Tissue Genomic DNA Extraction Kit (TIANGEN® DP304). After DNA extraction, the quality of the DNA was assessed using a Nanodrop 2000 spectrophotometer. The acceptable parameters for sample DNA quality were defined as an OD ratio (260/280) ranging from 1.8 to 2.0 and a concentration exceeding 100 ng/μl. Subsequently, prepared 2% agarose gel electrophoresis was conducted to verify the integrity of the DNA samples, ensuring satisfactory banding patterns. Finally, the DNA samples meeting the quality requirement were stored at −80 °C in a Hair DW-86L728J freezer for future use. For total RNA extraction from the tissues samples, the procedure strictly adhered to the instructions provided by the Tiangen TRNzol Universal Total RNA Extraction Kit (TIANGEN® DP424). Following RNA extraction, the concentration and purity of the RNA were determined using appropriate methods. Once the RNA samples met the acceptable criteria, including adequate concentration and purity, they were stored at −80 °C in the Hair DW-86L728J freezer to maintain their integrity and stability for subsequent analysis.
Library construction and sequencing
The genomic DNA from the samples underwent third-generation long-read sequencing following the standard protocol provided by Oxford Nanopore Technologies (ONT). Initially, the genomic DNA was randomly fragmented using Megaruptor (Diagenode, USA). Subsequently, adapter preparation and ligation were performed using the Nanopore SQK-LSK 109 kit (Oxford Nanopore Technologies, USA). The ligated DNA library was then quantified using the Qubit 3.0 Fluorometer. Finally, the prepared sample was loaded onto Nanopore Flow cells R9.4 and sequenced on the PromethION platform. The sequencing statistics are summarized in Table 1. In total, 577,228 reads were obtained, accumulating a substantial base count of 52,490,712,237 bp. The average read length was 90,935.9 bp, with an N50 length of 100,823 bp. Moreover, the GC content of reads was calculated to be 42.82%.
The samples underwent HiFi sequencing using the Pacific Biosciences single-molecule real-time (SMRT) circular consensus sequencing (CCS) library preparation method. Initially, a total of 100 μg high-quality genomic DNA was sheared into fragments with a target size of approximately 20 kb using Covaris g-TUBEs (Covaris). The size distribution of the sheared genomic DNA was assessed using the Agilent 2100 Bioanalyzer DNA 12000 chip (Agilent Technologies) to ensure they met the required specifications. Subsequently, the PacBio DNA Template Prep Kit 2.0 (Pacific Biosciences of California, Inc., CA) was utilized for constructing sequencing libraries suitable for HiFi sequencing on the PacBio RS II instrument (Pacific Biosciences of California, Inc.). The prepared libraries were then loaded onto a SMRT CELL for sequencing. In total, 4,261,430 reads were generated as sequencing data as summarized in Table 1, with a cumulative base count of 71,413,769,333 bp. The average read length was calculated to be 16,758 bp, with an N50 length of 16,838 bp. Additionally, the GC content of the reads was determined to be 42.61%.
The genomic library for second-generation short-read sequencing of the samples was constructed as follows: Initially, high-quality genomic DNA was randomly sheared using a Covaris sonicator (Covaris, USA). Subsequently, the TruSeq Nano DNA HT Library Preparation Kit (Illumina, USA) was employed to generate an Illumina sequencing library with a target insert size of 350 bp. Finally, the purified library was loaded onto the Illumina NovaSeq 6000 platform for sequencing, which generated a total of 385,826,042 sequences. The cumulative sequencing data obtained from this process amounted to 57,873,906,300 bp. The GC content of the sequences was calculated to be 43.51%.
Hi-C sequencing library construction and sequencing
The construction and sequencing of the Hi-C libraries followed a standard protocol with certain modifications12. Initially, pectoral muscle tissue samples were cross-linked using a 4% formaldehyde solution at room temperature. Subsequently, the nuclear pellets were re-suspended in 20 μl of lysis buffer, followed by re-suspension of nuclei using 100 μl of NEB buffer. The dissolved nuclei were then lysed using a diluted SDS lysis buffer. DNA was subsequently digested using the four-base cutter MboI, and the DNA ends were labeled using biotin-14-dCTP. Biotin was later removed using T4 DNA polymerase. Followed by DNA ligation using T4 DNA ligase. Finally, after DNA purification, paired-end 150 bp sequencing was conducted on the Illumina HiSeq platform. The sequencing results are summarized in Table 1: A total of 1,075,285,592 reads were obtained, accumulating a substantial base count of 161,292,838,800 bp. The average read length was determined to be 90,935.80 bp, with an N50 length of 100,823 bp. Additionally, the average GC content of the reads was calculated to be 42.82%.
RNA Library construction and sequencing
For the construction and sequencing of RNA libraries for the six samples, total RNA was isolated from the brain, heart, liver, spleen, lung, and pectoral muscle tissues using the EasyPure RNA Kit (Transgen). The NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, Ipswich, MA, USA) was then employed to prepare sequencing libraries from the isolated RNA samples. Finally, paired-end (2 × 125 bp) sequencing was performed on the Illumina HiSeq Xten platform. The sequencing results were detailed in Table 1. Among the samples, the heart tissue yielded the highest number of reads, with a total of 45,882,692 reads obtained. Conversely, the spleen tissue had the lowest number of reads with 38,462,044 reads obtained. The average total number of reads across the six tissues was 6,393,354,550 bp, with a calculated GC content of 46.04%.
For the construction and sequencing of full-length transcriptome libraries from mixed samples, we employed the PacBio Sequel system (Pacific Biosciences, CA, USA) for Isoform Sequencing (Iso-Seq). Following the Iso-Seq protocol, cDNA synthesis and amplification were performed using the NEBNext Single Cell/Low Input cDNA Synthesis & Amplification Module. Subsequently, the PacBio SMRTbell Express Template Prep Kit 2.0 was utilized for sample processing, including adapter ligation and addition of SMRTbell sequences. To ensure high-quality and complete transcripts, size-selective purification was conducted using the ProNex® Size-Selective Purification System to remove low-quality and short fragment sequences, completing the preparation of the Iso-Seq library. Finally, high-quality full-length transcriptome sequencing was carried out on the Sequel System (Pacific Biosciences) to obtain accurate and comprehensive full-length transcript sequences. In total, 48,373,842 reads were obtained, resulting in a cumulative data size of 84,725,302,734 bp. The average read length was determined to be 1,751.50 bp, with an N50 length of 2,447 bp. Moreover, the GC content of the reads was calculated to be 46.05%.
Genome sequence assembly
Genome size estimation
In this study, the genome size of the Taihu goose was estimated using the K-mer method based on second-generation short-read sequencing data13. The distribution of K-mers was analyzed by examining the paired-end sequencing library data and utilizing the Jellyfish tool14. The K-mer distribution was further modeled using GenomeScope (v2.0) to gain preliminary insights into the characteristics of the Taihu goose genome15. In Fig. 1C, the observed distribution of K-mers in the sequenced genomic sequences of the Taihu goose is represented by the blue line. Additionally, the brown line represents the K-mers resulting from sequencing errors, which typically have lower frequencies as these errors occur randomly during sequencing. Based on the analysis of the K-mer distribution, GenomeScope was applied to model and estimate the length of the Taihu goose genome to be approximately 1.12 Gb, with a genome heterozygosity of around 0.5%.
Genome assembly
In this study, a comprehensive stategy combining Hifiasm (v0.18.5)16 and NextDenovo (v2.4.0)17 software was employed for genome assembly. Hifiasm was utilized to assemble the genome using various datasets, including Hifi data alone, Hifi + Hi-C data, and Hifi + ONT long reads + Hi-C data. Additionally, NextDenovo was used for assembly using the Hifi + ONT long reads + Hi-C data. The assembly results are summarized in Table 2, where NextDenovo demonstrated superior performance with 244 contigs and an N50 length of 33,928,929 bp, which was selected for downstream analysis. To refine the assembly, the run_purge_dups.py (v1.2.4)18 tool was employed to eliminate duplicate contigs. Given the lower accuracy of ONT third-generation long-read sequencing, error correction was performed using Hifi data. This involved counting k-mer occurrences using the meryl software (v1.4)19, aligning the assembled genome with the Hifi data using winnowmap software (v2.03)20, and subsequent secondary filtering and removal of chimeric alignments using the falconc software (v1.15.0)21. Following these steps, three rounds of correction were conducted using the racon software (v1.5.0)22 to obtain the final genome assembly sequence after Hifi correction. For high-quality genome assembly and complete scaffold sequences, the Chromap software (v0.2.5)23 and yahs (v1.2a.1)24 software were utilized in conjunction with Hi-C data. The assembled scaffold sequences were then compared with the known Swan Goose genome (GCA_025388735.1) to identify and align them. This comparison enabled the determination of correspondence between the scaffold sequences and each chromosome of the Swan Goose genome, leading to the renaming of the scaffold sequences based on the matching chromosomes 1–38 and the Z chromosome.
To obtain more comprehensive sequence information of the goose W chromosome, we conducted additional targeted assembly work specifically focusing on the W chromosome. In the initial published version of the goose genome, the sequence information for the W chromosome was incomplete. Therefore, we employed the W chromosome of a closely related species, the duck, as a reference. By utilizing the “scaffold” module of the ragtag.py software (v2.1.0)25, we merged and joined previously unconnected scaffolds to construct the W chromosome of the goose. We assembled a W chromosome with a length of 17.35 Mb. The W chromosome consists of 18 scaffolds, with scaffold_42 representing the primary portion of the W chromosome, accounting for 9.63% of its total length, and the average depth of coverage is 0.996. The detailed coverage results were shown in Table S1. Overall, our efforts resulted in the successful assembly of 38 autosomes, as well as the W and Z sex chromosomes, providing the most complete and comprehensive goose genome assembly achieved to date (Fig. 2). This achievement holds significant academic value for further research on the sex determination mechanism and genetic characteristics of geese.
A circos plot of 40 chromosomes of Taihu goose genome assembly. The rings from outside to inside indicate (a) chromosomes of the Taihu goose genome, (b) GC density, (c) exon density, (d) CDS density, (e) lncRNA density, (f) mRNA density, and (g) gene density, b-g were drawn in 100 kb; The innermost circle is a collinear plot of homologous genes on different chromosomes.
Gap filling
To address any remaining gaps in the assembled scaffold sequences, we employed the quarTeT software (v1.1.3)26 for gap filling. The specific parameters used were “-GapFiller -g *fasta -t 30 -l 5000 -i 60”. The quarTeT tool utilizes quartet alignment information to fill the gaps and enhances the accuracy of the filling process by incorporating additional known genome information. Following the gap filling procedure, we successfully closed all 33 autosomes, with the exception of a few remaining gaps on the two sex chromosomes. The distribution of these gaps across the chromosomes is depicted in Fig. 3.
Genome completeness assessment
The Taihu goose genome assembly was subjected to gene structures prediction using the metaeuk software (v6.a5d39d9)27. Predicted gene sequences were then compared to a reference dataset of eukaryotic avian species using HMMER (v3.3.2)28. By analyzing the alignment and coverage information between the predicted gene sequences and the reference sequences, we assessed the completeness of the Taihu goose genome assembly in terms of conserved gene content. Through statistical analysis of the alignment results, we identified both single-copy genes (S) and multi-copy genes (D) present in the assembled genome. Notably, 96.5% of the single-copy genes aligned completely to the genome. Similarly, 0.4% of the multi-copy genes were found to be entirely present in the genome. To further evaluate the quality of the genome assembly, the Quast software (v5.2.0) was utilized to assess key metrics. The Taihu goose genome size was determined to be 1,197,991,206 bp, with a scaffold N50 of 81,007,908 bp. Compared to previously published chromosome-level goose genomes4,5,6, our assembly exhibited a significant reduction in the number of scaffolds, totalling only 73. Importantly, the scaffold N50 exceeded 80 Mb, signifying a substantial improvement in assembly continuity compared to previous genome versions. Detailed comparative results can be found in Table 3.
Gene annotation
The RepeatMasker software (v4.1.5)29 was utilized to annotate repetitive sequences in the genome of the goose. According to Table 4, dispersed repeats accounted for 8.92% of the total length of the goose genome, with a cumulative length of approximately 106.89 Mb. Among these repeats, retrotransposons constituted about 77.17 Mb (6.44%), while DNA transposons accounted for 3.66 Mb. Notably, Long Interspersed Nuclear Elements (LINEs), comprised around 4.87% of the Taihu goose genome sequences, representing the most abundant type of repetitive sequence. It is worth mentioning that the avian-specific retrotransposon CR1 (Chicken Repeat 1) exhibited the highest abundance, constituting nearly 100% of all LINEs. In addition, Long Terminal Repeats (LTRs) accounted for 1.49% of the Taihu goose genome sequences, while Small Interspersed Nuclear Elements (SINEs) comprised 0.08%. To annotate protein-coding genes and mRNA in the Taihu goose genome, the Liftoff software (v1.6.3)30 was employed following the masking of repetitive sequences. The NCBI goose genome reference (GCF_002166845.1), along with its annotation information and transcriptome dataset was used for this purpose. The annotation results revealed a total of 34,898 genes, 436,162 mRNAs, 806,158 Exons, 743,910 Introns, 651,148 CDS and 135,622 UTRs that were annotated in the Taihu goose genome.
Telomere and centromere identification
In our study, we employed the telomere recognition “TTAGGG,” which is specific to animals, to identify telomere in the goose genome. The TeloExplorer function of the quarTeT software (v1.1.3)25 was utilized for telomere identification. The results revealed that chromosome 3 exhibited the highest number of telomere repeat sequences within a 10,000 bp window at each end, with 1,101 and 1,793 repeats, respectively. A visual representation of the telomere distribution can be seen in Fig. 3. To identify centromere, we used the centromics software which is available at https://github.com/ShuaiNIEgithub/Centromics. The assembled genome was analyzed using ont and hifi datasets, along with Hi-C data, to identify the centromeres. By analyzing the peak values in the HiC and TR-CL2 data, we determined the positions of the centromeres on the chromosomes. The locations of the centromeres have been indicated on the chromosome ideogram, as shown in Fig. 3.
Data Records
The PacBio HiFi, Nanopore, Hi-C and Illumina sequencing data have been deposited in the GSA at CNCB-NGDC CRR93493231, CRR93493332, CRR93493433, CRR93493534, and CRR93493635 respectively.
The transcriptomic sequencing data of liver, lung, kidney, brain, spleen, heart, and mix-tissue were deposited in the GSA at CNCB-NGDC CRR93493736, CRR93493837, CRR93493938, CRR93494039, CRR93494140, CRR93494241 and CRR93494342, respectively.
The T2T genome assembly data have been deposited on GenBank under accession number JBECYW01000000043.
Technical Validation
We employed the 3 C criterion, encompassing Contiguity, Completeness, and Correctness, to comprehensively evaluate the quality of our genome assembly. The resulting chromosome-level genome assembly measured 564.5 Mb, with a scaffold N50 value of 81 Mb. In terms of quantitative assessment, the BUSCO(v5.4.5)44 analysis demonstrated the completeness of genomes was 96.90% (Table 3), indicating a highly comprehensive assembly of the goose genome.
The Hi-C heatmap revealed a well-organized pattern of interaction contacts along the diagonals within and around the chromosome inversion region (Fig. 1D), thereby indirectly confirming the accuracy of the chromosome assembly.
Code availability
All software and pipelines were executed according to the manual and protocols of the published bioinformatic tools. The version and code/parameters of software have been described in Methods. No custom scripts or code were used.
References
Lu, L. et al. The goose genome sequence leads to insights into the evolution of waterfowl and susceptibility to fatty liver. Genome Biol 16, 89 (2015).
Luo, R. et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1, 18 (2012).
Gao, G. et al. Genome and metagenome analyses reveal adaptive evolution of the host and interaction with the gut microbiota in the goose. Sci Rep 6, 32961 (2016).
Li, Y. et al. Pacific Biosciences assembly with Hi-C mapping generates an improved, chromosome-level goose genome. Gigascience 9 (2020).
Ouyang, J. et al. Chromosome-level genome and population genomics reveal evolutionary characteristics and conservation status of Chinese indigenous geese. Commun Biol 5, 1191 (2022).
Zhao, Q. et al. Chromosome-level genome assembly of goose provides insight into the adaptation and growth of local goose breeds. Gigascience 12 (2022).
Shay, J. W. & Wright, W. E. Telomeres and telomerase: three decades of progress. Nat Rev Genet 20, 299–309 (2019).
Wu, M. et al. Segrosome assembly at the pliable parH centromere. Nucleic Acids Res 39, 5082–5097 (2011).
Piras, F. M. et al. Molecular Dynamics and Evolution of Centromeres in the Genus Equus. Int J Mol Sci 23 (2022).
Nurk, S. et al. The complete sequence of a human genome. Science 376, 44–53 (2022).
Huang, Z. et al. Evolutionary analysis of a complete chicken genome. Proc Natl Acad Sci USA 120, e2216641120 (2023).
Belton, J. M. et al. Hi-C: a comprehensive technique to capture the conformation of genomes. Methods 58, 268–276 (2012).
Salzberg, S. L. et al. GAGE: A critical evaluation of genome assemblies and assembly algorithms. Genome Res 22, 557–567 (2012).
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Vurture, G. W. et al. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics 33, 2202–2204 (2017).
Cheng, H., Concepcion, G. T., Feng, X., Zhang, H. & Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods 18, 170–175 (2021).
Hu, J., Fan, J., Sun, Z. & Liu, S. NextPolish: a fast and efficient genome polishing tool for long-read assembly. Bioinformatics 36, 2253–2255 (2020).
Roach, M. J., Schmidt, S. A. & Borneman, A. R. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinformatics 19, 460 (2018).
Rhie, A., Walenz, B. P., Koren, S. & Phillippy, A. M. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol 21, 245 (2020).
Jain, C., Rhie, A., Hansen, N. F., Koren, S. & Phillippy, A. M. Long-read mapping to repetitive reference sequences using Winnowmap2. Nat Methods 19, 705–710 (2022).
Wenger, A. M. et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat Biotechnol 37, 1155–1162 (2019).
Vaser, R., Sović, I., Nagarajan, N. & Šikić, M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res 27, 737–746 (2017).
Zhang, H. et al. Fast alignment and preprocessing of chromatin profiles with Chromap. Nat Commun 12, 6566 (2021).
Zhou, C., McCarthy, S. A. & Durbin, R. YaHS: yet another Hi-C scaffolding tool. Bioinformatics 39 (2023).
Alonge, M. et al. RaGOO: fast and accurate reference-guided scaffolding of draft genomes. Genome Biol 20, 224 (2019).
Lin, Y. et al. quarTeT: a telomere-to-telomere toolkit for gap-free genome assembly and centromeric repeat identification. Hortic Res 10, 127 (2023).
Levy Karin, E., Mirdita, M. & Söding, J. MetaEuk-sensitive, high-throughput gene discovery, and annotation for large-scale eukaryotic metagenomics. Microbiome 8, 48 (2020).
Potter, S. C. et al. HMMER web server: 2018 update. Nucleic Acids Res 46, w200–w204 (2018).
Tempel, S. Using and understanding RepeatMasker. Methods Mol Biol 859, 29–51 (2012).
Shumate, A. & Salzberg, S. L. Liftoff: accurate mapping of gene annotations. Bioinformatics 37, 1639–1643 (2021).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934932 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934933 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934934 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934935 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934936 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934937 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934938 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934939 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934940 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934941 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934942 (2024).
NGDC GSA https://ngdc.cncb.ac.cn/gsa/browse/CRA013344/CRR934943 (2024).
Zhao, H., Wang, J. & Meng, H. Taihu goose T2T genome assembly. GenBank https://identifiers.org/ncbi/insdc:JBECYW010000000 (2024).
Seppey, M., Manni, M. & Zdobnov, E. M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol Biol 1962, 227–245 (2019).
Acknowledgements
This research was supported by the JBGS Project of Seed Industry Revitalization in Jiangsu Province (JBGS[2021]030), the Key and General Project of Modern Agriculture in Jiangsu Province (BE2022359). The funders had no role in the design of the study, the collection, analyses, or interpretation of data and in writing of the manuscript. We acknowledge Professor Zhengkui Zhou (Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, 100193, China) for giving valuable comments.
Author information
Authors and Affiliations
Contributions
J.W. and H.M. designed the project. J.W. and D.Q.G. coordinated the study. H.C.Z., G.B.S., B.D., W.Q.Z., X.H.M., X.M.L., J.W., M.L.Z., G.S.Z., and R.C.J. conducted the sampling; H.C.Z. and H.Z. analyzed the genome size; W.H.Y annotated the genome; H.C.Z. performed the chromosomal synteny analysis, H.C.Z. drafted the manuscript, H.Z. and T.Y.G. improved and revised the manuscript. H.C.Z. and H.Z. conducted genome assembly, data processing, and statistical analysis. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, H., Zhou, H., Sun, G. et al. Telomere-to-telomere genome assembly of the goose Anser cygnoides. Sci Data 11, 741 (2024). https://doi.org/10.1038/s41597-024-03567-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-024-03567-8
- Springer Nature Limited