Abstract
The endoscopic examination of subepithelial vascular patterns within the vocal fold is crucial for clinicians seeking to distinguish between benign lesions and laryngeal cancer. Among innovative techniques, Contact Endoscopy combined with Narrow Band Imaging (CE-NBI) offers real-time visualization of these vascular structures. Despite the advent of CE-NBI, concerns have arisen regarding the subjective interpretation of its images. As a result, several computer-based solutions have been developed to address this issue. This study introduces the CE-NBI data set, the first publicly accessible data set that features enhanced and magnified visualizations of subepithelial blood vessels within the vocal fold. This data set encompasses 11144 images from 210 adult patients with pathological vocal fold conditions, where CE-NBI images are annotated using three distinct label categories. The data set has proven invaluable for numerous clinical assessments geared toward diagnosing laryngeal cancer using Optical Biopsy. Furthermore, given its versatility for various image analysis tasks, we have devised and implemented diverse image classification scenarios using Machine Learning (ML) approaches to address critical clinical challenges in assessing laryngeal lesions.
Similar content being viewed by others
Background & Summary
Artificial intelligence (AI) algorithms have been introduced in the last years to otolaryngology and other disciplines to assist the clinical interpretation of endoscopic image or video data, establishing the new field of videomics1. The different approaches include image processing, Machine Learning (ML), and Deep Learning (DL) algorithms based on Convolutional Neural Networks (CNN) and require large data sets of endoscopic images to train and test the AI models. The use of videomics in laryngeal endoscopy is particularly challenging because of the complex anatomy and dynamic nature of the vocal folds and the lack of large data sets of high-definition images2,3.
From the clinical point of view, most patients with glottic or vocal fold pathologies are presented to Otolaryngologists due to persistent hoarseness or dysphonia. Hoarseness can be attributed to various causes, including local inflammation, functional voice disorders, and benign, premalignant or malignant laryngeal lesions. The differentiation between these possible diagnoses based on the endoscopic examination of the larynx defines the therapeutic modality. In contrast, the early diagnosis of laryngeal cancer or precancerous lesions is paramount for the patient’s prognosis and preservation of laryngeal function. In cases of suspected malignancy, surgical intervention with biopsy or complete excision is necessary for the histopathological examination of the lesion4,5.
The standard method for the clinical evaluation of the vocal folds is laryngoscopy using rigid or flexible scopes in the context of White Light Endoscopy (WLE). This procedure allows the recording of endoscopic videos and images for future reference, teaching or research purposes4,6. During laryngoscopy, the evaluation of vascular changes in the vocal folds is crucial for taking clinical diagnostic decisions . Over the years, several classification systems have been proposed for this purpose7,8,9. Recently, image Enhanced Endoscopy (EE) techniques like Narrow Band Imaging (NBI) or Enhanced Contact Endoscopy (ECE) have been introduced and have shown to be clinically helpful in differentiating between benign and malignant laryngeal lesions with higher accuracy compared to WLE10,11,12. By providing higher contrasted vascular patterns, NBI can help overcome the ‘umbrella effect’ caused by vocal fold leukoplakia, a white plaque covering the mucosa that can obscure vascular changes12,13. However, the evaluation of vascular patterns largely depends on the classification system used and is subject to the individual experience of each endoscopist14,15.
The use of AI can reduce subjectivity in evaluating endoscopic images and provide valuable assistance to Otolaryngologists in making a clinical diagnosis. Currently, most applications focus on differentiating between normal vocal folds and benign or malignant lesions using WLE or NBI laryngoscopy of the vocal folds based on image texture or vasculature analysis2,3. The proposed approaches vary considerably in image pre-processing methods and the number of images used to train and test the algorithm. In distinguishing between benign and malignant lesions, a recent meta-analysis by Zurek et al. reported a diagnostic sensitivity of 0.80 to 0.95 and a specificity of 0.86 to 0.99, concluding that the diagnostic accuracy of ML-based methods is comparable to that of experienced professionals. Therefore, AI can be a valuable assistant tool for young or inexperienced professionals16.
During the last few years, we have tested the combined use of NBI with intraoperative Contact Endoscopy (CE-NBI) for a more detailed examination of highly contrasted vascular patterns and detection of minute vascular changes through high magnification. Enhanced visualization of vascular patterns through CE-NBI allows better differentiation between benign and malignant lesions. Examining the vascularization network on the edge of the lesion is beneficial in the presence of vocal fold leukoplakia. This approach has been tested in the clinical setting, leading to high diagnostic accuracy values and interrater reliability14,15. We have further proposed several feature extraction methods combined with ML-based methods for an automated vascular pattern categorization on CE-NBI images17,18. Moreover, a DL-based approach was developed based on the Transfer Learning concept to reach a more objective assessment of the laryngeal lesions using CE-NBI images19. Given that there are only a few public data sets in the field of laryngeal endoscopy with a limited number of images available20,21,22, in this paper, we aim to introduce the public CE-NBI data set and share the value of this kind of medical data we have collected through the years with the scientific community. The provided laryngeal endoscopy data set contains 11144 labeled images of 210 patients, the most extensive published data set on CE-NBI images. The present data set with three types of labeling is suitable for various clinical investigations and computer-based algorithm developments. The publication of such data could promote multi-center cooperation and further studies on the usefulness of videomics in laryngeal diagnostic and treatment procedures.
Methods
Ethical consideration
All patients were required to give informed and written consent to participate in the study. Moreover, all patients’ data were anonymized, and a random name was assigned to each file before it was exported from Magdeburg University Hospital’s central server. The local ethics committee reviewed and approved the study protocol, which meets the criteria of the latest version of the Declaration of Helsinki (report no. 49/18), and the Data Safety Office of Magdeburg University Hospital approved the release of the CE-NBI images under an open license.
Data acquisition
The CE-NBI video scenes of adult patients with suspicious benign, premalignant, and malignant lesions in the vocal fold have been captured during the endoscopy-based diagnostic procedure before performing micro-laryngoscopy surgery. The procedure was conducted in the Department of Otorhinolaryngology, Head and Neck Surgery in Magdeburg University Hospital, Germany, from 1 January 2015 to 31 December 2021. Following micro-laryngoscopy surgery, the tissue samples collected from the vocal fold were sent for histopathological examination. The final diagnostic result was recorded and was used later to label the data. The video acquisition setup included the Evis Exera II /Evis Exera III Video Systems with a xenon light source plus an integrated NBI filter (Olympus Medical Systems, Hamburg, Germany) as well as a rigid 30-degree contact endoscope (Karl Storz, Tuttlingen, Germany). The magnification degree of the endoscopic system was adjusted in combination with 60x or 150x magnification of the contact endoscope to reach the optimum visualization of the vascular patterns during the procedure.
All the video scenes were acquired before performing an excisional biopsy or cordectomy of the vocal fold in Audio Video Interleave (AVI) format with 30 frames per second (fps).
Data preparation
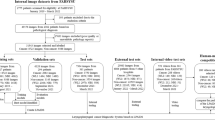
The CE-NBI images were extracted and selected from the captured video scenes in a three-step process. For each video, an experienced person working in the field of medical video and image processing (Ph.D. student) manually selected the time intervals where the video’s quality was good and acceptable to see the blood vessels (good resolution without blur and artifacts). Then, one in every three frames was extracted from the selected intervals in the videos using an automatic algorithm in MATLAB R2019a and MATLAB R2020a. In the last step, two experienced Otolaryngologists in joint sessions visually evaluated the series of extracted CE-NBI images per patient and selected images with unique and non-redundant vascular patterns in the mucosa of the target lesion for the data set.
The data annotations were followed in two paths, resulting in three different labels per image. First, the histopathology results from the surgical biopsy were used to label the images of each patient to:
-
The diagnosed laryngeal histopathology label (multi-class labeling).
-
The lesion-type benign-malignant label (binary labeling).
Second, the macroscopic appearance of the vocal fold, independent of the histopathology results, was assessed visually by two experienced Otolaryngologists to generate:
-
The leukoplakia diagnosis label, according to the presence or absence of the vocal fold leukoplakia (binary labeling).
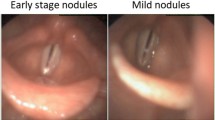
In total, the laryngeal histopathologies were divided into 16 different categories. As it is presented in Fig. 1 and according to World Health Organization (WHO) classification, the benign lesions included histopathologies such as cyst, polyp, reinke’s edema, bamboo nodes, nodule, granuloma, amyloidosis, inflammation, hemangioma, papillomatosis, hyperplasia, hyperkeratosis. Regarding the premalignant lesions, low grade dysplasia was labeled benign because of its low malignant transformation potential, while high grade dysplasia and carcinoma in situ were labeled malignant. Hence, the malignant lesions contained histopathologies as high grade dysplasia, carcinoma in situ, and squamous cell carcinoma (SCC).
Data Records
The full data set, including all images and labels, is available from Zenodo Repository (https://zenodo.org/) and can be downloaded from23. In total, the data set consists of two main data categories: benign images and malignant images. In each category, the images of every patient are ordered according to the laryngeal histopathology classes. In this way, the images belonging to each class are stored in the corresponding folder of the category it belongs to. For example, the cyst folder in the benign category includes total CE-NBI images of all patients diagnosed with a cyst or the SCC folder in the malignant category contains all CE-NBI images of patients with SCC. Figure 2 shows one example of CE-NBI images for every histopathology. Moreover, one general Excel file is provided to map the image files of each patient to image labels (diagnosed laryngeal histopathology, lesion-type benign-malignant, and leukoplakia diagnosis) and image dimension. The data set has a total size of 1.34 GB, 945 MB for benign images and 422 MB for malignant images.
The example of CE-NBI images for every histopathology. (a) Cyst, (b) Polyp, (c) Reinke’s edema, (d) Hemangioma (e) Granuloma, (f) Bamboo nodes, (g) Amyloidosis, (h) Inflammation, (i) Nodule, (j) Hyperplasia, (k) Hyperkeratosis, (l) Papillomatosis, (m) Low grade dysplasia, (n) High grade dysplasia, (o) Carcinoma in situ, and (p) SCC.
The CE-NBI data set contains 11144 labeled images of 210 patients with a resolution of 96 dpi stored in JPEG (Joint Photographic Experts Group) format that can have different dimensions as specified in Table 1.
Table 2 represents an overview of the total number of CE-NBI images and patients based on the type of laryngeal lesions and histopathologies. Additionally, a more detailed visualization of the number of images per 16 different histopathology classes and two lesion-type classes is presented in Figs. 3, 4. The benign class with 7657 CE-NBI images of 150 patients from 13 different histopathologies contains 69% of total data. In this class, reinke’s edema with 2661 images of 45 patients has the highest number of images among other histopathologies. The remaining 31% of the total data belongs to the malignant class with 3487 CE-NBI images of 60 patients with 3 different histopathologies. SCC with 1906 images of 30 patients has the highest number of images among other malignant histopathologies.
As was mentioned before, all images have the leukoplakia diagnosis label. Leukoplakia can be associated with a broad spectrum of histopathological diagnoses, from hyperplasia to malignant transformation. From all the images in the CE-NBI data set, 3400 images of 65 patients belong to leukoplakia cases. Table 3 summarizes the number of images and patients with leukoplakia per type of laryngeal lesion and histopathology. As is shown in Fig. 5, low grade dysplasia with 775 and SCC with 938 images have the highest number of CE-NBI images in benign and malignant categories for leukoplakia cases, respectively.
Technical Validation
Several studies were conducted to provide a better vision and understanding of the technical aspects of the CE-NBI data set. While the data set is well suited for various image processing and ML-based tasks, we have implemented multiple image classification scenarios on parts of this data set. The proposed approaches addressed the main clinical issues in the area of laryngeal cancer diagnosis and performed well on CE-NBI image classification based on the type of laryngeal lesion and histopathology. The value of each study is briefly explained below, along with their results as summarized in Table 4.
In the first study, we used a set of hand-crafted features in combination with supervised ML classifiers to evaluate the geometrical characteristics of vascular patterns in CE-NBI images. The proposed approach confirmed the relevance of the vascular structures to the laryngeal histopathology as well as to the type of laryngeal lesion17,24. This evaluation was continued by performing a classification scenario similar to the routine clinical procedure, where the performance of the proposed approach was compared to the diagnosis decision of Otolaryngologists regarding the type of laryngeal lesion based on vascular patterns in CE-NBI images25,26. The presented results showed the efficiency of a computer-based solution to assist Otolaryngologists when there are disagreements regarding the final diagnosis.
In the next round of analyzing CE-NBI images, the textural aspects of these images were studied using a novel set of features called Cyclist Effort Features (CyEfF)18. The presented results demonstrated the importance of textural characteristics in CE-NBI images because the CyEfF, in combination with the previously discussed Geometrical Features (GF), could improve the classification performance of ML classifiers. Additionally, the combination of these two sets of hand-crafted features showed high performance in the supervised classification of CE-NBI images of leukoplakia cases according to the type of laryngeal lesion27.
In another evaluation that included around 73% of the current data set, a pre-trained Residual Networks (ResNet50) architecture28 was combined with the cut-off layer technique as a fully automatic approach for the CE-NBI image classification based on benign and malignant lesions19. The given results could prove the significance of labeled data in the ML-based image classification task. Besides, the proposed model could be a solution for the subjective assessment of laryngeal lesions in clinical settings.
In the current study, we have developed a new DL-based approach using the ensemble modeling technique to combine the power of different architectures for CE-NBI image classification. We have validated and tested this approach on the entire publicly available CE-NBI data set to perform an automatic image classification based on benign and malignant classes. First, we have used the Transfer Learning concept and selected three pre-trained architectures, including Dense Convolutional Neural Network (DenseNet121)29, Efficient Network (EfficientNetB0V2)30, and Residual Networks (ResNet50V2) for this classification task. We have applied fine-tuning strategy on every architecture to reach the optimum performance, speed up the training, and overcome the problem with the small data set size. The final parameters after fine-tuning for each architecture were set as follows: the batch size of 32, the number of epochs equals 30 to 50, the input shape of the image as 224 × 224 pixels, and early stopping with the patience of 5 epochs. After training each fine-tuned architecture, an ensemble model was constructed using parallel topology, and predictions were made using model averaging. Model averaging is a well-known method of ensemble learning in which ensemble prediction is calculated as an average of the number of predictions, and each model makes an equal contribution to the final prediction. For the evaluation, we employed the hold-out approach to split the data into 80% training and 20% testing. In this way, we ensured that there was no overlap between the two sets and that images of every patient were just conjoined to separate groups. Furthermore, we have applied image augmentation techniques on the training set, including geometric transformations such as vertical and horizontal flips, to solve the issue of unbalanced data between two classes and avoid the possible occurrence of over-fitting. After this process, the number of images in the training set was increased to 11,680 data, with 6080 benign and 5600 malignant images. We have trained and validated three fine-tuned DenseNet121, EfficientNetB0V2, and ResNet50V2 on the training set where Fig. 6 shows the accuracy track for each model. Then, we tested each fine-tuned model along with the final ensemble model on the unseen data in the testing set, where Fig. 7 represents the confusion matrix of this experiment for each model. As presented in Table 4, the proposed ensemble model indicated a higher performance than the other approaches developed based on the Transfer Learning concept in CE-NBI image classification, showing the significance of the data in developing DL-based methods and solving complex image classification tasks.
It is important to highlight that we mainly explored the CE-NBI image data set for classification tasks based on laryngeal lesion type (benign versus malignant classes). Therefore, further technical research studies could be beneficial to explore the CE-NBI data set for multi-class image classification scenarios based on diagnosed laryngeal histopathology.
Usage Notes
The presented data set is a worthwhile source of data with 16 different diagnoses of various patients that can play a crucial role in the future advancement of laryngeal lesion diagnosis and treatment. The CE-NBI data set is generated from the data acquired in the existing clinical setting. Therefore, it has some limitations for clinical and technical research applications that are outlined as follows:
As the data acquisition strategy was focused on patients with suspicious benign, premalignant, and malignant lesions, the data set does not include a control group with healthy cases. Therefore, the data set can mainly be used in diagnostic studies, such as differentiation between benign and malignant pathologies, and it is not a suitable source for studies focusing on detecting laryngeal tissue changes.
The distribution of CE-NBI images of various histopathologies in the data set represents their numerical occurrence in the actual clinical setting. Nevertheless, it causes an imbalanced distribution of data among different classes. In the given data set, there are nearly double the number of images in the benign category compared to the malignant group. This issue may pose challenges in the development of ML-based algorithms. For example:
-
the algorithms could tend to be biased towards the majority class because they have more data to learn from,
-
the trained models could generalize poorly to new and unseen data because the minority class is not well-represented in the training data,
-
and the final models could overfit the training data to correctly classify the minority class because they have learned non-valuable patterns from the images in the training set.
The problem of imbalanced data is a common issue in clinical and technical studies that can be addressed with data resampling. The clinicians usually study 5 to 10 images per patient to arrive at the diagnosis decision. Therefore, the undersampling of the patient images in the majority class could be a potential solution to the imbalanced data issue. On the other hand, the development of ML-based approaches benefits from the considerable amount of data. Thus, the oversampling of data in minority groups using different techniques, such as data augmentation, could deal with this problem. Moreover, designing cost-sensitive learning algorithms and applying ensemble modeling techniques could be other solutions to improve the performance of ML-based approaches on the imbalanced CE-NBI image data set.
The small number of occurrences for certain histopathologies in the existent clinical setting has resulted in a limited number of CE-NBI images for these cases in the data set. Some classes, such as inflammation, nodule, amyloidosis, granuloma, bamboo nodes, and hemangioma, contain less than 100 CE-NBI images which may not be sufficient for the DL-based model to learn the underlying patterns of the data. This issue could hinder the development of ML-based methods for multi-class classification tasks based on diagnosed laryngeal histopathology. However, conducting a multi-class classification scenario with three classes, including benign, premalignant, and malignant groups, could be the next step toward histopathological diagnosis.
The application of CE-NBI images for laryngeal lesion assessment is limited to the research area where the data has to be acquired intraoperatively and under general anesthesia. In this condition, there is no standard and defined protocol for data acquisition. We could handle this issue by creating a study protocol in Magdeburg University Hospital to perform the data collection task. However, the lack of standard workflow in this step could result in data variability among different clinical centers because each hospital can follow an individual workflow where the characteristics of the images, such as magnification and resolution, could differ from the other centers. Future studies could overcome this limitation by proposing a universal data acquisition protocol for CE-NBI application or focusing on data collection for examination methods that are part of the routine clinical procedure, such as flexible endoscopy.
Code availability
The CE-NBI image data set can be used without any other code for technical and clinical image classification and assessment purposes. However, the algorithms already developed on this data set are available (https://github.com/NazilaEsmaeili/CE-NBI-Classification) to compare the performance of the newly developed approaches with the existing methods. The provided codes are available for public access only for research purposes.
References
Paderno, A., Holsinger, F. C. & Piazza, C. Videomics: bringing deep learning to diagnostic endoscopy. Current opinion in otolaryngology & head and neck surgery 29, 143–148 (2021).
Bensoussan, Y., Vanstrum, E. B., Johns, M. M. III & Rameau, A. Artificial intelligence and laryngeal cancer: From screening to prognosis: A state of the art review. Otolaryngology–Head and Neck Surgery 01945998221110839 (2022).
Paderno, A. et al. Artificial intelligence in clinical endoscopy: insights in the field of videomics. Frontiers in Surgery 1361.
Mannelli, G., Cecconi, L. & Gallo, O. Laryngeal preneoplastic lesions and cancer: challenging diagnosis. qualitative literature review and meta-analysis. Critical reviews in oncology/hematology 106, 64–90 (2016).
Mehlum, C. S. et al. Value of pre-and intraoperative diagnostic methods in suspected glottic neoplasia. European Archives of Oto-Rhino-Laryngology 277, 207–215 (2020).
Odell, E. et al. European laryngological society position paper on laryngeal dysplasia part i: aetiology and pathological classification. European Archives of Oto-Rhino-Laryngology 278, 1717–1722 (2021).
Ni, X. et al. Endoscopic diagnosis of laryngeal cancer and precancerous lesions by narrow band imaging. The Journal of Laryngology & Otology 125, 288–296 (2011).
Puxeddu, R., Sionis, S., Gerosa, C. & Carta, F. Enhanced contact endoscopy for the detection of neoangiogenesis in tumors of the larynx and hypopharynx. The Laryngoscope 125, 1600–1606 (2015).
Arens, C. et al. Proposal for a descriptive guideline of vascular changes in lesions of the vocal folds by the committee on endoscopic laryngeal imaging of the european laryngological society. European Archives of Oto-Rhino-Laryngology 273, 1207–1214 (2016).
Tibbetts, K. M. & Tan, M. Role of advanced laryngeal imaging in glottic cancer: early detection and evaluation of glottic neoplasms. Otolaryngologic Clinics of North America 48, 565–584 (2015).
Kim, D. H., Kim, Y., Kim, S. W. & Hwang, S. H. Use of narrowband imaging for the diagnosis and screening of laryngeal cancer: A systematic review and meta-analysis. Head & Neck 42, 2635–2643 (2020).
Saraniti, C., Chianetta, E., Greco, G., Mat Lazim, N. & Verro, B. The impact of narrow-band imaging on the pre-and intra-operative assessments of neoplastic and preneoplastic laryngeal lesions. a systematic review. International archives of otorhinolaryngology 25, 471–478 (2021).
Klimza, H., Jackowska, J., Tokarski, M., Piersiala, K. & Wierzbicka, M. Narrow-band imaging (nbi) for improving the assessment of vocal fold leukoplakia and overcoming the umbrella effect. PLoS One 12, e0180590 (2017).
Davaris, N. et al. Evaluation of vascular patterns using contact endoscopy and narrow-band imaging (ce-nbi) for the diagnosis of vocal fold malignancy. Cancers 12, 248 (2020).
Mehlum, C. S. et al. Interrater variation of vascular classifications used in enhanced laryngeal contact endoscopy. European Archives of Oto-Rhino-Laryngology 277, 2485–2492 (2020).
Żurek, M., Jasak, K., Niemczyk, K. & Rzepakowska, A. Artificial intelligence in laryngeal endoscopy: Systematic review and meta-analysis. Journal of Clinical Medicine 11, 2752 (2022).
Esmaeili, N. et al. Novel automated vessel pattern characterization of larynx contact endoscopic video images. International journal of computer assisted radiology and surgery 14, 1751–1761 (2019).
Esmaeili, N. et al. Cyclist effort features: A novel technique for image texture characterization applied to larynx cancer classification in contact endoscopy—narrow band imaging. Diagnostics 11, 432 (2021).
Esmaeili, N. et al. Deep convolution neural network for laryngeal cancer classification on contact endoscopy-narrow band imaging. Sensors 21, 8157 (2021).
Moccia, S. et al. Confident texture-based laryngeal tissue classification for early stage diagnosis support. Journal of Medical Imaging 4, 034502 (2017).
Laves, M.-H., Bicker, J., Kahrs, L. A. & Ortmaier, T. A dataset of laryngeal endoscopic images with comparative study on convolution neural network-based semantic segmentation. International journal of computer assisted radiology and surgery 14, 483–492 (2019).
Yin, L. et al. Laryngoscope8: Laryngeal image dataset and classification of laryngeal disease based on attention mechanism. Pattern Recognition Letters 150, 207–213 (2021).
Esmaeili, N. et al. Contact Endoscopy – Narrow Band Imaging (CE-NBI) Data Set for Laryngeal Lesion Assessment. Zenodo https://doi.org/10.5281/zenodo.6674034 (2022).
Esmaeili, N. et al. A preliminary study on automatic characterization and classification of vascular patterns of contact endoscopy images. In 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2703–2706 (IEEE, 2019).
Esmaeili, N. et al. Laryngeal lesion classification based on vascular patterns in contact endoscopy and narrow band imaging: manual versus automatic approach. Sensors 20, 4018 (2020).
Esmaeili, N. et al. Manual versus automatic classification of laryngeal lesions based on vascular patterns in ce+ nbi images. Current Directions in Biomedical Engineering 6, 70–73 (2020).
Davaris, N. et al. Use of artificial intelligence (ai) for the intraoperative evaluation of vocal fold leukoplakias. Laryngo-Rhino-Otologie 100 (2021).
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE conference on computer vision and pattern recognition, 770–778 (2016).
Huang, G., Liu, Z., Van Der Maaten, L. & Weinberger, K. Q. Densely connected convolutional networks. In Proceedings of the IEEE conference on computer vision and pattern recognition, 4700–4708 (2017).
Tan, M. & Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. In International conference on machine learning, 6105–6114 (PMLR, 2019).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
N.E. wrote the manuscript, initiated and performed the data preparation, generated the data set, and designed and developed the technical concepts for the data processing. N.D. wrote the manuscript, acquired the data, coordinated the data preparation with data annotation, and performed clinical data analysis. A.B. contributed to the writing and proofreading of the manuscript, coordinated the data preparation, and contributed to the design and development of technical concepts for data processing. A.I. contributed to the editing and proofreading of the manuscript, coordinated the data preparation, and contributed to the design and development of technical concepts for the data processing. N.N. contributed to the editing and proofreading of the manuscript, coordinated the design and development of technical concepts for data processing, and supervised the project. M.F. contributed to the editing and proofreading of the manuscript, coordinated the design and development of technical concepts for data processing, and supervised the project. C.A. contributed to the editing and proofreading of the manuscript, acquired the data, coordinated the data preparation with data annotation, performed clinical data analysis, and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esmaeili, N., Davaris, N., Boese, A. et al. Contact Endoscopy – Narrow Band Imaging (CE-NBI) data set for laryngeal lesion assessment. Sci Data 10, 733 (2023). https://doi.org/10.1038/s41597-023-02629-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-023-02629-7
- Springer Nature Limited