Abstract
The present database provides demographic (age and sex), clinical (hearing loss and acoustic properties of tinnitus), psychometric (based on Tinnitus Handicapped Inventory and Hospital Anxiety and Depression Scale) and electroencephalographic information of 89 tinnitus sufferers who were semi-randomly treated for eight weeks with one of five acoustic therapies. These were (1) placebo (relaxing music), (2) tinnitus retraining therapy, (3) auditory discrimination therapy, (4) enriched acoustic environment, and (5) binaural beats therapy. Fourteen healthy volunteers who were exposed to relaxing music and followed the same experimental procedure as tinnitus sufferers were additionally included in the study (control group). The database is available at https://doi.org/10.17632/kj443jc4yc.1. Acoustic therapies were monitored one week after, three weeks after, five weeks after, and eight weeks after the acoustic therapy. This study was previously approved by the local Ethical Committee (CONBIOETICA19CEI00820130520), it was registered as a clinical trial (ISRCTN14553550) in BioMed Central (Springer Nature), the protocol was published in 2016, it attracted L’Oréal-UNESCO Organization as a sponsor, and six journal publications have resulted from the analysis of this database.
Measurement(s) | brain electrical signals |
Technology Type(s) | Electroencephalography |
Factor Type(s) | EEG |
Similar content being viewed by others
Background & Summary
Tinnitus is a multifactorial and heterogeneous condition highly associated with hearing loss (HL), age, sex, marital status, education, and even, employment. Furthermore, a large number of tinnitus sufferers (around 65%) eventually present sleep disorders, emotional and cognitive distress (e.g., stress, anxiety or depression) or any other mental condition1. In fact, around 70% of tinnitus cases are preceded by a mental disorder. Patients with highly distressing tinnitus can additionally have cardiovascular, endocrine or metabolic diseases2.
Tinnitus is a heterogeneous affection with no standard effective treatment. A recent study3 based on a survey conducted by the web platform Tinnitus Hub, where 5017 tinnitus sufferers participated, seven of the 25 most widely used treatment were sound based therapies. From the most to the least applied therapies, these were: 1st self sound stimulation, 6th masker, 11th Tinnitus Retraining Therapy (TRT), 18th notched music, 19th soundcure, 20th acoustic neuromodulation, and 21st neuromonics. As can be seen, almost one third of the current available treatments for tinnitus depends on sound effect on body functioning. It is well-established that sound brings about physiological, cognitive and psychological changes4. However, it is still unknown how sound-based treatment alleviate or palliate tinnitus, and what is the appropriate therapy for each clinical case due to its heterogeneous nature.
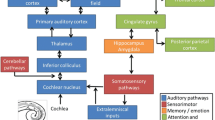
To measure the sound effects of acoustic therapies on tinnitus, electroencephalographic (EEG) monitoring has been proposed. In5, authors presented a review of computational methods to analyse EEG signals for evaluating the sound effects of acoustic therapies at a cortical level. Over the past two years, the acoustic therapies that have been assessed are (1) neuromodulation, (2) auditory residual inhibition, (3) binaural beats therapy (BBT), and (4) auditive discrimination therapy (ADT). In the first case6, neuromodulation therapy was applied for 75 days, and EEG monitoring was undertaken every two weeks. It was found that the level of EEG connectivity diminished considerably. In the second case, auditory residual inhibition did not reduce significantly the tinnitus loudness, and this finding was supported by EEG evidence since band power in alpha and gamma increased in magnitude7. Similarly, alpha band power in auditory areas increased during auditory residual inhibition in the study conducted in8. In the third case, BBT reduced stress (23% of patients) and tinnitus perception (15% of patients), and it slightly reduced EEG synchronicity over the right frontal lobe9. In the last case, the level of EEG synchronicity due to auditory processing decreased due to the attentional redirection achieved by ADT10.
As can be seen from previous studies, EEG analysis is a helpful tool to evaluate the sound effects of acoustic therapies, in addition to psychoacoustic and clinical assessments. For this reason, the present database provides demographic, clinical, psychometric, and EEG information of 89 tinnitus sufferers who were randomly treated for eight weeks with one of five acoustic therapies. These were (1) relaxing music (placebo), (2) tinnitus retraining therapy (TRT), (3) ADT, (4) enriched acoustic environment (EAE), and (5) BBT. TRT seeks to reduce tinnitus by reducing the loudness perception of the unreal sound11,12. ADT intends to redirect the patient attention towards the therapy by presenting a composed sound of standard and deviant pulses in a random way, and thus reducing tinnitus perception13. EAE intends to prevent HL, and subsequent plastic tonotopic cortical map changes after acoustic trauma. EAE is based on a sequence of random frequency tones with amplitude proportional to the HL reported on the patient audiometry14. BBT consists of two pure tones presented on each ear, having a difference in frequency according to the target oscillatory EEG band. The use of this therapy has reported reduction of stress levels by reducing activation of areas in the sympathetic system15, what in turn reduce tinnitus perception. In addition to the 89 tinnitus sufferers, 14 healthy volunteers were recruited, exposed to relaxing music (same as control group), and followed the same experimental procedure, as tinnitus sufferers.
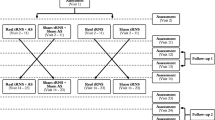
In total, the database contains four types of information sources of 103 participants. See Table 1. First, demographic information refers to residence place, gender, age (higher than 18-year-old), and nationality. Second, clinical history includes HL level, and frequency, laterality, and intensity of tinnitus perception. Finally, psychometric and EEG monitoring of the acoustic therapies is included, as well. It is important to note that the initial cohort was of 108 participants, 103 of them were included in the study, and 71 of them completed the experimental procedure. See Fig. 1.
Psychometric monitoring was based on the Spanish version of the Hospital Anxiety and Depression Stress (HADS), and the Tinnitus Handicap Inventory (THI), which provided psychoacoustic effect of acoustic therapies. On one hand, HADS questionnaire evaluates anxiety and depression impact of tinnitus16. On the other hand, THI questionnaire measures the degree of tinnitus perception; emotional, physical, and social response to tinnitus; and tinnitus intrusiveness on audition17. Further details concerning the questionnaire-based monitoring of this study protocol can be found in18.
EEG information gives an insight into neuroplastic changes (if any) of acoustic therapies. The available EEG datasets make possible to undertake signal analysis in three modes: spontaneous, evoked, and induced EEG activity. In most of previous studies, only spontaneous activity has been analyzed, namely, participants were either at resting state or at listening to their acoustic therapy. However, EEG signals provide more in-depth neural information when they are related to specific motor, emotional, sensory, perceptual and/or cognitive events. This refers to evoked and induced EEG activity. Traditional patterns for evoked and induced EEG activity are event-related potentials and event-related (de-) synchronization, what can be estimated from the present databank. Spontaneous activity analysis helps to evaluate nature of ongoing activity. In contrast, evoke and induced activity analysis allows studying brain responses when individuals perceive the sound, pay attention to the stimulus, make a decision, and act accordingly. In brief, both bottom-up and top-down mechanisms associated with neuroplastic changes due to acoustic rehabilitation can be studied by using the present databank.
The present database was created by following a protocol formerly approved by the Ethical Committee of the National School of Medicine of the Tecnologico de Monterrey, described and published in19, and registered under the trial number: ISRCTN14553550. The database is available at20.
The study protocol was conducted as follows. All of the participants were informed about the experimental procedure and signed a consent form, where they authorized the publication of their collected data and the results of their follow-ups. Most of patients were recruited from the National Rehabilitation Institute, and they were additionally notified that their head physician was also following-up the investigation. Head physicians provided clinical histories and demographic information of patients.
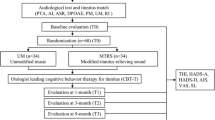
All the patients who accepted to participate in the study were assigned to one of five groups: (1) TRT, (2) EAE, (3) ADT, (4) BBT and (5) placebo. Patients with no severe HL were randomly assigned to placebo and EAE groups. On the other hand, patients with a well-identified tinnitus pitch were included in TRT or ADT groups. Patients with tinnitus lower that 1 kHz were assigned to BBT. Control group (healthy volunteers) was asked to use the same relaxing music as placebo group. On average, 17 volunteers were assigned per group (see Table 1). Note that every acoustic therapy was adjusted to the HL level, tinnitus frequency and tinnitus intensity, expect for the relaxing music that was used as placebo.
All of the participants (both with and without tinnitus) were instructed to use the corresponding acoustic therapy for one hour every day, at any time of the day, and for eight weeks. The therapy was monitored four times along the two months: the first (session 1), the third (session 2), the fifth (session 3), and the eighth (session 4) week after having initiated the therapy. At each monitoring session, the THI and HADS questionnaires were applied, and an EEG recording was made, as illustrated in Fig. 2. It can be seen from Fig. 1 that only 71 of the 103 participants completed the experimental procedure. Although the repository has 103 files, not all of them are complete. This information is specified in the Excel file found in20.
Methods
Device for acoustic therapy
Audio players were provided for patients. These audio players had a 4 Gb memory, and included a pair of headphones, and a battery charger. The frequency response of headphones was tested to guarantee the proper reproduction of acoustic therapies, and volume limit was adjusted to the patient audiometry. However, some patients preferred to use their own devices. To record the daytime and the daily duration of acoustic therapies, they are currently offered in an online mode21.
EEG data adquisition
To record EEG activity, a g.USBamp amplifier of EEG signals was used. This system is a high-performance and high-accuracy biosignal amplifier with sixteen EEG channels (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, C4, T4, T5, Pz, T6, O1, and O2). These channels can be acquired up to 32 700 samples per second within a bandwidth between 0 and 100 Hz. For this study, the amplifier was configured to record the EEG signals at a sampling frequency of 256 Hz. The Cz channel was used to reference the rest of the channels, and left lobe ear worked as ground. The montage of the EEG channels was according to the 10/20 International System and is depicted in Fig. 3a. EEG electrodes were connected to the amplifier via the g.GAMMAbox, which refers to a battery system for electrode power supply, and patient electrical isolation. See Fig. 3b.
(A) Electrode montage for EEG monitoring. Two pre-frontal (FP1, FP2), five frontal (F7, F3, Fz, F4, F8), four temporal (T3, T4, T5, T6), two central (C3, C4), one parietal (Pz) and two occipital (O1, O2) recording sites are provided in the present database. Reference (blue) and ground (yellow) electrodes are not present in the EEG recording. (B) g.tec system for EEG recording. The g.tec system consisted of an electrode cap of medium size, a biosignal amplifier (g.USBamp), and an electrical isolation system for electrode power supply (g.GAMMAbox).
The g.USBamp was connected to the computer via OpenViBE platform, where experimental paradigm was implemented. OpenViBE was configured to digitally filter EEG signals by a band-pass, and a notch filters. The band-pass filter was a Butterworth design of 8th order with cut-off frequencies between 0.1 and 100 Hz. The notch filter was similarly a Butterworth design of 4th order at 60 Hz. Before each recording, OpenViBE was used to measure electrode impedance, which was equal to or lower than 5kohms for being acceptable.
Experimental procedure for psychometric monitoring and EEG recording
Experimental procedure was undertaken in a well-equipped research laboratory with sufficient conditions to attend volunteers, and record EEG data. Laboratory conditions refer to a quiet atmosphere, water and toilet services, washing facilities, quiet air conditioner and parking place. The laboratory had a low background noise around 35 dBA.
As was mentioned above, acoustic therapies were applied for eight weeks, and monitored four times. THI and HADS questionnaires were completed in session 2 (S2), session 3 (S3) and session 4 (S4). EEG signals at resting state (baseline) for six minutes were recorded in session 1 (S1) and S4. In addition, three minutes of EEG recording was undertaken in S4 when participants were listening to their corresponding acoustic therapy. Finally, evoked/induced activity was recorded as well in two different modes: passive and active. Passive mode (PM) for 2.5 minutes was recorded in all of the four sessions, and active mode (AM) was recorded from S2 to S4. EEG recording procedure is described in detail below and illustrated in Fig. 4.
Experimental procedure. Acoustic therapies were monitored for eight weeks, along with four follow-ups. THI and HADS questionnaires were completed in S2, S3 and S4. EEG signals at resting state (baseline) for six minutes were recorded in S1 and S4. In addition, three minutes of EEG recording was undertaken in S4 when participants were listening to their corresponding acoustic therapy. PM for 2.5 minutes was recorded in all of the four sessions, and AM was recorded from S2 to S4.
Baseline
Baseline was recorded in S1 and S4. The participants were asked to sit down on a comfort chair with their eyes open at looking at a fixed point on a computer screen for three minutes. After this three-min period, they listened a beep sound once, and they had been previously instructed to close their eyes for three minutes. In total, six minutes for baseline were registered.
Therapy
This mode was recorded at S4, when the assigned acoustic therapy had been already applied for eight weeks. For this mode, participants sat down with their eyes close (EC) and listened to their corresponding acoustic therapy for three minutes.
Passive mode (PM)
PM was the only condition recorded in the four sessions. Participants were stimulated around two minutes with a sequence of 50 auditory stimuli of one second with an inter-stimulus interval of two seconds. Participants were asked to keep their eyes closed during the auditory stimulation. Every auditory stimulus was in line with the acoustic therapy in use.
Active mode (AM)
AM was recorded at S2, S3, and S4, and EC was requested as possible. Three different soundscapes were played, while five associated auditory stimuli were randomly played. In order to identify auditory stimuli, participants pressed a keyboard button. The soundscapes, and their related auditory stimuli to be identified for each monitoring session were: (1) restaurant: (i) human sound (tasting food), (ii) microwave sound, (iii) glass breaking, (iv) door closing, and (v) soda can being opened; (2) park: (i) camera clicking, (ii) turning a book page, (iii) cards shuffling, (iv) human laughing, and (v) human whistling; and (3) construction in progress: (i) human sound (yelling), (ii) police siren, (iii) mobile dialing, (iv) bang and (v) hit. All the stimuli lasted 1 s and were repeated 50 times at a random rate.
Processing
Raw signals are provided in the present database, in two formats: GDF and SET.
Data Records
Repository
This database was published on September 17th, 2021 at Mendeley Data20.
Data files
On one hand, demographic, clinical and psychometric data of participants is provided in an Excel-file. This file must be read from left to right, and the top of the columns as headers. Each row represents a participant. In some cases, the information would not be available, and unavailability was represented as ‘- ’. EEG-files are provided in GDF and SET formats. Both of them can be imported by using the MATLAB open-source toolbox, EEGLAB.
Data organization
Excel-file and EEG-files are provided in the main folder. EEG-files are organized in three levels: (1) group, (2) patient, and (3) recording condition. See Fig. 5. Each patient folder contained six different conditions: (1) open- and close-eyes (‘OECE.gdf’), (2) PM (‘Passive.gdf’), (3) at listening therapy (‘Therapy.gdf’), (4) AM at restaurant soundscape (‘A1-Restaurant.gdf’), (5) AM at park soundscape (‘A2-Park.gdf’), and (6) AM at construction soundscape (‘A3-Construction.gdf’).
EEG recording events
Interactions with and instructions given to participants during the EEG recording were marked with event numbers. In total, 13 events were marked along the GDF-files. Depending on the type of file, the events used in each experimental condition changed. Then, events per file type are defined in Table 2.
Technical Validation
Study design
A person-centred and needs-based approach that is a common practice in rehabilitation audiology was followed in the present study design. That is, each acoustic therapy was adjusted to the HL and acoustic tinnitus characteristics of each participant, what are indeed reported in Fig. 6. Furthermore, the selection of the therapy for each participant was not randomly allocated. The allocation was in line with HL level, and acoustic properties of tinnitus. For example, participants with low and moderate HL were allocated to placebo and EAE groups, those with a well-identified tinnitus pitched were treated with TRT and ADT groups, and those with a tinnitus frequency lower that 1 kHz were in BBT group. See Fig. 2.
Hearing condition and tinnitus characteristics. On the left, HL level of participants in left (y-axis) and right (x-axis) ears is presented. The HL level is categorized in seven impairment levels: (1) no impairment (0–15 dB), (2) slight HL (15–25 dB), (3) mild HL (25–40 dB), (4) moderate HL (45–55 dB), (5) significant HL (55–70 dB), (6) severe HL (70–90 dB), and (7) profound HL (>90 dB). On the right, acoustic characteristics of the tinnitus in terms of intensity, frequency and laterality are presented.
On the other hand, only patients with subjective tinnitus were recruited. Tinnitus can be objective, when sound is generated in the body, or subjective, when no specific inner body sound source. To avoid recruiting patients with objective tinnitus, all of those with any history of otitis, cerebellopontine angle tumours, psychiatrist pathologies, demyelinating diseases of the nervous system, or epilepsy were excluded from the study protocol.
Finally, it is important to note that an age-matched control group, which followed the same procedure that experimental group, was included. The daily exposure to any kind of sound can lead to habituation not related to tinnitus palliation. Therefore, it was relevant to have a control group to monitor unrelated habituation changes.
Methodological assessment
The present study was previously approved by the local Ethical Committee (CONBIOETICA19CEI00820130520), it was registered as a clinical trial (ISRCTN14553550) in BioMed Central (Springer Nature), the protocol was published in19, it attracted L’Oréal-UNESCO Organization as a sponsor, and six publications have resulted from the analysis of this database5,9,10,18,19,22.
Sample tracking system
The sample tracking system adopted for this study was in four different directions: (1) a clinical support by the head-physician, (2) demographic information collection, (3) psychometric evaluation, and (4) EEG monitoring. Sample age and gender are presented in Fig. 7. Age was categorized in youth (18–29 years), adultness (30–59 years) and elderly (>60 years). As can be seen from the figure, most of participants were adults, and a well-balanced gender sample was recruited.
Demographic information of the sample. On the left, sample age in three categories (young, adult, elderly) is presented, where can be seen that most of participants were in adult age (30–59 years). On the right, sample gender is presented, where can be seen that majority of participants were females.
In addition, a psychometric evaluation was undertaken during the acoustic treatment at three different stages of the procedure. This evaluation concerned tinnitus perception assessment (based on THI), and stress/anxiety monitoring (based on HADS). THI-evaluation revealed light, mild, moderate, severe or catastrophic impairment due to tinnitus along the acoustic treatment. See Fig. 8. Similarly, HADS-evaluation allowed detecting the changes of stress and anxiety due to tinnitus in three different ranges: normal, abnormal, and borderline abnormal. See Fig. 9.
HADS outcome for each group (placebo, BBT, TRT, EAE and ADT) in the three monitoring sessions (S2, S3 and S4). This questionnaire outcome is one of three conditions: normal, abnormal and borderline abnormal. On the left, the stress-monitoring outcome is presented. On the right, the anxiety-monitoring outcome is shown.
Finally, an EEG-based monitoring throughout the acoustic therapies was carried out. EEG information is a window to understand the nature of tinnitus, and the possible neuroplastic changes provoked by acoustic therapies. EEG signals provided in this database can be analysed in three different modalities: (1) spontaneous, (2) evoked and (3) induced modality. From those modalities, neuro-markers can be estimated from spontaneous activity, and/or codification and retrieval of auditory material masked by tinnitus perception can be analysed from evoked and induced activity (among many other analyses).
As a case in point, individual alpha frequency (IAF) was estimated from EEG signals in spontaneous modality (EO and EC condition), and shown in Fig. 10. Numerically, IAF refers to the peak value of the alpha band spectrum (8–13 Hz) at resting state over the occipital lobe23. IAF is around 10 Hz in healthy adult individuals, and reflects the functionality of the Central Nervous System, the mental health and the cognitive functions24. Several studies have demonstrated that IAF diminished when neuroplastic changes occurred in traumatic lesion, schizophrenia, attention deficit disorder, and post-traumatic stress. From the figure, it can be seen that mean IAF is below 10 Hz, as is expected owing to the neuroplastic changes provoked by refractory and chronic tinnitus. However, this result is not conclusive since control group has an IAF below 10 Hz, as the rest of the groups. This result is not surprising since IAF slowly decreases in elderly individuals, as well25. The main purpose of results in Fig. 10 is to show that EEG signals provided in this database are meaningful and technically valid for any research purpose pursued by the scientific community. Obviously, to obtain conclusive results, extensive analyses are necessary.
References
Langguth, B., Kreuzer, P. M., Kleinjung, T. & De Ridder, D. Tinnitus: causes and clinical management. The Lancet Neurology 12(9), 920–30 (2013).
Brüggemann, P. et al. Impact of multiple factors on the degree of tinnitus distress. Frontiers in Human Neurosciences 10(1), 341 (2016).
Simoes, J. P. et al. Towards personalized tinnitus treatment: An exploratory study based on internet crowdsensing. Frontiers in Public Health 1(7), 157 (2019).
Koelsch, S. A neuroscientific perspective on music therapy. Annals of the New York Academy of Sciences 1169(1), 374–384 (2009).
Ibarra-Zarate, D. & Alonso-Valerdi, L. M. Acoustic therapies for tinnitus: The basis and the electroencephalographic evaluation. Biomedical Signal Processing and Control 59(101900), 1–11 (2020).
Zhang, X. et al. Correlation Analysis of EEG Brain Network With Modulated Acoustic Stimulation for Chronic Tinnitus Patients. IEEE Transactions on Neural Systems and Rehabilitation Engineering 29(1), 156–162 (2021).
King, R. O. C. et al. The Effect of Auditory Residual Inhibition on Tinnitus and the Electroencephalogram. Ear and Hearing 42(1), 130–141 (2021).
Schoisswohl, S., Schecklmann, M., Langguth, B., Schlee, W. & Neff, P. Neurophysiological correlates of residual inhibition in tinnitus: Hints for trait-like EEG power spectra. Clinical Neurophysiology 132(7), 1694–1707 (2021).
Ibarra-Zarate, D. I., Naal-Ruiz, N. E. & Alonso-Valerdi, L. M. Binaural sound therapy for tinnitus treatment: A psychometric and neurophysiological evaluation. American Journal of Otolaryngology 43(1), 1–6 (2022).
Rodríguez-León, I. G. et al. Effect of Auditory Discrimination Therapy on Attentional Processes of Tinnitus Patients. Sensors 22(3), 937 (2022).
Kim, B. J., Chung, S. W., Jung, J. Y. & Suh, M. W. Effect of different sounds on the treatment outcome of tinnitus retraining therapy. Clinical and Experimental Otorhinolaryngology 7(2), 87 (2014).
Jastreboff, P. J. & Jastreboff, M. M. Tinnitus retraining therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. Journal of the American Academy of Audiology 11(3), 162–77 (2000).
Herraiz, C., Diges, I., Cobo, P., Aparicio, J. M. & Toledano, A. Auditory discrimination training for tinnitus treatment: the effect of different paradigms. European Archives of Oto-Rhino-Laryngology 267(7), 1067–74 (2010).
Tyler, R. S., Deshpande, A. K., Lau, C. C. & Kuk, F. The effectiveness of the progression of Widex Zen tinnitus therapy: A pilot study. American Journal of Audiology 26(3), 283–92 (2017).
da Silva, M. Junior, R. C. de Freitas, W. P. dos Santos, W. W. da Silva, M. C. Rodrigues and E. F. Conde, Exploratory study of the effect of binaural beat stimulation on the EEG activity pattern in resting state using artificial neural networks. Cognitive Systems Research 54(1), 1–20 (2019).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67, 361–370 (1983).
Newman, C. W., Jacobson, G. P. & Spitzer, J. B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 122, 143–148 (1996).
Alonso-Valerdi, L. M., González-Salazar, J. I. & Ibarra-Zarate, D. I. Neuropsychological monitoring of current acoustic therapies as alternative treatment of chronic tinnitus. American Journal of Otolaryngology 42(6), 1–10 (2021).
Alonso-Valerdi, L. M., Ibarra-Zarate, D. I., Tavira-Sánchez, F. J., Ramírez-Mendoza, R. A. & Recuero, M. Electroencephalographic evaluation of acoustic therapies for the treatment of chronic and refractory tinnitus. BMC Ear, Nose and Throat Disorders 17(1), 9 (2017).
Ibarra-Zarate, D. I., Alonso-Valerdi, L. M., Cuevas-Romero, A. R. & Intriago-Campos, L. A. Acoustic Therapies for Tinnitus Treatment: An EEG Database. Mendely Data https://doi.org/10.17632/kj443jc4yc.1 (2021).
Ibarra-Zárate, D. I. and Alonso-Valerdi, L. M., Neuroacoustics Mindoro, Mexico: https://mindoro.com.mx/ 2022.
Alonso-Valerdi, L. M., Torres-Torres, A. S., Corona-González, C. E. & Ibarra-Zárate, D. I. Clustering approach based on psychometrics and auditory event-related potentials to evaluate acoustic therapy effects. Biomedical Signal Processing and Control 76(1), 10371 (2022).
Ramsay, I. S., Lynn, P. A., Schermitzler, B. & Sponheim, S. R. Individual alpha peak frequency is slower in schizophrenia and related to deficits in visual perception and cognition. Scientific Reports 11(1), 1–9 (2021).
Grandy, T. H. et al. Individual alpha peak frequency is related to latent factors of general cognitive abilities. Neuroimage 79(1), 10–18 (2013).
Chiang, A. K. et al. Age trends and sex differences of alpha rhythms including split alpha peaks. Clinical Neurophysiology 122(8), 1505–1517 (2011).
Alonso-Valerdi, L. M. Variety of applications for BCI research, https://github.com/LuzAlondra/BrainComputerInterfaces: GitHub, 2015.
Alonso-Valerdi, L. M. Python Executable Script for Estimating Two Effective Parameters to Individualize Brain-Computer Interfaces: Individual Alpha Frequency and Neurophysiological Predictor. Frontiers in Neuroinformatics 10(22), 1–8 (2016).
Author information
Authors and Affiliations
Contributions
A.R.C.R. organized and revised all the EEG-files. She estimated the preliminary results of the EEG signals (IAF). She also produced all the graphic material presented in the paper, and provided the first draft of the paper. L.M.A.V. collected the information shared in this database, supervised the information organization and wrote the manuscript. L.A.I.C. retrieved and organized the information collected from H.A.D.S., T.H.I., the demographic survey and the clinical histories. He also provided the first draft of the manuscript. D.I.I.Z. collected the information shared in this database, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cuevas-Romero, A.R., Alonso-Valerdi, L.M., Intriago-Campos, L.A. et al. An Electroencephalography-based Database for studying the Effects of Acoustic Therapies for Tinnitus Treatment. Sci Data 9, 500 (2022). https://doi.org/10.1038/s41597-022-01622-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-022-01622-w
- Springer Nature Limited