Abstract
Physical exercise both during and after curative cancer treatment has been shown to reduce side effects. Evidence in the metastatic cancer setting is scarce, and interventions that improve health-related quality of life (HRQOL) are much needed for patients with metastatic breast cancer (MBC). The multinational randomized controlled PREFERABLE-EFFECT trial assessed the effects of exercise on fatigue and HRQOL in patients with MBC. In total, 357 patients with MBC and a life expectancy of ≥6 months but without unstable bone metastases were recruited at eight study centers across five European countries and Australia. Participants were randomly assigned (1:1) to usual care (control group, n = 179) or a 9-month supervised exercise program (exercise group, n = 178). Intervention effects on physical fatigue (European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-FA12 scale) and HRQOL (EORTC QLQ-C30 summary score) were determined by comparing the change from baseline to 3, 6 (primary timepoint) and 9 months between groups using mixed models for repeated measures, adjusted for baseline values of the outcome, line of treatment (first or second versus third or higher) and study center. Exercise resulted in significant positive effects on both primary outcomes. Physical fatigue was significantly lower (−5.3 (95% confidence interval (CI), −10.0 to −0.6), Bonferroni–Holm-adjusted P = 0.027; Cohen's effect size, 0.22) and HRQOL significantly higher (4.8 (95% CI, 2.2–7.4), Bonferroni–Holm-adjusted P = 0.0003; effect size, 0.33) in the exercise group than in the control group at 6 months. Two serious adverse events occurred (that is, fractures), but both were not related to bone metastases. These results demonstrate that supervised exercise has positive effects on physical fatigue and HRQOL in patients with MBC and should be recommended as part of supportive care.
ClinicalTrials.gov Identifier: NCT04120298.
Similar content being viewed by others
Main
Breast cancer is currently the most commonly diagnosed cancer worldwide. With about 2.3 million new cases in 2020, it poses a significant burden to public health1,2. In 2020, almost 700,000 people worldwide died from breast cancer, with advanced disease being the main cause1. Owing to therapeutic advances for patients with MBC, survival time has improved (it ranges from a median of 23 to 64 months, depending on tumor receptor status)3,4,5. Consequently, optimizing MBC patients’ HRQOL is a crucial aim, as indicated by 80% of the patients6. Nevertheless, there is no evidence of improvement in HRQOL in MBC patients over the past decade, and in certain cases, a decline has even been observed6. Patients often experience debilitating cancer-related and treatment-related side effects, including fatigue, decreased physical fitness, anxiety and depression, neuropathy and pain7,8. Of these side effects, fatigue is the most reported and burdensome symptom9, and has the largest negative impact on HRQOL6. Given that patients with metastatic disease typically receive ongoing treatment, these side effects may worsen over time. Cumulative symptom burden can be associated with treatment adjustments or discontinuation, which may negatively affect survival10. Therefore, interventions that can improve HRQOL by alleviating fatigue and other side effects are much needed for patients with MBC.
Recent evidence-based international guidelines recommend physical exercise both during and after curative cancer treatment to reduce side effects11,12. However, the guidelines acknowledge that, in the context of MBC, the evidence of the effects of exercise is scarce, and no recommendations can be provided. Two systematic reviews in patients with advanced cancer, including patients with MBC, concluded that exercise interventions are safe and feasible13,14, and that this is also true for patients with bone metastases15. The studies in these reviews suggest that exercise improves physical fitness and functioning in patients with MBC. However, insufficient data are available to draw any conclusions regarding the effects of exercise on fatigue, HRQOL and other cancer-related and treatment-related side effects13. Hence, a high-quality and adequately powered study is needed to assess the effects of exercise in patients with MBC.
The randomized controlled PREFERABLE-EFFECT study was designed to assess the effects of a structured and individualized 9-month exercise intervention on fatigue and HRQOL in patients with MBC. The secondary aims were to investigate the effects of exercise on other cancer-related and treatment-related side effects. Here, we report on the primary outcomes and a range of secondary outcomes of the PREFERABLE-EFFECT study.
Results
Patient characteristics
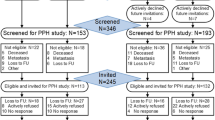
Between 8 January 2020 and 3 August 2022, a total of 856 patients were invited to take part in the PREFERABLE-EFFECT study. Of these, 357 provided consent and were enrolled (recruitment rate, 41.7%), with 178 randomized to the exercise group and 179 to the control group (Fig. 1). The overall dropout rates were 12.3% at 3 months, 18.5% at 6 months and 24.1% at 9 months. The median (interquartile range (IQR)) attendance rate for supervised exercise sessions was 77% (48–92%) (see Extended Data Table 1 for reasons for missed exercise sessions). Median compliance with the exercise protocol was 70% for the balance component, 59%–83% for the aerobic components and 63%–100% for the resistance components (Extended Data Table 2).
The exercise and control groups had similar sociodemographic and clinical characteristics at baseline (Table 1). The mean age of the participants was 55.4 years (s.d., 11.1), and the majority were female (99.4%), were receiving first-line or second-line treatment (74.8%) and had bone metastases (67.2%). At baseline, about half of all participants reported fatigue above the threshold for clinical importance (score on the QLQ-C30 fatigue scale ≥ 39; 49.9%), and more than half reported low physical functioning (score < 83; 56.0%), pain (score ≥ 25; 57.7%) and dyspnea (score ≥ 17; 57.4%) (Extended Data Fig. 1). These thresholds were determined in a previous study based on external criteria (for example, limitations in daily living and perceived need for care), which reflect the clinical importance of a health problem16.
Primary outcomes
The exercise group reported significantly better HRQOL than the control group at 6 months (between-group difference (BGD), 4.8 (95% CI, 2.2–7.4); Bonferroni–Holm-adjusted P = 0.0003; effect size (ES), 0.33). At 6 months, the exercise group also reported significantly lower physical fatigue levels compared to the control group (BGD, −5.3 (95% CI, −10.0 to −0.6); Bonferroni–Holm-adjusted P = 0.027; ES, 0.22) (Table 2 and Fig. 2a,b).
Secondary outcomes
Alongside the observed beneficial effects of exercise at 6 months regarding the primary outcomes, the exercise group reported significantly better HRQOL than the control group at 3 months (BGD, 3.9 (95% CI, 1.5–6.3); ES, 0.27) and 9 months (BGD, 4.3 (95% CI, 1.4–7.3); ES, 0.30). At 9 months, the exercise group also reported significantly lower levels of physical fatigue than the control group (BGD, −5.6 (95% CI, −10.9 to −0.4); ES, 0.24). At 6 months, we found positive exercise effects on physical fitness (BGD, 24.8 W (95% CI, 17.3–32.3); ES, 0.47) and on numerous QLQ-C30 scales, including global QOL (BGD, 4.7 (95% CI, 0.8–8.7); ES, 0.27), physical functioning (BGD, 7.0 (95% CI, 3.6–10.3); ES, 0.42), role functioning (BGD, 7.6 (95% CI, 2.1–13.1); ES, 0.29), social functioning (BGD, 5.5 (95% CI, 0.2–10.8); ES, 0.20), pain (BGD, −7.1 (95% CI, −12.1 to −1.9); ES, 0.28) and dyspnea (BGD, −7.6 (95% CI, −12.2 to −3.0); ES, 0.28) (Table 2 and Fig. 3a). Positive exercise effects on physical functioning and pain were already observed at 3 months (BGD, 3.9 (95% CI, 0.8–6.9) and ES, 0.23 for physical functioning; BGD, −4.9 (95% CI, −9.8 to −0.03) and ES, 0.20 for pain) and remained throughout the entire intervention period up to 9 months (BGD, 5.9, (95% CI, 2.2–9.6) and ES, 0.33 for physical functioning; BGD, −6.5 (95% CI, −12.0 to −1.0) and ES, 0.26 for pain). At 3 months, the exercise group reported significantly better cognitive functioning (BGD, 4.0, (95% CI, 0.4–7.5); ES, 0.16) and less insomnia (BGD, −5.7 (95% CI, −11.1 to −0.2); ES, 0.19) than the control group; however, these BGDs were no longer significant at 6 and 9 months. No beneficial effects of the exercise intervention were found on emotional functioning or emotional fatigue (Table 2 and Fig. 3a,b). Compared to the control group, the exercise group significantly increased self-reported vigorous-intensity aerobic exercise levels at 3, 6 and 9 months (BGD, 23 min per week (95% CI, 14–33) and ES, 0.73; BGD, 24 min per week (95% CI, 15–33) and ES, 0.77; and BGD, 15 min per week (95% CI, 5–24) and ES, 0.48, respectively), as well as resistance exercise levels (BGD, 30 min week (95% CI, 19–41) and ES, 1.17; BGD, 38 min per week (95% CI, 27–49) and ES, 1.49; and 16 min per week (95% CI, 5–26) and ES, 0.63, respectively) (Table 2 and Supplementary Table 1). Similarly, compared to the control group, the exercise group significantly increased objectively measured ‘very active’ physical activity levels at 6 months (BGD, 7 min per day (95% CI, 2–12); ES, 0.36) and 9 months (BGD, 7 min per day (95% CI, 1–13); ES, 0.36), whereas sedentary time was significantly decreased at 9 months (BGD, −85 min per day, (95% CI, −166 to −4); ES, 0.32). At 9 months, the exercise group also increased self-reported moderate-intensity aerobic exercise levels (BGD, 28 min per week (95% CI, 7–49); ES, 0.35) compared to the control group. No significant BGDs were found for other physical activity levels. Within-group differences for all outcomes are shown in Extended Data Tables 3–5.
A radar plot for all quality of life outcomes (a) and a radar plot for all fatigue dimensions (b). It should be noted that the scale of all quality of life symptom outcomes and fatigue outcomes were inverted to facilitate interpretability. An increase from baseline to 6 months post baseline now indicates an improvement for all outcomes. Asterisk, statistically significant BGD.
Safety
In total, two exercise-related serious adverse events (SAEs) were reported: a wrist fracture and a sacral stress fracture. These were not related to bone metastases. Both participants continued the exercise program after an interruption and/or a modification to the program. Frequently reported adverse events (AEs) (n = 80), which required modifications to the exercise program, included pain (51.3%), dizziness (12.5%), muscle soreness and/or cramps (11.3%) and fatigue (8.9%).
Exploratory outcomes
Regarding effect modification, exercise effects on HRQOL and physical fatigue did not vary significantly as a function of clinical characteristics or baseline values of physical fitness or patient-reported outcomes (PROs) (Fig. 4a,b). Greater effects on HRQOL were found for participants who were younger (BGD, 8.4 (95% CI, 3.2–13.6) for those <50 years old (31%) versus BGD, 3.3 (95% CI, 0.2–6.5) for those ≥50 years old) or who reported pain above the clinically important threshold at baseline (58%) compared to participants without pain (BGD, 6.0 (95% CI, 2.0–10.0) versus BGD, 2.5 (95% CI, −0.8–5.7).
Sensitivity analysis
The sensitivity analysis, with multiple types of imputed data, yielded similar results; that is, the exercise group reported significantly better HRQOL (BGD, 4.8, (95% CI, 2.3–7.3); ES, 0.33) and lower physical fatigue levels than the control group at 6 months (BGD, −5.6 (95% CI, −10.1 to −1.0), ES, 0.24).
Discussion
In the PREFERABLE-EFFECT study, we have demonstrated that patients with MBC were able to participate in a 9-month supervised exercise program that resulted in improvements in fatigue and HRQOL at 6 months, which were maintained for up to 9 months. In addition, beneficial effects on clinically relevant outcomes such as physical functioning, role functioning, physical fitness, dyspnea and pain were observed.
Current American College of Sports Medicine (ACSM)11 and American Society of Clinical Oncology12 guidelines recommend exercise during and after curative cancer treatment and report a lack of studies in the metastatic setting. Our results may contribute to future updates to these guidelines. Furthermore, our results may facilitate the inclusion of more detailed exercise recommendations in the current European School of Oncology–European Society for Medical Oncology international consensus guidelines for advanced breast cancer17. Currently, the guidelines do not include specific exercise recommendations for patients with metastatic disease owing to a lack of supporting empirical data. So far, only six randomized controlled exercise trials have been conducted in patients with MBC, with sample sizes ranging from 14 to 101 patients18,19,20,21,22,23. These were primarily feasibility studies and were not powered to detect statistically significant differences in fatigue or HRQOL between groups. Only two randomized controlled trials (n = 38 and n = 40) reported statistically significant positive effects of exercise on fatigue and HRQOL compared to a control group21,23. Recently, an adequately powered (n = 313) randomized controlled trial investigated the effects of a remotely supervised, 16-week home-based exercise program on HRQOL in patients with advanced pancreatic ductal adenocarcinoma24. Although the exercise program in that study was considered safe and feasible, no statistically significant beneficial effects of exercise on various dimensions of HRQOL, including global health status, fatigue and physical functioning, were found. The lack of an effect might be attributed, in part, to the underlying disease, which is characterized by a more aggressive clinical disease course and often involves multiple nutrition-related symptoms requiring a different type of behavioral intervention beyond exercise only. In addition, the exercise program differed from our EFFECT exercise program in several ways, including the intensity of the program (low-to-moderate intensity versus moderate-to-high intensity), the duration of the intervention (16 weeks versus 9 months) and the setting (home-based versus hospital-based or community-based).
Current guidelines for cancer survivors recommend exercise programs of at least 12 weeks in duration during or after curative cancer treatment to improve HRQOL and fatigue11. In the present study, we decided to offer a 9-month exercise program, as patients with metastatic disease typically undergo continuous treatment, often leading to an exacerbation of side effects over time, and experience disease progression, leading to treatment changes and physical deterioration. This might explain why we only observed beneficial effects after 6 months for some outcomes such as physical fatigue, role functioning and social functioning. Based on this observation, we recommend exercise programs of a longer duration for patients with metastatic disease. We also reasoned that a longer intervention would improve the self-efficacy of patients, enabling them to shift from a supervised setting to an unsupervised setting. Therefore, after 6 months, we substituted one supervised session with an unsupervised session and investigated the sustainability of effects at 9 months. Furthermore, we propose that best practice should include supervision by a qualified exercise professional with expertise in the cancer setting, and preferably with experience in working with patients with metastatic cancer. At least initially, in-person supervision is preferred to ensure safety. In addition, given the complexities of the disease and its possible complications, we recommend that patients receive medical approval from their treating physician before engaging in exercise, including a risk evaluation to assess the chance of a skeletal complication arising from exercise. Information provided by the physician can help to further tailor the exercise program to accommodate the patient’s condition and ensure safety. For example, certain exercises may not be suitable for patients with bone metastases in specific locations. The International Bone Metastases Exercise Working Group advises that exercises should be avoided or adjusted based on the location of the bone metastases. Furthermore, this working group recommends that exercise prescriptions for patients with bone metastases should follow the general exercise guidelines for cancer survivors, with a focus on postural alignment, controlled movement and technique, while taking the location and clinical presentation of the bone metastases into account. This, in fact, was the procedure that was followed in our intervention protocol, which can also be applied in clinical practice25.
In this study, the attendance rate of 77% and the compliance rates ranging from 59 to 100% show that the patients were highly motivated and able to complete the different components of the exercise program. In previous trials, adherence to an exercise program after a cancer diagnosis ranged from 62 to 78% (ref. 26), which underscores the high acceptability of the supervised EFFECT exercise program. Further, the number of reported SAEs and/or AEs (n = 2) is comparable to what has been reported in previous studies in patients with bone metastases, and the SAEs in our study were judged to be unrelated to the bone metastases15. In these studies, it was concluded that exercise appeared to be safe in this patient population, but predefined safety analyses are not yet available. Despite uncertainties among healthcare professionals about recommending exercise to individuals with metastatic disease27,28,29, our findings indicate that a supervised exercise regimen like the EFFECT intervention is well tolerated by patients with MBC with stable bone metastases.
In the current study, we found statistically significant effects of exercise on several HRQOL outcomes, including fatigue. The corresponding ESs were small to moderate. These ESs are comparable to those observed in exercise–oncology trials in the curative setting30,31, which serve as the basis for the current exercise guidelines11,12. It has been argued that HRQOL outcomes in cancer studies should be interpreted based on their clinical relevance instead of their statistical significance. For this reason, minimally important differences (MIDs) have been estimated in patients with advanced breast cancer, indicating the smallest change in a HRQOL outcome that is perceived as important by a patient32. Although MIDs are not available for our primary outcomes (that is, the summary QLQ-C30 score and the QLQ-FA12 physical fatigue score), they are available for other HRQOL outcomes assessed in our trial. The BGDs observed in our study for fatigue, social functioning and role functioning were larger than the published MIDs. This underscores the clinical relevance of our findings. Interestingly, we did not observe any effects of exercise on emotional functioning or emotional fatigue. Based on other studies, additional interventions, such as cognitive behavioral therapy, may be needed to improve these outcomes33,34.
Our results indicate that the exercise intervention not only had a positive effect on physical fatigue and HRQOL but also on pain and dyspnea. This suggests that exercise may also be used as a supportive treatment for these symptoms. Indeed, recent observational data also showed that cancer survivors who are physically active experience less pain compared to those who are less active35. In our multinational PREFERABLE-PERSPECTIVE survey study (n = 420), patients with MBC frequently mentioned fatigue and pain as a barrier to start or continue exercising, and some respondents even expected exercise to worsen these symptoms36. The current results could therefore be used for educational and motivational purposes in helping patients with MBC to overcome exercise barriers.
Considering the acknowledged clinical thresholds of EORTC-QLQ measures, the majority of our participants reported clinically low levels of physical functioning and high levels of fatigue, pain and dyspnea at baseline16. In comparison to reference values of European MBC patients, our study participants reported better physical functioning but higher levels of pain, dyspnea and fatigue9. This indicates that patients who took part in our study were in need of an intervention to alleviate these symptoms.
This multinational randomized controlled trial is the first adequately powered study investigating the effectiveness of exercise on HRQOL and fatigue in patients with MBC. Further strengths of the study are the long duration of the exercise program and the program's supervision in different clinical settings by different exercise professionals, which resembles clinical practice in the participating countries and thereby increases the generalizability of the findings.
Our study also has some limitations. We allowed patients to enter the study at any time during their treatment, which resulted in a heterogeneous sample. However, this also increases the generalizability of our results. Conversely, we did not have detailed information on patients who declined to participate in the trial, which might hamper the generalizability of our study results. The recruitment rate was around 40%. Owing to self-selection by the patient and/or selection by the treating physician, patients who were enrolled might have had certain characteristics (for example, completed higher education) that made them more prone to participate, whereas patients in greatest need of an exercise intervention (that is, with higher fatigue levels and/or more comorbidities) might have been less likely to participate. We were also unable to blind participants to their respective study arm. This may have motivated patients in the control arm to voluntarily increase their physical activity levels, especially as all patients had received general advice on physical activity and a fitness tracker. This could have resulted in an underestimation of the intervention effect. However, based on a self-report questionnaire, we did not observe an increase in time spent on resistance exercises and vigorous-intensity aerobic exercises in the control group. It may also have led to patients in the intervention group to be more motivated during physical tests at follow-up assessments or to report more positively on PROs, which would lead to overestimation of the intervention effect. Assessment of measured physical activity was limited to aggregated data, as detailed analyses using raw Fitbit data were beyond the scope of the current analyses.
Overall, this large multinational trial demonstrated significant beneficial effects of a supervised exercise intervention offered during oncological treatment on MBC patients’ HRQOL, fatigue and other clinically relevant outcomes. Based on our findings, we recommend that supervised resistance and aerobic exercise be included as an integral part of supportive care for patients with MBC.
Methods
Study design and participants
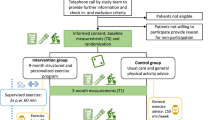
The PREFERABLE-EFFECT study design and methods have been published previously, and the full protocol is provided in the Supplementary information37. In brief, this multinational randomized controlled trial was undertaken at eight hospitals and study centers in Germany, the Netherlands, Poland, Spain, Sweden and Australia.
Eligible patients were 18 years of age or older, diagnosed with stage IV breast cancer, had an Eastern Cooperative Oncology Group performance status of ≤2, and were able and willing to participate in the exercise program and wear an activity tracker. Exclusion criteria were unstable bone metastases as determined by the local treating physician; untreated symptomatic brain metastases; estimated life expectancy of <6 months; serious active infection; excessive physical activity (>210 min per week of moderate-intensity to vigorous-intensity exercise) or current participation in an exercise training program comparable to the EFFECT exercise program; severe neurologic or cardiac impairment according to the ACSM criteria38; uncontrolled severe respiratory insufficiency or dependency on oxygen supplementation at rest or during exercise; uncontrolled severe pain; any other contraindications for exercise; any circumstances that would impede adherence to study requirements or the ability to give informed consent; or pregnancy. Patients were enrolled regardless of sex, which was collected according to the identity information provided by the patients. Patients were recruited by their clinical care or study teams or through social media (for example, national patient organizations). Medical eligibility criteria were assessed by a physician at the treating hospital.
Ethics statement
The study was conducted in accordance with standards of good clinical practice and the Declaration of Helsinki. The study was approved by the institutional review board of the University Medical Center Utrecht, the Netherlands (19-524/M), and by the local ethical review boards of all participating institutions. The study was registered with ClinicalTrials.gov on 9 October 2019 (NCT04120298). All patients provided written informed consent before enrollment.
Randomization and blinding
Patients who met the eligibility criteria and provided informed consent were randomly assigned (1:1), after completion of the baseline measurements, to participate in a 9-month structured and individualized exercise program in addition to usual care (exercise group) or to receive general physical activity advice in addition to usual care, but no structured exercise program (control group). All participants received an activity tracker. Randomization was performed centrally using a blocked computer-generated sequence and was stratified by study center and therapy line (first-line or second-line vs. third-line treatment or a later line of treatment). Owing to the nature of the intervention, participants, local clinicians and study nurses, and investigators were not blinded to group assignment after randomization.
Procedures
A 9-month structured and individualized exercise program was offered to participants randomized to the exercise group. Details of the exercise program have been published elsewhere37. In brief, the exercise program included supervised, multimodal exercise sessions of 1 h, two times per week for the first 6 months. For the last 3 months, one supervised session was replaced by one unsupervised session. Supervision was performed by qualified exercise professionals (for example, physiotherapists and exercise physiologists) in a community-based or hospital-based fitness center, or a physical therapy practice close to the participants’ home address. In addition to the in-person supervised exercise sessions, we offered live remote exercise sessions to participants using videoconferencing software (Zoom) if training facilities were closed owing to local COVID-19 regulations or if previously enrolled participants felt unsafe exercising at a local training facility because of the COVID-19 threat.
The multimodal exercise program consisted of resistance, aerobic and balance exercises (Extended Data Table 6). Resistance exercise intensity was individualized using 12-repetition maximum muscle strength testing. For participants with bone metastases, 12-repetition maximum testing was not performed for exercises that loaded the parts of the skeleton with bone metastases (see Extended Data Table 7). During the exercise sessions, resistance exercises that loaded the affected region were either omitted or performed according to the ‘start low (that is, low weight and more repetitions), go slow (that is, gradual increase)’ principle25, depending on patient characteristics and the experience of the involved exercise professional. Aerobic exercise intensity was tailored to the participants’ fitness levels using the maximal short exercise capacity (MSEC) and estimated peak power output (Wpeak) with the steep ramp test at baseline. The intensity of both the aerobic and resistance exercises gradually increased during the exercise program; however, the intensity was continuously adjusted, depending on the health status of the participant and the participant's perceived exertion.
In addition to the supervised exercise program, participants were encouraged to be physically active for at least 30 min per day on all remaining days of the week. To support this, participants received an activity tracker (that is, Fitbit Inspire HR) and an exercise app specifically designed for the PREFERABLE-EFFECT study. The app included exercises that participants learned during the supervised exercise program and that could be performed at home. All exercises were illustrated with simple animations and contained clear instructions (see Supplementary Fig. 1 for screenshots of the app). The exercise app was also used to support the unsupervised sessions during the last 3 months of the intervention period.
Participants randomized to the control group received care as usual, supplemented with written information on the current physical activity guidelines (that is, 150 min of aerobic exercise and resistance exercise two to three times per week). They were advised to avoid inactivity and to be as physically active as their health status allowed11. They also received an activity tracker and an explanation of the basic functions of the tracker. The control group did not receive a structured exercise intervention, as this is not yet part of routine care.
All participants visited the study center for measurements at baseline, and at 3 and 6 months post baseline. This included the assessment of functional performance and physical fitness. At all visits as well as at 9 months post baseline, PROs were assessed using online questionnaires. Participants were asked to complete them without conferring with others. For participants undergoing intravenous chemotherapy, the measurements took place at least 3 days after chemotherapy administration. PROs, including HRQOL and fatigue, were assessed using the EORTC QLQ-C30 and the EORTC QLQ-FA12, respectively39,40. The QLQ-C30 is a 30-item questionnaire, including a global HRQOL score, five functional scales (physical, role, emotional, cognitive and social), three symptom scales (fatigue, nausea and vomiting, and pain) and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties). A summary HRQOL score can be calculated using 13 subscales, excluding the global QOL and financial difficulties items41. The QLQ-FA12 is a 12-item questionnaire that assesses different dimensions of fatigue (physical, emotional, cognitive and total fatigue). For both EORTC questionnaires, scores range from 0 to 100. For the summary score, global QOL score and functional scales, higher scores indicate a higher HRQOL or a higher function, whereas for symptom scales, higher scores indicate a greater symptom burden. To assess higher levels of physical functioning, four items from the EORTC questionnaire item bank were added to the physical function scale (see Supplementary Table 2). Subsequently, a domain-specific T-score was calculated for physical functioning using EORTC software. This T-score reflects the score of the participant relative to an age-matched and a gender-matched European reference population, with 50 representing average physical functioning.
Self-reported physical activity levels were assessed using a modified version of the Godin–Shephard Leisure-Time Exercise Questionnaire42,43. The Godin questionnaire is a four-item questionnaire that includes questions about the average frequency and duration of mild-, moderate- and vigorous-intensity aerobic exercise and resistance exercise in bouts of at least 10 min performed during leisure time in a typical week. In addition, the Fitbit Inspire HR was used to measure daily step count and minutes of physical activity (that is, minutes per day being sedentary or lightly, fairly or very active, as classified by the FitBit software), throughout the study period. For Fitbit data, only data were used for participants who had >4 valid wear days (defined as ≥10 h of activity registration) around the measurement timepoints (that is, baseline and 3, 6 and 9 months post baseline).
As a measure of physical fitness, the MSEC was assessed with the steep ramp test using a cycle ergometer44. After 3 min of unloaded cycling, the test started at 25 W and was increased by 2.5 W s−1 or 25 W per 10 s, depending on the available settings of the cycle ergometer used, until exhaustion. Participants were instructed to cycle between 70 and 90 r.p.m. The test ended when the cycling cadence dropped below 60 r.p.m. or when the participant experienced pain or discomfort. After termination, the participant was asked to continue cycling at an easy cadence and with minimal load to promote recovery. The outcome was recorded as the highest achieved output in W and is referred to as the MSEC. From the MSEC, peak power output (Wpeak) was estimated using a regression equation45. Before physical fitness testing, resting heart rate and blood pressure were measured for safety reasons.
Body weight and height were measured in light clothing without shoes. Demographic and clinical data were extracted from questionnaires and medical records, respectively. Adherence to the supervised exercise program was recorded by the exercise professional in a case report form. Safety was assessed by the reporting of AEs and SAEs related to exercise or physical fitness testing. Participants in both groups were asked by the study personnel about exercise-related and physical fitness testing-related AEs and SAEs in a standardized manner during all follow-up visits. In addition, for participants allocated to the exercise group, the exercise professionals assessed any potential exercise-related AEs and SAEs that had occurred since the previous exercise session or during the current session and recorded this on standardized training documentation forms.
Adherence
Adherence to the supervised exercise program was measured in terms of attendance and compliance. Attendance rates were computed as the number of supervised exercise sessions attended divided by the number of sessions offered. Compliance rates were calculated as the number of supervised exercise sessions in which participants performed all prescribed balance, resistance and aerobic exercises, divided by the number of sessions prescribed.
Outcomes
The study had two primary outcomes: HRQOL and cancer-related physical fatigue, which were assessed using the summary score of the QLQ-C30 and the physical fatigue dimension of the QLQ-FA12, respectively. We assessed the primary outcomes at the fully supervised intervention period (that is, at 6 months) and defined the period from 6 to 9 months as the maintenance period.
Secondary outcomes reported in this paper include the primary outcomes assessed at 3 and 9 months, as well as a range of other variables: the QLQ-C30 global QOL score, and all other QLQ-C30 function and symptom scales and single items, all other QLQ-FA12 fatigue dimensions, self-reported and measured physical activity, and the MSEC.
The study included pre-planned modifier analyses for the following covariates: age (<50 vs. ≥50 years), baseline fatigue levels (QLQ-C30 fatigue scale score of <39 vs. ≥39)16, baseline depression levels (PHQ-4 depression subscore of <3 vs. ≥3), history of psychological disorders (any report vs. none), baseline insomnia (PSQI global score of 0–4, 5–8 or ≥9), baseline body mass index (<25 vs. ≥25 and <30 vs. ≥30), baseline fitness level (MSEC, continuous), type of therapy (chemotherapy vs. other), type of metastasis at baseline (bone only vs. mixed (visceral and non-visceral) vs. non-visceral only) and primary tumor receptor status (triple-negative vs. human epidermal growth factor receptor 2 (HER2)-positive vs. HER2-negative and hormone receptor-positive). In addition, the following subgroup analyses were prespecified: female patients only, all patients excluding those who never started the exercise program or dropped out within a month, all patients excluding those who did not adhere to the exercise program (that is, attendance of <80% of scheduled exercise sessions), all patients excluding those who began chemotherapy (intravenous or oral) between baseline and 6 months post baseline. A subgroup analysis based on baseline pain levels (QLQ-C30 pain scale score of <25 vs. ≥25)16 was not prespecified but became of interest during the study.
Statistical analysis
An improvement in either or both primary outcomes in the exercise group from baseline to 6 months post baseline relative to the control group was of primary relevance. Based on a pooled analysis of six randomized controlled exercise trials in patients with breast cancer receiving adjuvant treatment, we anticipated an ES of 0.35 (ref. 46). With n = 139 patients per group (n = 278 in total), a mean standardized ES of at least 0.35 could be detected with a power of at least 78% or 82% at a nominal two-sided significance level of 2.5% for each outcome separately using an analysis of covariance adjusted for baseline values of the outcome variable, assuming a correlation between pre-invervention and post-intervention levels of ρ = 0.3 or ρ = 0.4, respectively47. To account for a potential drop-out rate of approximately 20%, the target sample size was 350 participants (n = 175 per study arm).
A statistical analysis plan was written before the analysis was performed and included in the study protocol. Descriptive statistics were used to characterize the study population at baseline. Questionnaire scores were calculated according to published scoring manuals. All primary analyses were performed according to the intention-to-treat principle. For the primary outcomes, linear mixed-effects models were used to assess exercise effects on physical fatigue and HRQOL separately while taking the hierarchical structure of the data into account. Models were adjusted for the baseline value of the outcome and stratification factors (that is, center and therapy line) and included participants for whom the outcome was observed at two or more timepoints. Models with different covariance structures were compared on the basis of Akaike’s information criterion. Modeling assumptions were examined and met. The same approach was used for the analysis of secondary outcomes.
Cohen’s standardized ESs were calculated by dividing the adjusted BGD of the 3-month, 6-month and 9-month post-intervention means by the pooled standard deviation at baseline. For the primary outcome, a two-tailed Bonferroni–Holm-adjusted P value was calculated to indicate statistical significance. For all secondary outcomes, ESs and 95% CIs are reported without P values. These confidence intervals are intended to express precision of the effect estimate and should not be used to infer statistical significance, as they do not account for multiple comparisons.
Prespecified intervention effect modifiers were individually added to the model as a covariate main effect and interaction effect with group allocation. Covariates that appeared to be intervention effect modifiers (Pinteraction ≤ 0.10) gave rise to subgroup analyses. Prespecified subgroup analyses were performed, irrespective of interaction effects, to assess whether the intervention effect was consistent across subgroups. All modifier and subgroup analyses were treated as exploratory.
Missing values of the primary outcome variables as well as all other PROs were considered as missing at random and dealt with using linear mixed-effects models. A sensitivity analysis, using multiple imputation (m = 100, R package ‘MICE’)48, was carried out to explore potential bias and demonstrate the robustness of our results.
All statistical analyses were performed using R v4.2.2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are not yet openly available owing to reasons of confidentiality. Researchers can request current data from the corresponding author (a.m.may@umcutrecht.nl). Pseudonymized data (including data dictionaries) will be made available through the Digital Research Environment, which is a trusted digital research environment that can be accessed at https://mydre.org. This will be carried out after the review and approval of a methodologically sound proposal by the General Assembly of PREFERABLE, with a signed data access agreement, which is in line with Ethics Committee requirements (The Ethics Committee of University Medical Center Utrecht, The Netherlands). Requests will be processed within 6 weeks. These files will be available from the date of publication until the date stated in the approved request. Once the PREFERABLE project has been fully completed, the database will be anonymized and shared using DataverseNL. The study protocol is available as an open access publication (https://doi.org/10.1186/s13063-022-06556-7)37.
Code availability
The statistical coding is available on GitHub at https://github.com/AnoukHiensch/PREFERABLE-EFFECT.git49.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Arnold, M. et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66, 15–23 (2022).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 21, 519–530 (2020).
Cortes, J. et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 387, 217–226 (2022).
Hortobagyi, G. N. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 386, 942–950 (2022).
Cardoso, F. et al. Global analysis of advanced/metastatic breast cancer: decade report (2005–2015). Breast 39, 131–138 (2018).
Butt, Z. et al. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J. Natl Compr. Canc. Netw. 6, 448–455 (2008).
Mosher, C. E. et al. Living with metastatic breast cancer: a qualitative analysis of physical, psychological, and social sequelae. Breast J. 19, 285–292 (2013).
Mierzynska, J. et al. Reference values for the EORTC QLQ-C30 in early and metastatic breast cancer. Eur. J. Cancer 125, 69–82 (2020).
Kawahara, T. et al. Association of change in health-related quality of life and treatment discontinuation in metastatic breast cancer: a post hoc, exploratory analysis of two randomized clinical trials. Support. Care Cancer 30, 8367–8375 (2022).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 51, 2375–2390 (2019).
Ligibel, J. A. et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J. Clin. Oncol. 40, 2491–2507 (2022).
De Lazzari, N., Niels, T., Tewes, M. & Götte, M. A systematic review of the safety, feasibility and benefits of exercise for patients with advanced cancer. Cancers 13, 4478 (2021).
Toohey, K. et al. The effects of physical exercise in the palliative care phase for people with advanced cancer: a systematic review with meta-analysis. J. Cancer Surviv. 17, 399–415 (2023).
Weller, S. et al. Exercise for individuals with bone metastases: a systematic review. Crit. Rev. Oncol. Hematol. 166, 103433 (2021).
Giesinger, J. M. et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J. Clin. Epidemiol. 118, 1–8 (2020).
Cardoso, F. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 31, 1623–1649 (2020).
Scott, J. M. et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: a randomized controlled trial. Cancer 124, 2552–2560 (2018).
Oh, B. et al. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann. Oncol. 21, 608–614 (2010).
Ligibel, J. A. et al. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer 122, 1169–1177 (2016).
Headley, J. A., Ownby, K. K. & John, L. D. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol. Nurs. Forum 31, 977–983 (2004).
Yee, J. et al. Physical activity for symptom management in women with metastatic breast cancer: a randomized feasibility trial on physical activity and breast metastases. J. Pain Symptom Manage. 58, 929–939 (2019).
Sheean, P. et al. Every day counts: a randomized pilot lifestyle intervention for women with metastatic breast cancer. Breast Cancer Res. Treat. 187, 729–741 (2021).
Neuzillet, C. et al. Effect of adapted physical activity in patients with advanced pancreatic cancer: the APACaP GERCOR randomized trial. J. Natl Compr. Cancer Netw. 21, 1234–1242.e17 (2023).
Campbell, K. L. et al. Exercise recommendation for people with bone metastases: expert consensus for health care providers and exercise professionals. JCO Oncol. Pract. 18, e697–e709 (2022).
Kampshoff, C. S. et al. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int. J. Behav. Nutr. Phys. Act. 11, 80 (2014).
Sheill, G., Guinan, E., O Neill, L., Hevey, D. & Hussey, J. Physical activity and advanced cancer: the views of chartered physiotherapists in Ireland. Physiother. Theory Pract. 34, 534–541 (2018).
Ten Tusscher, M. R. et al. Education needs of Dutch physical therapists for the treatment of patients with advanced cancer: a mixed methods study. Phys. Ther. 100, 477–486 (2020).
Sheill, G., Guinan, E., Neill, L. O., Hevey, D. & Hussey, J. Physical activity and advanced cancer: the views of oncology and palliative care physicians in Ireland. Ir. J. Med. Sci. 187, 337–342 (2018).
van Vulpen, J. et al. Moderators of exercise effects on cancer-related fatigue: a meta-analysis of individual patient data. Med. Sci. Sports Exerc. 52, 303–314 (2020).
Buffart, L. M. et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 52, 91–104 (2017).
Musoro, J. Z. et al. Minimally important differences for interpreting EORTC QLQ-C30 scores in patients with advanced breast cancer. JNCI Cancer Spectr. 3, pkz037 (2019).
Ye, M. et al. A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psychooncology 27, 1695–1703 (2018).
Poort, H. et al. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann. Oncol. 31, 115–122 (2020).
Swain, C. T. V., Masters, M., Lynch, B. M., Patel, A. V. & Rees-Punia, E. Physical activity and pain in people with and without cancer. Cancer 130, 2042–2050 (2024).
Sweegers, M. G. et al. Perspectives of patients with metastatic breast cancer on physical exercise programs: results from a survey in five European countries. Support. Care Cancer 31, 694 (2023).
Hiensch, A. E. et al. Design of a multinational randomized controlled trial to assess the effects of structured and individualized exercise in patients with metastatic breast cancer on fatigue and quality of life: the EFFECT study. Trials 23, 610 (2022).
Riebe, D. et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med. Sci. Sports Exerc. 47, 2473–2479 (2015).
Aaronson, N. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 85, 365–376 (1993).
Weis, J. et al. International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12). J. Natl Cancer Inst. 109, djw273 (2017).
Giesinger, J. M. et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 69, 79–88 (2016).
Amireault, S. & Godin, G. The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Skills. 120, 604–622 (2015).
Amireault, S., Godin, G., Lacombe, J. & Sabiston, C. M. Validation of the Godin-Shephard leisure-time physical activity questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J. Cancer Surviv. 9, 532–540 (2015).
De Backer, I. C. et al. Exercise testing and training in a cancer rehabilitation program: the advantage of the steep ramp test. Arch. Phys. Med. Rehabil. 88, 610–616 (2007).
Stuiver, M. M. et al. Validation and refinement of prediction models to estimate exercise capacity in cancer survivors using the steep ramp test. Arch. Phys. Med. Rehabil. 98, 2167–2173 (2017).
van Vulpen, J. K., Peeters, P. H. M., Velthuis, M. J., van der Wall, E. & May, A. M. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas 85, 104–111 (2016).
Borm, G. F., Fransen, J. & Lemmens, W. A. J. G. A simple sample size formula for analysis of covariance in randomized clinical trials. J. Clin. Epidemiol. 60, 1234–1238 (2007).
van Buuren, S. & Groothuis-Oudshoorn, K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Hiensch, A. E. AnoukHiensch/PREFERABLE-EFFECT. GitHub https://github.com/AnoukHiensch/PREFERABLE-EFFECT.git (2024).
Acknowledgements
The authors would like to thank all participants, all involved exercise professionals and all professional staff at all participating centers in Germany (Heidelberg University Hospital, German Cancer Research Center, National Center for Tumor Diseases Heidelberg, and German Sport University Cologne), the Netherlands (University Medical Center Utrecht, the Netherlands Cancer Institute, Diakonessenhuis Utrecht and Alexander Monro Ziekenhuis), Poland (Greater Poland Cancer Center), Spain (Onkologikoa), Sweden (Karolinska Institutet and Karolinska University Hospital) and Australia (Australian Catholic University, Peter MacCallum Cancer Centre, Royal Melbourne Hospital and Cabrini Health) for their invaluable participation in this trial.
This study received funding from the European Union’s Horizon 2020 research and innovation program (no. 825677; main applicant, A.M.M.) and the National Health and Medical Research Council of Australia (2018/GNT1170698; main applicant, E.M.Z.). The funders of the study (the European Union (Horizon 2020) and the National Health and Medical Research Council of Australia) had no role in study design, data collection, data analysis, data interpretation or the writing of the report.
Author information
Authors and Affiliations
Contributions
M.E.S., P.Z., J.B., H.R., E.v.d.W., N.K.A., E.S., A.U., E.M.Z., W.B., M.M.S., Y.W., K.S. and A.M.M. conceived the study. A.M.M., K.S., Y.W., M.M.S., W.B., E.M.Z., A.U., E.S. and J.W. were principal investigators of the study at their local study center. A.M.M. was the main principal investigator of the PREFERABLE project. C.F., R.A., R.B., L.v.L.S., D.t.B.H., G.S., A.L., G.B.M., P.A.F., G.R., W.M. and E.v.d.W. recruited participants for the study. A.E.H., J.D., M.E.S., E.M.M., M.P., D.C., N.G., J.B., M.T., H.R., P.Z., J.W., J.M., M.G. and M.G.S. were responsible for all study measurements. A.E.H. and J.D. were responsible for the data analyses and wrote the first draft of the manuscript. All authors contributed to the interpretation and provided critical feedback on the analyses and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Percentage of participants scoring above (orange) or below (green) the threshold for clinical importance for the functional and symptom scales of the EORTC QLQ-C30 at baseline16.
Supplementary information
Supplementary Information
Supplementary Information (that is, study protocol), Supplementary Fig. 1 and Supplementary Tables 1 and 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hiensch, A.E., Depenbusch, J., Schmidt, M.E. et al. Supervised, structured and individualized exercise in metastatic breast cancer: a randomized controlled trial. Nat Med (2024). https://doi.org/10.1038/s41591-024-03143-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-024-03143-y
- Springer Nature America, Inc.