Abstract
Tap water isotopic compositions could potentially record information on local climate and water management practices. A new water isotope tracer 17O-excess became available in recent years providing additional information of the various hydrological processes. Detailed data records of tap water 17O-excess have not been reported. In this report, monthly tap water samples (n = 652) were collected from December 2014 to November 2015 from 92 collection sites across China. The isotopic composition (δ2H, δ18O, and δ17O) of tap water was analyzed by a Triple Water Vapor Isotope Analyzer (T-WVIA) based on Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) technique and two second-order isotopic variables (d-excess and 17O-excess) were calculated. The geographic location information of the 92 collection sites including latitude, longitude, and elevation were also provided in this dataset. This report presents national-scale tap water isotope dataset at monthly time scale. Researchers and water resource managers who focus on the tap water issues could use them to probe the water source and water management strategies at large spatial scales.
Measurement(s) | Isotope • oxygen-17 atom • oxygen-18 atom • stable hydrogen isotopes • tap water isotopic composition |
Technology Type(s) | laser absorption spectroscopy • stable isotope analysis |
Factor Type(s) | tap water collection site • month of tap water sample collection |
Sample Characteristic - Environment | tap water |
Sample Characteristic - Location | China |
Machine-accessible metadata file describing the reported data: https://doi.org/10.6084/m9.figshare.12923681
Similar content being viewed by others
Background & Summary
Stable isotopes of hydrogen and oxygen have been widely used to identify plant water uptake depths, partition evapotranspiration, and separate hydrographs1,2,3,4,5,6,7. Such applications rely on different isotopic compositions of different water pools and the isotope difference is fundamentally caused by isotope fractionation. There are two major isotope fractionation processes: equilibrium fractionation and kinetic fractionation when water vapor, liquid, or ice crystals are converted into each other. Equilibrium fractionation is mainly affected by different saturation vapor pressure (e.g., liquid condensation)8,9 and kinetic fractionation is mainly affected by diffusivities (e.g., evaporation and solid condensation at supersaturation)9,10.
17O is the least abundant (0.038%) oxygen isotope and can be used as a new tracer in meteorological and hydrological studies. Due to the advances of high-precision analytical methods11,12,13, 17O-excess (17O-excess = ln (δ17O + 1) − 0.528 x ln (δ18O + 1)), another important second-order isotope like d-excess (d-excess = δ2H – 8 x δ18O), becomes available to probe hydrological processes11,12,14. Taking precipitation formation as an example, the δ2H, δ18O, δ17O, and d-excess are all sensitive to both temperature and relative humidity10,15,16. However, 17O-excess is theoretically not affected by temperature and only affected by relative humidity between 10 °C to 45 °C because of the similar temperature sensitivity between δ18O and δ17O17,18. Therefore, combing 17O-excess and 18O measurements could separate the temperature (not affecting 17O-excess) and relative humidity (affecting both 17O-excess and 18O) effect on oxygen isotopes. 17O-excess can also be used to identify spectral contamination and improve direct vapor equilibration in plant and soil analysis19. According to the relationship between δ′18O and δ′17O (i.e., the slope of 1000 x ln (δ18O + 1) and 1000 x ln (δ17O + 1)), synoptic drought related to EI Nino and local drought is distinguishable20. Fog and dew are also differentiated using the δ′18O and δ′17O relationship at the Namib Desert21. Moreover, based on the conceptual evaporation model, the relationship between δ′18O and δ′17O, and the relationships between 17O-excess and δ′18O (or d-excess)) are used to estimate whether water (e.g., precipitation, river waters, and lake waters) is affected by equilibrium fractionation or kinetic fractionation associated with evaporation14,17,22,23,24,25,26,27,28. Up to now, the studies of water 17O-excess variations at large spatiotemporal distribution have mainly focused on snow and ice cores in high-latitude regions29,30,31,32,33,34,35,36, where 17O-excess of snow is sensitive to temperature because of kinetic fractionation associated with supersaturation conditions under extremely cold condition (−80 to −15 °C)29,31,32. There are only few studies focused on the mid-latitude regions24,25,37,38.
The Intergovernmental Panel on Climate Change reported extending durations of severe droughts, increasing surface temperatures, and decreasing rainfall39,40. Thus, tap water, as an essential part of the domestic water use, should be paid more attention due to the trend of water scarcity and severe water pollution. The isotope variations of tap water could reveal the regional water supply sources, and reflect water-resource management strategies that integrate human geography, climate and socio-economic development1,41. The tap water in some regions can be used as a precipitation proxy to study the local precipitation41,42, while other regions may be supplied from inter-basin water transfers, deep groundwater or montane snowmelt1,43. The water resources in the north of China are less than those in the south due to special geographical location, climate change, extensive water-intensive economic activities, and population growth44,45,46. Therefore, the spatiotemporal distribution of tap water isotopes in China are needed to better understand water sources, thus informing water resource management.
To our best knowledge, there is no monthly tap water isotope dataset including 17O-excess publicly available. Here, we provide monthly isotope dataset (δ2H, δ18O, δ17O, d-excess, and 17O-excess) of tap water in China collected between December 2014 to November 2015. The instrument operation (δ2H, δ18O, and δ17O) using Triple Water Vapor Isotope Analyzer (T-WVIA-45-EP; Los Gatos Research Inc. (LGR), Mountain View, CA, USA) based on Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) technique has been described in details in our previous studies24,37, as well as the detailed description of 17O-excess quality control method. We have published the tap water isotopic variations in Tian et al.47. In this new dataset, we present the first publicly available monthly tap water isotope dataset to fill the gap in global tap water isotope datasets, especially for 17O-excess, which would be used to study water resource issues in the sustainable development of human societies.
Methods
Sample collections
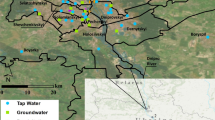
The monthly tap water samples across China were collected in 2015 (from December 2014 to November 2015) by Zhao et al.48, and conventional isotopes (δ2H and δ18O) were measured in Hydrology Laboratory of Tsinghua University. To obtain 17O-excess values, the samples were delivered to the IUPUI (Indiana University-Purdue University Indianapolis) Ecohydrology Lab to measure δ18O and δ17O (δ2H was also measured simultaneously). 652 samples from 92 sites in China were measured (Online-only Table 1), which have been reported by Tian et al.47. In here, we reported the detailed geographical location and monthly isotopic variations especially for 17O-excess values.
Isotope measurements and 17O-excess data processing
The details of the measurement process have been described by Tian et al.37,47. In brief, each sample was run at 1 Hz for 2 min under 13000 ppm to attain 120 data points using a Triple Water Vapor Isotope Analyzer (T-WVIA-45-EP, Los Gatos Research Inc. (LGR), Mountain View, CA, USA; preheated to 50 °C) coupled to a Water Vapor Isotope Standard Source (WVISS, LGR, Mountain View, CA, USA; preheated to 80 °C)49. To avoid memory effects between samples, the WVISS nebulizer was first purged for at least two minutes, and then the “stabilize” option of the device was turned on for two minutes to expel residual air inside the vaporizing chamber. The operation is different from the liquid water analyzer as described in other studies50,51. LGR#1 to LGR#5, as working standards with known and wide range of isotopic composition, were analyzed after every five tap water samples to ensure the accuracy of the T-WVIA performance. Furthermore, normalizing all of the isotope ratios using Vienna Standard Mean Ocean Water (VSMOW) and Standard Light Antarctic Precipitation (SLAP) to reduce differences between laboratories once a day12,52.
Accurate 17O-excess value of each sample (120 data points) require two steps for quality control. Firstly, calculated λ value (λ = ln (δ17O + 1)/ln (δ18O + 1) of each data point, the same as theoretical kinetic and equilibrium fractionation coefficient (θ) between liquid and vapor, should be between 0.506 and 0.5302,53. Secondly, the calculated 17O-excess value of each data point should be between −100 per meg and +100 per meg (1 per meg = 0.001‰), which is the range for almost all of the 17O-excess values of global precipitation2,17,23,25,54. The data points that meet the above two conditions were averaged to obtain the 17O-excess value for that sample.
Data Records
Monthly tap water isotope database is archived in PANGAEA in a single table including 652 rows and 10 columns55. Each row presents a monthly tap water event at one site. Each column corresponds to the geographic location information (including latitude, longitude, and elevation) and isotope variables including three measured individual stable isotopes (δ2H, δ18O, and δ17O) and two calculated second-order isotopic variables (d-excess and 17O-excess). A summary of the tap water in 2015 for 92 sites in China is presented in Table 1. The database spanned over 30.21° in latitude (from 20.03°N to 50.24°N) and 51.50° in longitude (from 75.99°E to 127.49°E). The elevation varied from 5 m to 3657 m with a mean value of 708 m. Fig. 1 depicts the distribution of monthly stable isotopes. The δ2H values varied from −132.40‰ to −22.36‰ with a mean value of −60.52 ± 19.54‰ (Table 1). The δ18O values varied from −17.74‰ to −3.8‰ with a mean value of −8.72 ± 2.49‰. The δ17O values varied from −9.38‰ to −1.97‰ with a mean value of −4.58 ± 1.32‰. The d-excess values varied from −5.9‰ to 20.8‰ with a mean value of 9.2 ± 4.5‰. The 17O-excess values varied from 19 to 66 per meg with a mean value of 39 ± 8 per meg. The tap water line (TWL) in China between δ18O and δ2H based on the 652 tap water samples within one year was δ2H = 7.65 (±0.07) x δ18O + 6.15 (±0.63) (R2 = 0.95, p < 0.001), which is close to the Global Meteoric Water Line (GMWL, δ2H = 8 x δ18O + 10) (Fig. 2a). The tap water line (TWL) between δ′18O and δ′17O was ln (δ17O + 1) = 0.5290 (±0.0001) x ln (δ18O + 1) + 0.000048 (±0.000001) (R2 = 1, p < 0.001), similar to the GMWL for oxygen (ln (δ17O + 1) = 0.528 x ln (δ18O + 1) + 0.000035, normalized to the VSMOW-SLAP scale25,54 (Fig. 2b).
Technical Validation
The precision of our measurement (δ2H, δ18O, δ17O, and 17O-excess) have been described in our previous studies using two international standards (SLAP and Greenland Ice Sheet Precipitation) and the five working standards from LGR, as well as comparing the reported precision in others literature37,47. They demonstrated that the precision of our OA-ICOS technique is comparable with other methods including IRMS technique25,31,32,34,52,54, CRDS method12,38, and other type of OA-ICOS water analyzer11.
References
Jameel, Y. et al. Tap water isotope ratios reflect urban water system structure and dynamics across a semiarid metropolitan area. Water Resour. Res. 52, 5891–5910 (2016).
Angert, A., Cappa, C. D. & DePaolo, D. J. Kinetic 17O effects in the hydrologic cycle: Indirect evidence and implications. Geochim. Cosmochim. Acta 68, 3487–3495 (2004).
Wang, L. et al. Partitioning evapotranspiration across gradients of woody plant cover: Assessment of a stable isotope technique. Geophys. Res. Lett. 37 (2010).
Zhao, L. et al. Factors controlling spatial and seasonal distributions of precipitation δ18O in China. Hydrol. Process. 26, 143–152 (2012).
Crawford, J., Hughes, C. E. & Parkes, S. D. Is the isotopic composition of event based precipitation driven by moisture source or synoptic scale weather in the Sydney Basin, Australia? J. Hydrol. 507, 213–226 (2013).
Putman, A. L. & Bowen, G. J. A global database of the stable isotopic ratios of meteoric and terrestrial waters. Hydrol. Earth Syst. Sc. 23, 4389–4396 (2019).
Lanning, M., Wang, L., Benson, M., Zhang, Q. & Novick, K. A. Canopy isotopic investigation reveals different water uptake dynamics of maples and oaks. Phytochemistry 175, 112389 (2020).
Griffis, T. J. Tracing the flow of carbon dioxide and water vapor between the biosphere and atmosphere: A review of optical isotope techniques and their application. Agr. Forest Meteorol. 174, 85–109 (2013).
Winkler, R. et al. Interannual variation of water isotopologues at Vostok indicates a contribution from stratospheric water vapor. Proc. Natl. Acad. Sci. 110, 17674–17679 (2013).
Jouzel, J. et al. Water isotopes as tools to document oceanic sources of precipitation. Water Resour. Res. 49, 7469–7486 (2013).
Berman, E. S., Levin, N. E., Landais, A., Li, S. & Owano, T. Measurement of δ18O, δ17O and 17O-excess in water by Off-Axis Integrated Cavity Output Spectroscopy and Isotope Ratio Mass Spectrometry. Anal. Chem. 85, 10392–10398 (2013).
Steig, E. et al. Calibrated high-precision 17O-excess measurements using cavity ring-down spectroscopy with laser-current-tuned cavity resonance. Atmos. Meas. Tech. 7, 2421–2435 (2014).
Coplen, T. B. et al. Isotope-abundance variations of selected elements. Pure Appl. Chem. 74, 1987–2017 (2002).
Barkan, E. & Luz, B. Diffusivity fractionations of H216O/H217O and H216O/H218O in air and their implications for isotope hydrology. Rapid Commun. Mass Spectrom. 21, 2999–3005 (2007).
Dansgaard, W. Stable isotopes in precipitation. Tellus 16, 436–468 (1964).
Guan, H., Zhang, X., Skrzypek, G., Sun, Z. & Xu, X. Deuterium excess variations of rainfall events in a coastal area of South Australia and its relationship with synoptic weather systems and atmospheric moisture sources. J. Geophys. Res. 118, 1123–1138 (2013).
Barkan, E. & Luz, B. High precision measurements of 17O/16O and 18O/16O ratios in H2O. Rapid Commun. Mass Spectrom. 19, 3737–3742 (2005).
Cao, X. & Liu, Y. Equilibrium mass-dependent fractionation relationships for triple oxygen isotopes. Geochim. Cosmochim. Acta 75, 7435–7445 (2011).
Nehemy, M. F. et al. 17O‐excess as a detector for co‐extracted organics in vapor analyses of plant isotope signatures. Rapid Commun. Mass Spectrom. 33, 1301–1310 (2019).
Kaseke, K. F. et al. Precipitation origins and key drivers of precipitation isotope (18O, 2H, and 17O) compositions over Windhoek. J. Geophys. Res. 123, 7311–7330 (2018).
Kaseke, K. F., Wang, L. & Seely, M. K. Nonrainfall water origins and formation mechanisms. Sci. Adv. 3, e1603131 (2017).
Criss, R. E. Principles of stable isotope distribution. (Oxford University Press on Demand, 1999).
Landais, A. et al. Combined measurements of 17O-excess and d-excess in African monsoon precipitation: Implications for evaluating convective parameterizations. Earth Planet. Sci. Lett. 298, 104–112 (2010).
Tian, C., Wang, L., Kaseke, K. F. & Bird, B. W. Stable isotope compositions (δ2H, δ18O and δ17O) of rainfall and snowfall in the central United States. Sci. Rep. 8, 6712 (2018).
Li, S., Levin, N. E. & Chesson, L. A. Continental scale variation in 17O-excess of meteoric waters in the United States. Geochim. Cosmochim. Acta 164, 110–126 (2015).
Surma, J., Assonov, S., Bolourchi, M. & Staubwasser, M. Triple oxygen isotope signatures in evaporated water bodies from the Sistan Oasis, Iran. Geophys. Res. Lett. 42, 8456–8462 (2015).
Surma, J., Assonov, S., Herwartz, D., Voigt, C. & Staubwasser, M. The evolution of 17O-excess in surface water of the arid environment during recharge and evaporation. Sci. Rep. 8, 4972 (2018).
Passey, B. H. & Ji, H. Triple oxygen isotope signatures of evaporation in lake waters and carbonates: A case study from the western United States. Earth Planet. Sci. Lett. 518, 1–12 (2019).
Landais, A., Ekaykin, A., Barkan, E., Winkler, R. & Luz, B. Seasonal variations of 17O-excess and d-excess in snow precipitation at Vostok station, East Antarctica. J. Glaciol. 58, 725–733 (2012).
Landais, A. et al. Triple isotopic composition of oxygen in surface snow and water vapor at NEEM (Greenland). Geochim. Cosmochim. Acta 77, 304–316 (2012).
Pang, H. et al. Spatial distribution of 17O-excess in surface snow along a traverse from Zhongshan station to Dome A, East Antarctica. Earth Planet. Sci. Lett. 414, 126–133 (2015).
Schoenemann, S. W., Steig, E. J., Ding, Q., Markle, B. R. & Schauer, A. J. Triple water-isotopologue record from WAIS Divide, Antarctica: Controls on glacial-interglacial changes in 17O-excess of precipitation. J. Geophys. Res. 119, 8741–8763 (2014).
Winkler, R. et al. Deglaciation records of 17O-excess in East Antarctica: reliable reconstruction of oceanic normalized relative humidity from coastal sites. Clim. Past 8, 1–16 (2012).
Steen-Larsen, H. et al. What controls the isotopic composition of Greenland surface snow? Clim. Past 10, 377–392 (2014).
Risi, C. et al. Understanding the 17O-excess glacial-interglacial variations in Vostok precipitation. J. Geophys. Res. 115 (2010).
Landais, A., Barkan, E. & Luz, B. Record of δ18O and 17O-excess in ice from Vostok Antarctica during the last 150,000 years. Geophys. Res. Lett. 35 (2008).
Tian, C. & Wang, L. Stable isotope variations of daily precipitation from 2014-2018 in the central United States. Sci. Data 6, 190018 (2019).
Affolter, S., Häuselmann, A. D., Fleitmann, D., Häuselmann, P. & Leuenberger, M. Triple isotope (δD, δ17O, δ18O) study on precipitation, drip water and speleothem fluid inclusions for a Western Central European cave (NW Switzerland). Quat. Sci. Rev. 127, 73–89 (2015).
Kidron, G. J. & Starinsky, A. Measurements and ecological implications of non-rainfall water in desert ecosystems–A Review. Ecohydrology, 12, e2121 (2019).
Solomon, S. et al. Climate change 2007-the physical science basis: Working group I contribution to the fourth assessment report of the IPCC. Vol. 4 (Cambridge university press, 2007).
West, A., February, E. & Bowen, G. Spatial analysis of hydrogen and oxygen stable isotopes (“isoscapes”) in ground water and tap water across South Africa. J. Geochem. Explor. 145, 213–222 (2014).
Bowen, G. J., Ehleringer, J. R., Chesson, L. A., Stange, E. & Cerling, T. E. Stable isotope ratios of tap water in the contiguous United States. Water Resour. Res. 43, W03419 (2007).
Good, S. P. et al. Patterns of local and nonlocal water resource use across the western U.S. determined via stable isotope intercomparisons. Water Resour. Res. 50, 8034–8049 (2014).
Nickum, J. E. Is China living on the water margin? The China Quarterly 156, 880–898 (1998).
Piao, S. et al. The impacts of climate change on water resources and agriculture in China. Nature 467, 43–51 (2010).
McDonald, R. I. et al. Urban growth, climate change, and freshwater availability. Proc. Natl. Acad. Sci. 108, 6312–6317 (2011).
Tian, C., Wang, L., Tian, F., Zhao, S. & Jiao, W. Spatial and temporal variations of tap water 17O-excess in China. Geochim. Cosmochim. Acta, 1–14 (2019).
Zhao, S. et al. Divergence of stable isotopes in tap water across China. Sci. Rep. 7, 43653 (2017).
Tian, C., Wang, L. & Novick, K. A. Water vapor δ2H, δ18O and δ17O measurements using an off-axis integrated cavity output spectrometer-sensitivity to water vapor concentration, delta value and averaging-time. Rapid Commun. Mass Spectrom. 30, 2077–2086 (2016).
Penna, D. et al. Evaluation of between-sample memory effects in the analysis of δ2H and δ18O of water samples measured by laser spectroscopes. Hydrol. Earth Syst. Sc. 16, 3925–3933 (2012).
Cui, J., Tian, L., Gerlein‐Safdi, C. & Qu, D. The influence of memory, sample size effects, and filter paper material on online laser‐based plant and soil water isotope measurements. Rapid Commun. Mass Spectrom. 31, 509–522 (2017).
Schoenemann, S. W., Schauer, A. J. & Steig, E. J. Measurement of SLAP2 and GISP δ17O and proposed VSMOW-SLAP normalization for δ17O and 17O-excess. Rapid Commun. Mass Spectrom. 27, 582–590 (2013).
Meijer, H. & Li, W. The use of electrolysis for accurate δ17O and δ18O isotope measurements in water. Isotopes Environ. Health Studies 34, 349–369 (1998).
Luz, B. & Barkan, E. Variations of 17O/16O and 18O/16O in meteoric waters. Geochim. Cosmochim. Acta 74, 6276–6286 (2010).
Tian, C. et al Triple isotope variations of monthly tap water in China. PANGAEA, https://doi.org/10.1594/PANGAEA.919079 (2020).
Acknowledgements
Funding for this work was made available from the Indiana University-Purdue University Indianapolis Research Support Funds Grant, U.S. National Science Foundation (EAR-1554894) and the National Science Foundation of China (51825902).
Author information
Authors and Affiliations
Contributions
C.T. analyzed the samples and wrote the manuscript. L.W. conceived the idea and edited the manuscript. W.J. and F.L. commented on the manuscript. F.T. and S.Z. collected the samples.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Online-only Table
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files associated with this article.
About this article
Cite this article
Tian, C., Wang, L., Jiao, W. et al. Triple isotope variations of monthly tap water in China. Sci Data 7, 336 (2020). https://doi.org/10.1038/s41597-020-00685-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-020-00685-x
- Springer Nature Limited
This article is cited by
-
High-elevation Tibetan Plateau before India–Eurasia collision recorded by triple oxygen isotopes
Nature Geoscience (2023)
-
Variation of tap-water isotope ratios and municipal water sources across Kyiv city, Ukraine
Discover Water (2022)