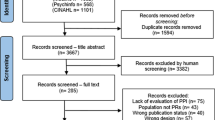
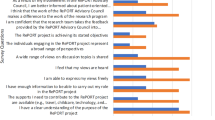
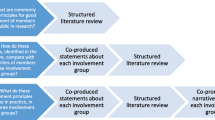
Abstract
Patient and public involvement and engagement (PPIE) can provide valuable insights into the experiences of those living with and affected by a disease or health condition. Inclusive collaboration between patients, the public and researchers can lead to productive relationships, ensuring that health research addresses patient needs. Guidelines are available to support effective PPIE; however, evaluation of the impact of PPIE strategies in health research is limited. In this Review, we evaluate the impact of PPIE in the ‘Therapies for Long COVID in non-hospitalised individuals’ (TLC) Study, using a combination of group discussions and interviews with patient partners and researchers. We identify areas of good practice and reflect on areas for improvement. Using these insights and the results of a survey, we synthesize two checklists of considerations for PPIE, and we propose that research teams use these checklists to optimize the impact of PPIE for both patients and researchers in future studies.

Similar content being viewed by others
References
Staniszewka, S. A patient–researcher partnership for rare cancer research. Nat. Med. 26, 164–165 (2020).
Ryll, B. From good to great: what patients can do for your medical research. Nat. Med. 26, 1508 (2020).
Manna, E. My disability has become an ability. Nat. Med. 26, 1317 (2020).
Murray, M. Alzheimer’s: put patients and researchers in touch. Nature 488, 157 (2012).
NIHR. Patient and public involvement in health and social care research: a handbook for researchers 2014. https://www.rds-yh.nihr.ac.uk/wp-content/uploads/2015/01/RDS_PPI-Handbook_2014-v8-FINAL-11.pdf
NIHR. Standards for public involvement in research—better public involvement for better health and social care research 2019. https://www.invo.org.uk/wp-content/uploads/2019/11/UK-standards-for-public-involvement-v6.pdf
NIHR. Strategies for diversity and inclusion in public involvement: supplement to the briefing notes for researchers 2012. https://www.invo.org.uk/wp-content/uploads/2012/06/INVOLVEInclusionSupplement1.pdf
NIHR. The Race Equality Framework—a practitioner’s guide for public involvement in research. 2022. https://www.nihr.ac.uk/documents/Race%20Equality%20Framework%20-%2020%20April%202022.pdf
NIHR. Payment guidance for researchers and professionals 2022. https://www.nihr.ac.uk/documents/payment-guidance-for-researchers-and-professionals/27392?pr=
NIHR. Guidance on co-producing a research project 2018. https://www.invo.org.uk/wp-content/uploads/2019/04/Copro_Guidance_Feb19.pdf
Richards, T. Patient and public involvement in research goes global. BMJ https://blogs.bmj.com/bmj/2017/11/30/tessa-richards-patient-and-public-involvement-in-research-goes-global/ (2017).
Price, A. et al. Frequency of reporting on patient and public involvement (PPI) in research studies published in a general medical journal: a descriptive study. BMJ Open 8, e020452 (2018).
Chambers, R. Involving patients and the public—is it worth the effort? J. R. Soc. Med. 94, 375–377 (2001).
Cruz Rivera, S., Kyte, D. G., Aiyegbusi, O. L., Keeley, T. J. & Calvert, M. J. Assessing the impact of healthcare research: a systematic review of methodological frameworks. PLoS Med. 14, e1002370 (2017).
Edelman, N. & Barron, D. Evaluation of public involvement in research: time for a major re-think? J. Health Serv. Res Policy 21, 209–211 (2016).
Haroon, S. et al. Therapies for Long COVID in non-hospitalised individuals: from symptoms, patient-reported outcomes and immunology to targeted therapies (The TLC Study). BMJ Open 12, e060413 (2022).
World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (2021).
Aiyegbusi, O. L. et al. Symptoms, complications and management of Long COVID: a review. J. R. Soc. Med. 114, 428–42. (2021).
Subramanian, A. et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 28, 1706–1714 (2022).
Office of National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/3november2022
Health Research Authority. Public involvement in a pandemic: lessons from the UK COVID-19 public involvement matching service (2021).
Callard, F. & Perego, E. How and why patients made Long COVID. Soc. Sci. Med. 268, 113426 (2021).
Therapies for Long COVID in non-hospitalised individuals: the TLC Study 2022. https://www.birmingham.ac.uk/research/applied-health/research/tlc-study/index.aspx (2023).
Routen, A. et al. Patient and public involvement within epidemiological studies of ong COVID in the UK. Nat. Med. 29, 771–773 (2023).
Turner, G. M. et al. Co-production of a feasibility trial of pacing interventions for Long COVID. Res. Involv. Engagem. 9, 18 (2023).
Hughes, S. E. et al. Development and validation of the symptom burden questionnaire for Long COVID (SBQ-LC): Rasch analysis. BMJ 377, e070230 (2022).
Chandan, J. S. et al. Non-pharmacological therapies for post-viral syndromes, including Long COVID: a systematic review. Int. J. Environ. Res. Public Health 20, 3477 (2023).
Chandan, J. S. et al. Non-pharmacological therapies for postviral syndromes, including Long COVID: a systematic review and meta-analysis protocol. BMJ Open 12, e057885 (2022).
Crocker, J. C. et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ 363, k4738 (2018).
Turner, G., Aiyegbusi, O. L., Price, G., Skrybant, M. & Calvert, M. Moving beyond project-specific patient and public involvement in research. J. R. Soc. Med. 113, 16–23 (2020).
Hines, P. A. et al. Regulatory Science to 2025: an analysis of stakeholder responses to the European Medicines Agency’s Strategy. Front. Med. 7, 508 (2020).
Food and Drug Administration. FDA patient-focused drug development guidance series for enhancing the incorporation of the patient’s voice in medical product development and regulatory decision making 2020. https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical (2023).
Medicines and Healthcare products Regulatory Agency. Putting patients first: a new era for our agency. Delivery Plan 2021–2023 (2021).
Aiyegbusi, O. L. et al. The opportunity for greater patient and public involvement and engagement in drug development and regulation. Nat. Rev. Drug Discov. 22, 337–338 (2023).
NIHR. What is patient and public involvement and public engagement? https://www.spcr.nihr.ac.uk/PPI/what-is-patient-and-public-involvement-and-engagement (2023).
Health Data Research UK. Patient and public involvement and engagement. https://www.hdruk.ac.uk/about-us/patient-and-public-involvement-and-engagement/ (2023).
Morton, S. Progressing research impact assessment: a ‘contributions’ approach. Res. Evaluation 24, 405–419 (2015).
Popay, J. & Collins, M. PiiAF: The Public Involvement Impact Assessment Framework Guidance 2014. https://piiaf.org.uk/documents/piiaf-guidance-jan14.pdf (2014).
Staniszewska, S. et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 358, j3453 (2017).
Acknowledgements
We gratefully acknowledge Long COVID SOS, Long COVID Scotland and Long COVID Support who assisted with the recruitment of study participants and PPIE members and the clinicians who assisted with the recruitment of patient partners for the PPIE group. This work is independent research jointly funded by the NIHR and UK Research and Innovation (UKRI; Therapies for Long COVID in non-hospitalised individuals: from symptoms, patient-reported outcomes and immunology to targeted therapies (The TLC Study), COV-LT-0013). The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, the Department of Health and Social Care or UKRI. The funders had no role in the design and conduct of the study, including the collection, management, analysis and interpretation of the data, and preparation and review of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Y.A., L.A., L.B., J.C., A.C., F.J., S.K., K.L.M., P.M., J.O., G.P., M.S.-C. and D.S. are patient partners affiliated with CPROR. O.L.A. and M.J.C. conceptualized the study. O.L.A., M.J.C., Y.A., L.A., L.B., J.C., A.C., F.J., S.K., K.L.M., P.M., G.P., M.S.-C. and D.S. provided input on the survey design. O.L.A. analyzed the survey results and drafted the manuscript. C.M. created Fig. 1. S.E.H., C.M., G.M.T., A.S., R.H., E.H.D., C.F., S.H., M.J.C., Y.A., L.A., L.B., J.C., A.C., F.J., S.K., K.L.M., P.M., J.O., G.P., M.S.-C., D.S. and O.L.A. and members of the TLC Study Group read, revised and approved the final manuscript. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care.
Corresponding author
Ethics declarations
Competing interests
O.L.A. declares personal fees from Gilead Sciences, Merck and GlaxoSmithKline outside the submitted work and receives funding from the NIHR Birmingham Biomedical Research Centre, NIHR Applied Research Collaboration (ARC), West Midlands, NIHR Blood and Transplant Research Unit (BTRU) in Precision Transplant and Cellular Therapeutics at the University of Birmingham and University Hospitals Birmingham NHS Foundation, Innovate UK (part of UKRI), Gilead Sciences, Merck, Anthony Nolan and Sarcoma UK. S.E.H. receives funding from the NIHR ARC, West Midlands, NIHR BTRU in Precision Transplant and Cellular Therapeutics at the University of Birmingham and Anthony Nolan. S.E.H. declares personal fees from Cochlear and Aparito outside the submitted work. C.M. receives funding from the NIHR Surgical Reconstruction and Microbiology Research Centre, the NIHR BTRU in Precision Transplant and Cellular Therapeutics, Innovate UK and Anthony Nolan, and has received personal fees from Aparito outside the submitted work. K.L.M. is a Trustee and volunteer at Long COVID SOS. K.L.M. is on the Long COVID Advisory Board for Dysautonomia International and is employed by the NIHR. J.C. receives funding from NIHR on PPI from a study at UCL (NIHR132914) and a study at University Hospitals Bristol (NIHR203304). J.C. is a lay member on the NICE COVID-19 expert panel and a citizen partner on the COVID END Evidence Synthesis Global Horizon Scanning panel. J.C. declares personal fees from MEDABLE, GlaxoSmithKline and Roche Canada outside of the submitted work. S.H. receives funding from NIHR and UKRI. M.J.C. received personal fees from Astellas, Aparito, CIS Oncology, Takeda, Merck, Daiichi Sankyo, Glaukos, GSK and the Patient-Centered Outcomes Research Institute outside the submitted work. In addition, a family member owns shares in GSK. M.C. receives funding from the NIHR Birmingham Biomedical Research Centre, NIHR Surgical Reconstruction and Microbiology Research Centre, NIHR BTRU in Precision Transplant and Cellular Therapeutics, and NIHR ARC West Midlands at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, Innovate UK (part of UKRI), Macmillan Cancer Support, European Regional Development Fund—Demand Hub, SPINE UK, UKRI, UCB Pharma, GSK, Anthony Nolan and Gilead Sciences. All other authors declare no competing interests. This study was approved by the Health Research Authority and the West Midlands Solihull-Research Ethics Committee (IRAS project ID: 296374; REC reference: 21/WM/0203).
Peer review
Peer review information
Nature Medicine thanks Paul Wicks, Jan Geissler and Linzy Houchen-Wolloff for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aiyegbusi, O.L., McMullan, C., Hughes, S.E. et al. Considerations for patient and public involvement and engagement in health research. Nat Med 29, 1922–1929 (2023). https://doi.org/10.1038/s41591-023-02445-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02445-x
- Springer Nature America, Inc.
This article is cited by
-
An evaluation of a public partnership project between academic institutions and young people with Black African, Asian and Caribbean heritage
Research Involvement and Engagement (2024)
-
Parental Engagement in Identifying Information Needs After Newborn Screening for Families of Infants with Suspected Athymia
Journal of Clinical Immunology (2024)
-
Diabetes Research Matters: A Three-Round Priority-Setting Survey Consultation with Adults Living with Diabetes and Family Members in Australia
The Patient - Patient-Centered Outcomes Research (2024)
-
Evaluation of an integrated knowledge translation approach used for updating the Cochrane Review of Patient Decision Aids: a pre-post mixed methods study
Research Involvement and Engagement (2024)