Abstract
Methanogenic archaea are main actors in the carbon cycle but are sensitive to reactive sulfite. Some methanogens use a sulfite detoxification system that combines an F420H2-oxidase with a sulfite reductase, both of which are proposed precursors of modern enzymes. Here, we present snapshots of this coupled system, named coenzyme F420-dependent sulfite reductase (Group I Fsr), obtained from two marine methanogens. Fsr organizes as a homotetramer, harboring an intertwined six-[4Fe–4S] cluster relay characterized by spectroscopy. The wire, spanning 5.4 nm, electronically connects the flavin to the siroheme center. Despite a structural architecture similar to dissimilatory sulfite reductases, Fsr shows a siroheme coordination and a reaction mechanism identical to assimilatory sulfite reductases. Accordingly, the reaction of Fsr is unidirectional, reducing sulfite or nitrite with F420H2. Our results provide structural insights into this unique fusion, in which a primitive sulfite reductase turns a poison into an elementary block of life.

Similar content being viewed by others
Main
When cold seawater permeates through sediments or enters hydrothermal vent walls, a partial oxidation of sulfide (HS−, S2−) results in the formation of (bi)sulfite (HSO3−), SO32−, a highly reactive intermediate of the sulfur cycle1. Methanogenic archaea are extremely sensitive to this strong nucleophile, which results in the collapse of methanogenesis, their central energy metabolism2. Despite its toxic effects, many hydrogenotrophic methanogens thrive in environments where they are exposed to fluctuating SO32− concentrations, especially methanogens living in proximity to hydrothermal vents or in geothermally heated sea sediments3,4,5,6.
When exposed to SO32−, the hyperthermophile Methanocaldococcus jannaschii3 expresses high amounts of the Group I coenzyme F420-dependent sulfite reductase (referred to as MjFsr), which confers not only protection, but also the ability to grow on SO32− as sole sulfur source (for example, in the absence of S2−)5,7. Because of this trait, the fsr gene has been used as a genetic marker7,8.
Fsr is composed of an N-terminal half belonging to the F420-reducing hydrogenase β-subunit family (FrhB; Supplementary Fig. 1) and a C-terminal half made of a single sulfite/nitrite reductase repeat5,9 (S/NiRR, from here on referred to as sulfite reductase domain). All known sulfite reductases reduce SO32− using a magnetically coupled siroheme‒cysteine‒[4Fe‒4S] center10. This metallocofactor is also used by nitrite reductases to reduce nitrite (NO2−), a side reaction observed in many sulfite reductases11.
Until now, several groups of sulfite reductases have been identified, which are, depending on their biological function, spectroscopic properties and molecular composition, generally classified into assimilatory or dissimilatory ones, in addition to two biochemically uncharacterized predicted sulfite reductases (Supplementary Fig. 1)6,11,12. The only structural data obtained so far are from aSirs (assimilatory) and dSirs (dissimilatory, here, dSirs refer to DsrAB), and therefore this study will use them for comparison. While aSirs are monomeric enzymes that directly reduce SO32− to S2− for assimilation, dissimilatory enzymes are organized by the heterodimers DsrA/DsrB, in which DsrA harbors an inactive catalytic site (referred to as structural; Extended Data Fig. 1)11,12,13,14. Under physiological conditions, dSirs catalyze the first two-electron reduction step and transfer the sulfur species intermediate to the sulfur-carrier protein DsrC used for energy conservation (Extended Data Fig. 1)15. In the absence of DsrC, DsrAB releases some S2−, as well as the reaction intermediates trithionate and thiosulfate15,16,17.
Structural and evolutionary studies suggest that aSirs and dSirs originated from a common progenitor12,14, a primitive Sir that contained a catalytic siroheme‒[4Fe‒4S] and was operating by itself. The gene encoding this ancestral enzyme was duplicated, and in the dSir case, the duplicated version evolved into DsrB, while DsrA was retained for structural function. In the case of aSir, the original and duplicated genes fused and only one active siroheme‒[4Fe‒4S] was retained. On the basis of sequence and phylogenetic analyses, it has been suggested that fsr evolved before the duplication event and therefore represents a primordial sulfite reductase5,18,19. Alternatively, fsr could have arisen through lateral gene transfer followed by gene fusion events.
Besides its evolutionary importance, the electron-donor module of Fsr, the F420H2-oxidase, is directly fused to its sulfite reductase domain. This fusion allows the enzyme to perform the entire six-electron reduction of SO32− on its own via an unknown electronic relay, using electrons from reduced F420. The coenzyme F420 is a deazaflavin derivative present at high cytoplasmic concentrations in methanogens5,20,21,22 and can be reduced by the F420-reducing hydrogenase (FrhABG; Supplementary Fig. 1). Due to the difference in the redox potentials of the F420/F420H2 (∆E0′ = −350 mV) and HSO3−/HS− (∆E0′ = −116 mV) couples, the overall reaction is extremely exergonic (∆G0′ = −135 kJ mol−1 per converted SO32−) and promotes SO32− detoxification at very high rates5. Because of this efficiency and its temperature stability, Fsr is an attractive catalyst for chemists.
Here, we present the X-ray crystal structures of Fsr isolated from two Methanococcales as well as the electron paramagnetic resonance (EPR) spectroscopy characterization of its metallocofactors, providing the first snapshots and molecular insights, to our knowledge, into this prototypical sulfite reductase.
Results
Identification of Fsr in Methanothermococcus thermolithotrophicus
MjFsr, previously characterized5,7,19, turned out to be less suitable for our structural studies due to crystallization defects (see below). Therefore we took an alternative organism belonging to the same order (Methanococcales). Methanothermococcus thermolithotrophicus is a fast-growing thermophile isolated from geothermally heated marine sediments that has already demonstrated its advantages for structural biology23. It was previously shown that this archaeon can grow on 1 mM SO32− as a sole sulfur source4. The participation of Fsr in this process has not yet been investigated and the fsr gene appeared to be absent in the 55 contigs of the deposited shotgun genome (assembly number ASM37696v1, Bioproject: PRJNA182394). After adaptation, we confirmed that M. thermolithotrophicus could grow on SO32−, even at concentrations up to 40 mM (Extended Data Fig. 2a). When cell extracts of both organisms were passed on native PAGE, a distinct band at ≈300 kDa was observed for the cultures grown on SO32− (Extended Data Fig. 2b,c). Based on the band intensity, which is comparable to that of the methyl coenzyme M reductase (MCR, the main catabolic enzyme and one of the highest expressed in the cell), as in M. jannaschii5, we concluded that Fsr is present in M. thermolithotrophicus.
The closed circular genomic sequence of strain DSM 2095 was obtained and contains two entire fsr genes, one of which shares 80.4% sequence identity, and a second isoform that shares 75.6% sequence identity with MjFsr (Supplementary Fig. 2). The purified Fsr in this study has the closest sequence identity (80.4%) to MjFsr, as confirmed by mass spectrometry.
Fsr from both organisms was purified natively under anaerobic atmosphere and yellow light (Extended Data Fig. 2d,e). SDS–PAGE profiles and sulfite reductase activity assays were used to follow the enzyme during the purification. MtFsr exhibits the typical absorbance of [4Fe‒4S] clusters and siroheme‒[4Fe‒4S]-containing proteins, as shown for MjFsr (Extended Data Fig. 2f)5,24. Based on the native PAGE and gel filtration profiles, MtFsr is organized as a homotetramer in solution (Extended Data Fig. 2c,g), similar to MjFsr5.
The F420H2-oxidase domain flanks a sulfite reductase core
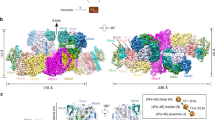
A single-wavelength anomalous dispersion experiment was performed to solve the MjFsr crystal structure. MtFsr was solved by molecular replacement, using MjFsr as a template. The crystal structures of both Fsr superpose well (Extended Data Fig. 3a) and were refined to 2.30 Å for MjFsr and 1.55 Å for MtFsr (Fig. 1 and Extended Data Table 1). Since MjFsr has pseudo-merohedral twinning and a lower resolution compared to MtFsr, the latter was used for the in-depth structural and biochemical analysis.
Visualization of MtFsr domains (top panel). The [4Fe‒4S] cluster-binding motif in the proximity of the siroheme is highlighted. The main panel shows the tetrameric arrangement of MtFsr. Three chains are represented in the surface and colored in white, black and cyan. One monomer of MtFsr is represented as a cartoon and colored according to the top panel. [4Fe‒4S] clusters are numbered on the basis of their position in the electron relay going from the FAD to the siroheme. The siroheme, FAD and the [4Fe‒4S] clusters are represented by balls and sticks. Carbon, nitrogen, oxygen, sulfur and iron atoms are colored as purple (siroheme)/light yellow (FAD), blue, red, yellow and orange, respectively. Fd and Sir stand for ferredoxin domain and sulfite reductase domain, respectively.
As shown in Fig. 1, Fsr is organized as follows: the N-terminal ferredoxin domain (MtFsr residues 1–57 containing two [4Fe‒4S] clusters) is linked to the F420H2-oxidase domain (MtFsr residues 58–336, harboring the flavin and one [4Fe‒4S] cluster), which is connected to the C-terminal sulfite reductase domain (MtFsr residues 339–484, 546–618) that binds the siroheme‒[4Fe‒4S] and has an inserted ferredoxin domain (MtFsr residues 485–545, containing two [4Fe‒4S] clusters). The tetrameric structure of the protein is established by a dimer of two homodimers over a large contact area through the two additional ferredoxin domains and the C-terminal part of the sulfite reductase domain (562–618 in MtFsr, 562–620 in MjFsr; Extended Data Fig. 3b). The homotetramer has the overall shape of a butterfly, composed of a sulfite reductase core flanked by the F420H2-oxidase domain. Notably, the asymmetric unit of MtFsr contains four tetramers (including 96 [4Fe–4S] clusters), providing insights on its natural flexibility (Extended Data Fig. 4a–c).
The F420H2-oxidase domain of Fsr is almost identical between MjFsr and MtFsr (root mean square deviation (r.m.s.d.) = 0.33 Å for 277-Cα aligned) and superposes well with FrhB from Methanothermobacter marburgensis (PDB 4OMF (ref. 25), with a r.m.s.d. = 0.92 Å for 179-Cα aligned) and Methanosarcina barkeri (PDB 6QGR (ref. 26), with a r.m.s.d. = 0.98 Å for 179-Cα aligned; Fig. 2a). The overall fold is perfectly conserved between the F420H2-oxidase domain of Fsr and FrhB, except for the helix α1 of FrhB, which became a loop in Fsr. The active site of the F420H2-oxidase domain of Fsr contains a flavin adenine dinucleotide (FAD; Supplementary Fig. 3), which is similarly bound in Fsr and FrhB (Supplementary Fig. 4). No electron density could be found despite cocrystallization with F420H2 (see Methods). Nevertheless, the reduced F420-binding site is presumably located in a positively charged cleft that would complement the charges of the acidic gamma-carboxy groups (Supplementary Fig. 3c)25,26.
a, Superposition of the F420H2-oxidase domain in Fsr (MjFsr in dark green, MtFsr in light green) with FrhB from M. barkeri (black, PDB 6QGR) and FrhB from M. marburgensis (white, PDB 4OMF). The extended loops 171–189 in MjFsr and MtFsr are highlighted, as well as the lid, which is static in the Frh structures, but more flexible in Fsr (Extended Data Fig. 4b,c). b, Representation of MtFsr F420H2-oxidase domain (green surface) and its N-terminal ferredoxin domain (blue cartoon residues 1–70). The N terminus of Fsr and C terminus from the F420H2-oxidase domain are highlighted by blue and red spheres, respectively. The inserted ferredoxin domain, provided by the opposing monomer (Fsr′), is shown in white cartoon representation. c, Arrangement of FrhB (green surface) with FrhG (cartoon) from M. marburgensis (PDB 4OMF). The N-terminal part (45–205) of FrhG is colored in white and its C-terminal part (206–275), structurally equivalent to the N-terminal ferredoxin domain of Fsr, is colored in blue. The cyan ball highlights the connection between both FrhG parts.
A [4Fe‒4S] cluster relay connects both active sites
The distance between the isoalloxazine ring from the FAD to the closest siroheme‒[4Fe‒4S] is approximately 40 Å. Electrons delivered by reduced F420 must therefore travel through an electron-transfer relay of metallocofactors. The first part of this relay, located in the N-terminal ferredoxin and F420H2-oxidase domains, shares high structural homologies with FrhBG. Indeed, FrhG and the N-terminal ferredoxin domain of Fsr are located at the same position of the F420-oxidoreductase domain (Fig. 2b,c), resulting in a similar electron relay. This homology suggests a common origin that may have evolved by fusion (for Fsr) or by duplication and fusion (for FrhG).
As illustrated in Fig. 3, the overall electronic path consists of five [4Fe‒4S] clusters connected by short edge-to-edge distances (<11.5 Å). Dimerization is critical because half of the relay is provided by the second protomer. An intraelectron transfer between both Fsr dimers is unlikely due to the long distance between the nearest clusters (that is, 18.9 and 19.5 Å).
MtFsr, shown as cartoon, has the same color code and numbering of its [4Fe‒4S] clusters (balls and sticks) as in the domain representation in Fig. 1. Edge-to-edge distances connecting the clusters are shown as dashes. The distances to the adjacent [4Fe‒4S] clusters of the opposite dimer are shown in red. The primes correspond to the second monomer forming the dimer. The residues binding the clusters are shown as balls and sticks. Carbon atoms are colored by their domain affiliation. Nitrogen, oxygen, sulfur and iron atoms are colored in blue, red, yellow and orange, respectively. Siroheme and FAD are shown as sticks with purple and yellow carbon atoms, respectively.
The electrons on the isoalloxazine ring can be transferred directly to the [4Fe‒4S] cluster 1, which is located in the F420H2-oxidase domain. From there they are passed on to the clusters 2 and 3 in the N-terminal ferredoxin domain. The extended loop 171–189 in Fsr serves as a platform to specifically bind both ferredoxin domains, and the Glu 180 coordinates the [4Fe‒4S] cluster 3 (monodentate, 2.22 Å; Fig. 3, Extended Data Fig. 5 and Supplementary Fig. 5). The electrons continue to flow through the clusters 4′ and 5′ in the inserted ferredoxin domain and finally reach the siroheme‒[4Fe‒4S].
Sequence analyses indicated four [4Fe‒4S] clusters and the one coupled to the siroheme5. But both Fsr structures revealed an additional cluster ([4Fe‒4S] cluster 1), which has a noncanonical binding sequence (PCX40CX54CX2C). Strikingly, the four predicted clusters have completely different binding residues compared to primary structural analysis (Extended Data Fig. 5). Each [4Fe‒4S] cluster has a divergent protein environment: cluster 1 is surrounded by basic residues; clusters 2 and 5 have a hydrophobic shell; clusters 4 and 6 are in a more polar environment; and cluster 3 has a glutamate ligand. These differences may reflect the need to establish a ‘redox potential ladder’ to allow a smooth one-way transfer of electrons. To investigate the electron-transfer path, electrochemical experiments followed by EPR spectroscopy were performed.
Redox properties of the metallocofactors
EPR spectroscopy at 10 K (Extended Data Fig. 6a–d) revealed that in as-isolated MtFsr high-spin (S = 5/2) and low-spin (S = 1/2) signals typical for the siroheme in sulfite reductases27,28 were absent, neglecting the sharp axial S = 5/2 EPR signal around g = 6, which, quantified by double integration of its simulation spectrum (g = 6.22, 5.92 and 1.98), is at most 3% of MtFsr. Apparently, on purification under strictly anaerobic conditions, the siroheme remains in its ferrous state. After methylene blue oxidation or on dye-mediated redox titration with Em,7.5 = −104 mV (all potentials refer to potentials versus the H2/H+ normal hydrogen electrode) an intense rhombic S = 5/2 EPR signal with g = 6.7 and 5.1 appeared (Fig. 4a,b). The spectrum could be simulated with three components: a main species with g = 6.70 and 5.10 (78%), a less abundant species (19%) with g = 6.80 and 5.08, but narrower linewidth, and the sharp axial g = 6 species already seen in as-isolated MtFsr. For both rhombic components g = 1.95 was taken as the third g value, as the experimental spectrum contained a weak [3Fe-4S]1+ signal from limited [4Fe‒4S]2+ breakdown upon oxidation. In sulfite reductase and other hemoproteins multiple high-spin species are common29. Addition of SO32− to methylene blue-oxidized MtFsr led to disappearance of the siroheme ferric high-spin signals and formation of a weak low-spin EPR signal, of which only the highest g value (2.8) was detectable, as in other sulfite reductases30.
a, EPR spectra of as-isolated, methylene blue-oxidized (MB-ox.) and, consecutively, Na2SO3 (10 mM)-treated MtFsr. b–d, Dye-mediated redox titrations of indicated EPR signals (or double integral in c). Representative spectra at three selected potentials are shown in the insets, including g values and simulations (see text). EPR spectra for all samples are in Extended Data Fig. 6a,b,d. Nernst fits for n = 1 with Em = −104 mV (b), −275, three times −350 and −435 mV (c) and −445 mV (d) are shown. NHE, normal hydrogen electrode. The fit for g = 2.064 used n = 1 (in red) for −275 mV and n = 2 for −350 mV (in black). EPR conditions: temperature, 10 K; modulation frequency, 100 kHz; modulation amplitude, 1.0 mT; microwave frequency 9.353 GHz; microwave power 20 mW except in c, where 0.2 mW. While one cluster indeed has a measured redox potential of −275 mV and three others are at −350 mV, one of them exhibits a lower potential of −435 mV. The presence of such a low redox potential cluster has already been seen in complex I and does not contradict our hypothesis regarding the electron flow.
In an enzyme approaching the complexity of the complex I, it is not feasible to determine all individual redox potentials of its five regular [4Fe‒4S]1+/2+ cubanes and the siroheme-bridged cubane. First, on the basis of distances in Fsr, extensive magnetic coupling31 between neighboring cubanes is anticipated, blurring individual EPR features. Second, the coupling between the ferrous siroheme and its cysteine-bridged reduced cubane leads to complex mixtures of sharp g = 1.94, broader g = 2.29 and very anisotropic S = 3/2 mimicking signals32. Third, we had to avoid sodium dithionite inherently containing SO32− and therefore used sodium borohydride-reduced F420, while following the solution potential with mediators. One [4Fe‒4S]1+/2+ cubane with simulated g values of 2.064, 1.927 and 1.85 was reduced at a relatively high potential and is also detected in as-isolated Fsr (Fig. 4a,c). From the amplitude of the second derivative of the experimental EPR spectrum at g = 2.064, Em,7.5 = −275 mV was estimated from fitting to the Nernst equation with n = 1 (Fig. 4c). The signal ‘disappeared’ on further reduction with Em,7.5 = −350 mV in a manner indicating cooperativity (n = 2). As super-reduction to [4Fe‒4S]0 is unlikely (Em = −790 mV (ref. 33)), we interpret this phenomenon as reduction of two neighboring clusters of the g = 2.064 cluster. This cluster thus is number 2, 3 or 4′ (the siroheme cubane typically has a very low potential27). In the absence of sufficiently differing EPR features below −350 mV we double integrated the EPR spectra. On the basis of iron content divided by 24 (siroheme does not release Fe ions in acid) we quantified 4.5 ± 0.5 spin/subunit at the lowest attainable potential (−526 mV), which most likely corresponds to the five regular clusters. A fit for the spin integral as a function of the redox potential included the experimental Em,7.5 = −275 mV and Em,7.5 = −350 mV for both neighboring clusters. Avoiding overfitting, we could satisfactorily reproduce the data for five redox transitions with three midpoint potentials: one at Em,7.5 = −275 mV (experimental), one at a low potential to represent the lowest potential region (Em,7.5 = −435 mV) and three times Em,7.5 = −350 mV for the other three clusters (which includes the two clusters leading to broadening of the g = 2.064 signal).
In the low-field region, a species with unusual g values was detected (simulated g values 5.05, 3.05 and 1.96) at very low potential (Fig. 4d). It was accompanied in some samples by an isotropic g = 4.3 signal. But, since the integrated intensity was maximally 5% of the g = 5.05 species and non-Nernstian behavior was seen, it was not considered physiologically relevant. It has previously been shown that such a g = 5.05 species is not from a S = 3/2 system but from transitions of the siroheme–Fe2+ exchange coupled to [4Fe‒4S]1+ (J/D ≈ −0.2 and E/D ≈ 0.11, in which J, D and E are the effective Heisenberg exchange coupling parameter and the spin Hamiltonian zero-field splitting parameters of the spin quintet, respectively; Extended Data Fig. 6c)32. In full agreement with findings on the Escherichia coli assimilatory reductase27 a very low potential (Em,7.5 = −445 mV) was estimated.
A prototypical sulfite reductase
The C-terminal domain of Fsr represents the simplest sulfite reductase crystallized so far. While Fsr shares the common fold of sulfite reductases (Extended Data Fig. 7a and Supplementary Fig. 6)9,13,14, it lacks the large N- and C-terminal extensions found in aSirs and dSirs, which presumably serve to strengthen dimerization and to interact with partners34 (Fig. 5a–c). Without these extensions, Fsr is much more compact—possibly a thermophilic trait. Each Fsr protomer contains one functional siroheme center. In comparison, dSirs harbor one functional and one structural siroheme center in each DsrAB heterodimer, while aSirs have lost one siroheme‒[4Fe‒4S] site (Extended Data Fig. 7b–d).
a–c, All structures are represented in surface, dimeric partners shown in white transparent and residues from the opposing monomer are labeled with a prime symbol. The black ovals and black dashed lines indicate the twofold symmetry axes. The inserted ferredoxin domains of DsrAB and MtFsr are colored in orange. a, aSir from Zea mays with its [2Fe‒2S] ferredoxin colored in light green (PDB 5H92). b, DsrAB from A. fulgidus (PDB 3MM5). c, MtFsr tetramer. For MtFsr, the green surface indicates the F420H2-oxidase position. d–f, Active site of sulfite reductases. Close-up of the active site and the functional siroheme surroundings in E. coli aSir (PDB 1AOP) (d), dSir of A. fulgidus (PDB 3MM5) (e) and MtFsr (f) in which HS− was tentatively modeled. Residues coordinating the [4Fe‒4S] cluster, the siroheme and the sulfur species are shown as balls and sticks, while sulfur and iron are depicted as spheres. Framed residues highlight the differences between the siroheme‒[4Fe‒4S] binding in aSirs and dSirs.
Although Fsr is phylogenetically more distant from aSirs than from dSirs, it superposes well with the first and second halves of aSirs (Supplementary Figs. 6–10). The position of the C terminus of Fsr coincides with the beginning of the linker connecting the two half domains in aSirs (Extended Data Fig. 7a,b and Supplementary Figs. 7 and 8). This detail corroborates the theory that modern aSirs evolved by duplication and fusion events.
The inserted ferredoxin domain in Fsr is at the same position as the ferredoxin domain in DsrA or DsrB (Extended Data Fig. 7a,c,d and Supplementary Figs. 9 and 10). There is a remarkable three-dimensional conservation of the electron connectors between Fsr, DsrA, DsrB and even the aSir from Zea mays, where the external [2Fe‒2S] ferredoxin sits on the core of the sulfite reductase35 (Fig. 5a–c). Such a conserved position suggests a common origin, but could also be due to the restricted access of the [4Fe–4S]–siroheme and the selection pressure towards an optimized distance for electron transfer.
Fsr has traits of assimilatory sulfite reductases
While the sirohemes of DsrAB are partially surface exposed to interact with DsrC (Extended Data Fig. 1)13, the Fsr sirohemes are buried but still accessible via a positively charged solvent channel (Extended Data Fig. 8). As in DsrAB, the two sirohemes within one Fsr dimer are in close proximity (9.4 Å; Supplementary Fig. 11)14.
The binding of the siroheme in MjFsr and MtFsr is highly conserved. It is mainly anchored by positively charged residues from one protomer, while the dimeric partner binds the adjacent [4Fe‒4S] cluster establishing the siroheme‒[4Fe‒4S] center, as reported for other sulfite reductases14. On the basis of the observed electron density, we tentatively modeled a SO32− bound to the siroheme iron (2.3 Å; Extended Data Fig. 8b) in MjFsr. In MtFsr, the axial ligand is a single atomic species at all sites of the asymmetric unit, which is in proximity but not covalently bound to the iron (2.9 Å; Extended Data Fig. 8c). The anion HS− was modeled in the electron density based on the pH 5.5 in the crystallization solution. This species could be the result of cocrystallizing Fsr with reduced F420, which might have forced the complete reduction of bound SO32−.
In MjFsr, four positively charged residues (Arg 355, Arg 423, Lys 460 and Lys 462), which are perfectly conserved across sulfite reductases (Fig. 5d,e and Supplementary Figs. 7 and 9), bind the SO32− and two water molecules. In MtFsr, the modeled HS− is bound by Arg 423, Lys 460 and Lys 462, and one water molecule is stabilized by Arg 355 (Fig. 5f). Group II Fsr found in the genome of anaerobic methanotrophic archaea6 (except for ‘Candidatus Methanoperedens nitroreducens’) and Methanosarcinales, should have a larger binding pocket and two arginines of Group I Fsr are replaced by a lysine and glycine. This suggests that the functionally uncharacterized Group II Fsr has a different substrate specificity6,17. Interestingly, the second isoform found in M. thermolithotrophicus harbors one arginine but exchanged the other one for a threonine (Thr 438; Supplementary Fig. 2), indicating an alternative physiological function.
The active site of Fsr shows the same traits as an assimilatory sulfite reductase: an arginine at position 388, and the coordination of the siroheme-coupled [4Fe‒4S] cluster by the canonical motif (CX5CXnCX3C; Fig. 5d,f). In comparison, DsrAs contain a conserved threonine where aSirs have arginine (αThr 136 in Desulfovibrio vulgaris and αThr 133 in Archaeoglobus fulgidus) and the catalytically active [4Fe‒4S] cluster coupled to the siroheme of DsrBs is coordinated by the canonical motif CXnCCX3C (Fig. 5e). Fsr must therefore follow the same catalytic path as aSirs; the six-electron reduction of SO32− to S2− should be unidirectional, without the formation or consumption of intermediates (for example, thiosulfate or trithionate). MtFsr did not accept thiosulfate as an electron acceptor, which is in agreement with the findings for MjFsr5. We also monitored F420-reduction by MtFsr with S2− as substrate (up to 10 mM) and observed no reaction. The addition of 10 mM S2− to 1.4 mM of Na2SO3 also had no effect on the F420H2 oxidation rate. Taken together, these results support that Fsr indeed acts like an aSir.
On the basis of its equal Vmax but six-fold lower Km value (Table 1), MtFsr prefers NO2− over SO32−, a property that may expand its role from sulfite detoxification to ammonium production, as M. thermolithotrophicus has been reported to grow on nitrate as a sole source of nitrogen36. If the archaeon uses a nitrate reductase, NO2− would accumulate and Fsr would be a suitable candidate for NO2− conversion. In addition, we have shown that MtFsr reduces selenite (SeO32−) in vitro with a relative activity of 20.7 ± 7.5% compared to SO32− (see Methods). These promiscuous activities could expand the physiological range of the enzyme, but also its biotechnological applications.
Discussion
Some methanogens show a remarkable tolerance to SO32−, one of the sulfur-reactive species that can cause oxidative damage to the methanogenic machinery. Besides the possibility that those methanogens can keep low intracellular SO32− concentrations through pumping mechanisms, the cytoplasmic Group I Fsr is used as a first line of defense to convert toxic SO32− into HS−, which can then be used for sulfur assimilation. The efficient SO32− detoxification strategy of Methanococcales relies on the enormous amount of expressed Fsr, which constitutes 5–10% of the cellular protein (Extended Data Fig. 2b,c and Methods), but also on the use of abundant F420H2, which can be rapidly regenerated via H2 oxidation by Frh22.
Fsr discloses a ‘cofactor swapping’ between two subunits forming a homodimer in a head-to-tail configuration, which dimerizes with a second homodimer, creating a butterfly-shaped tetramer. As a result, the centrally located sulfite reductase domains are surrounded by F420H2-oxidase domains. These shuttle electrons via three [4Fe‒4S] cluster from one subunit to the other two [4Fe‒4S] cluster and the siroheme‒cysteine‒[4Fe‒4S] cofactor of the other subunit within the functional dimer. In contrast to the bidirectional hydrogenase Frh, which maintains an isopotential of E′0 ≈ −400 mV (ref. 25), the different metalloclusters of Fsr must establish a downhill redox potential from the FAD to the siroheme‒[4Fe‒4S]. Our electrochemical and spectroscopic studies indicate that the electrons carried by F420H2 are immediately transferred to the siroheme‒[4Fe‒4S] (Fig. 4a,b and Extended Data Fig. 6a). The metallocofactors should ensure efficient electron transfer rather than serving as a transient storage, and a cascade of redox potential from −380 mV (F420/F420H2 redox potential under certain physiological conditions22) to −116 mV (E´0 of HSO3−/HS−) is expected.
Once reduced, the siroheme‒[4Fe‒4S] could transfer the electrons to the sulfur species covalently bound to its Fe. dSirs physiologically perform a two-electron reduction to allow the transfer of the sulfur intermediate to DsrC. In contrast, aSirs and Fsr perform a three times two-electron reduction to release HS−. A positively charged environment around the active site attracts SO32− and an organized water network has been proposed to provide fast proton transfer via the Grotthuss mechanism, allowing successive SO32− reduction (Extended Data Fig. 8a)16,37. Despite a strikingly similar position of the residues involved in substrate binding, aSirs/Fsr and dSirs react differently. With the possibility of genetically modifying M. maripaludis or M. jannaschii, it would be worthwhile to exchange the residues that confer aSir traits at the active site (Arg 388, Cys 428) with dSir ones and observe the effects on the phenotype7,8.
Throughout evolution, sulfite reductases have been kept to detoxify SO32− as well as to conserve energy by dissimilatory SO32−reduction or oxidation of H2S38. Based on sequence and structural similarity with enzymes from different superfamilies, it has been proposed that modern sulfite reductases originated from a primordial Sir/Nir that functioned as a self-complementary homodimer18. A snapshot of this progenitor can be derived from the Fsr structure, as the organization of its sulfite reductase domain is highly simplified (Extended Data Fig. 9). The evolution of Fsr is still a matter of debate but it needs to be thoroughly studied, as its discovery has reinforced the question of whether sulfate respiration or methanogenesis was the primeval means of energy conservation during the evolution of early Archaea39,40. Both metabolisms, related to each other, possibly coexisted or even coexist still6,18,41. Methanogens might have lost the genes required for complete sulfate dissimilation over time, but kept the sulfite reductase to adapt to environments where SO32− fluctuations do occur. However, M. thermolithotrophicus appears to use a complete sulfate-reduction pathway, as it is able to grow on sulfate as its sole sulfur source4. This assimilation pathway requires SO32− as an intermediate, and Fsr is expected to orchestrate its reduction. Although further studies need to investigate whether this methanogen can also express other enzymes of the sulfate-reduction pathway, the structural elucidation of Fsr provides the first snapshot of a sulfate reduction-associated enzyme in a methanogen.
Methods
Methanogenic archaea strains and cultivation medium
M. jannaschii (DSM 2661) and M. thermolithotrophicus (DSM 2095) cells were obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig) and cultivated in a previously described minimal medium with some modifications42.
Reagents used for this study
Lists of reagents and providers are provided in Supplementary Table 1.
Sulfur-free cultivation medium for Methanococcales
Per liter of medium: 558 mg KH2PO4 (final concentration 4.1 mM), 1 g KCl (13.4 mM), 25.13 g NaCl (430 mM), 840 mg NaHCO3 (10 mM), 368 mg CaCl2·2H2O (2.5 mM), 7.725 g MgCl2·6H2O (38 mM), 1.18 g NH4Cl (22.06 mM), 61.16 mg nitrilotriacetic acid (0.32 mM), 6.16 mg FeCl2·4H2O (0.031 mM), 10 µl 2 mM Na2SeO3·5H2O stock (0.02 µM), 3.3 mg Na2WO4·2H2O (0.01 mM) and 2.42 mg Na2MoO4·2H2O (0.01 mM) were dissolved under constant stirring in a measuring cylinder with 750 ml of deionized H2O (dH2O)42. Resazurin (1 ml, 1.5 mM) was added (0.0015 mM) and 10 ml of sulfur-free trace elements (see below) were added subsequently. For M. jannaschii, 30.24 g PIPES (100 mM final) was used as a buffer and a pH 7.0 was adjusted using sodium hydroxide pellets. For M. thermolithotrophicus the pH was set to either 7.6 with 50 mM Tris–HCl as buffer or to 6.2 with 50 mM MES. The media were filled up to a final volume of 1 liter by the addition of dH2O.
The cultivation media were transferred in a 1 l pressure-protected Duran laboratory bottle with a magnetic stirring bar. The Duran flask was closed with a butyl rubber stopper and degassed by applying 3 min of evacuation, followed by 30 seconds of ventilation with 1 × 105 Pa N2 atmosphere, under constant magnetic stirring. This was repeated 15 times and at the final ventilation step an overpressure of 0.3 × 105 Pa N2 was applied.
Trace element composition
A 100-fold-concentrated trace element solution was prepared by first dissolving 1.36 g nitrilotriacetic acid (7.1 mM) in 800 ml dH2O under magnetic stirring. The pH was shifted to 6.2 by adding NaOH pellets. Then, 89.06 mg MnCl2·4H2O (0.45 mM), 183.3 mg FeCl3·6H2O (0.68 mM), 60.27 mg CaCl2·2H2O (0.41 mM), 180.8 mg CoCl2·6H2O (0.76 mM), 90 mg ZnCl2 (0.66 mM), 37.64 mg CuCl2 (0.28 mM), 46 mg Na2MoO4·2H2O (0.19 mM), 90 mg NiCl2·6H2O (0.38 mM) and 30 mg VCl3 (0.19 mM) were added separately. The trace element mixture was filled up to a final volume of 1 liter with dH2O.
Anaerobic growth of Methanococcales
For all studied archaea, cell growth was measured spectrophotometrically by measuring the optical density at 600 nm (OD600). To control the purity of the culture, samples were taken and analyzed via light microscopy. Both methanogens were cultivated at 65 °C, unless stated otherwise, with 1 × 105 Pa of H2/CO2 in the gas phase. M. jannaschii was cultivated in flasks and M. thermolithotrophicus was cultivated in flasks or a fermenter.
Growth of M. jannaschii
Duran bottles (10× 1 liter) were sealed with butyl rubber stoppers and the gas phase was exchanged for H2/CO2 (80:20, 1 × 105 Pa). A 100-ml portion of anaerobic cultivation media was transferred into each bottle (ratio 1:10 of medium/gas phase), with 1 mM Na2SO3 as a sole sulfur source. A portion of 5 ml of overnight culture (OD600 of 0.9) was used as an inoculum for 100 ml media. No additional reductant was added. The cultures were placed at 65 °C, with standing for at least one hour, followed by overnight shaking at 180 rotations per minute without light. The cells were collected in exponential phase with a final OD600 of 1.83 by immediately transferring them in an anaerobic tent (N2/CO2 atmosphere at a ratio of 90:10), followed by anaerobic centrifugation for 30 min at 6,000g at 4 °C. The cell pellet was transferred in a sealed bottle gassed with 0.3 × 105 Pa N2 and flash frozen in liquid N2 to be stored at −80 °C.
Growth of M. thermolithotrophicus for Fsr crystallization
M. thermolithotrophicus was grown in a fermenter at 50 °C with 10 mM sulfate (SO42−) as sole sulfur substrate. Since SO32− could be an intermediate in the SO42− reduction pathway it would require the expression of Fsr. Therefore, 1.5 l of anaerobic cultivation medium with 10 mM SO42− were continuously bubbled with H2 and CO2 (80:20, 2 × 104 Pa) and inoculated with 100 ml preculture (OD600 of 4.2). Since the fermenter is an open system, we set a more alkaline pH (7.6) to prevent evaporation of produced S2−. Here, it should predominantly be present in the form of HS−, and not H2S, and therefore stay for longer time in the medium. The pH was checked every two hours by using a pH indicator. The cells were grown until late exponential phase (OD600 of 2.97) and then immediately transferred in an anaerobic tent (N2/CO2 atmosphere at a ratio of 90:10). Cells were collected by anaerobic centrifugation for 30 min at 6,000g at 4 °C. A 1.5-l culture with an OD600 of 2.97 yielded 19.25 g of cells (wet weight). The cell pellet was transferred in a sealed bottle, gassed with 0.3 × 105 Pa N2, flash frozen in liquid N2 and stored at −80 °C.
Growth of M. thermolithotrophicus for Fsr activity assays
To perform enzymatic activity assays, M. thermolithotrophicus was directly grown on 2 mM Na2SO3. The ten 1-l Duran bottles were sealed with butyl rubber stoppers and the gas phase was exchanged for H2 and CO2 (80:20, 1 × 105 Pa). A 100 ml of anaerobic cultivation media containing 50 mM MES at pH 6.2 was transferred in each bottle (ratio of 1:10 of medium/gas phase), with 2 mM Na2SO3 final as a sole sulfur source. A 5-ml portion of overnight-grown culture (OD600 of 1.7) was used as an inoculum for 100 ml of media. No additional reductant was added. The cultures were placed at 65 °C, with standing overnight. The cells were grown until early exponential phase (OD600 of 0.8), since we assumed that most SO32− has not been converted into HS− yet and that Fsr should be highly expressed and active. The cells were immediately collected by transferring them in an anaerobic tent (N2/CO2 atmosphere at a ratio of 90:10), followed by anaerobic centrifugation for 30 min at 6,000g at 4 °C. The cell pellet was transferred in a sealed bottle, gassed with 0.3 × 105 Pa N2, flash frozen in liquid N2 and stored at −80 °C.
Sulfite growth inhibition
M. thermolithotrophicus was grown on different Na2SO3 concentrations to determine the growth-inhibiting threshold. For this, 250-ml serum flasks were sealed with a butyl rubber stopper and the gas phase was exchanged for H2 and CO2 (80:20, 1 × 105 Pa). A 10-ml portion of anaerobic cultivation media with a pH set at 6.2 with 50 mM MES was transferred into each bottle. Then, different Na2SO3 concentrations (2 mM, 10 mM, 20 mM, 30 mM and 40 mM final) were added in triplicate as a sole sulfur source, and 2 mM Na2S was used as a control. The cultures grew at 65 °C for 22 hours, with standing. The three biological replicates for each setup are represented as dots in Extended Data Fig. 2a, with the standard deviation shown as bars.
Growth of M. thermolithotrophicus for titrations and EPR spectroscopy
Due to the high demand of MtFsr for titration and EPR spectroscopy experiments, M. thermolithotrophicus was grown in one 10-l fermenter with SO42− as a sole sulfur substrate and in another 10-l fermenter with SO32− as a sole sulfur source, to boost MtFsr natural expression. The fermenter containing SO42− was performed as described above with an inoculum of 350 ml (OD600 of 3.2). A 7.4-l culture with an OD600 of 4.8 yielded 74 g of cells (wet weight). In the SO32− fermenter, M. thermolithotrophicus was grown at 50 °C in 7 l anaerobic cultivation medium with a pH of 6.2 supplemented with 5 mM SO32− as a sole sulfur substrate, continuously bubbled with H2 and CO2 (80:20, 2 × 104 Pa). A 600-ml preculture (OD600 of 2.34) was used as inoculum. The cells were grown until an OD600 of 2.48 and then immediately transferred in an anaerobic tent (N2/CO2 atmosphere at a ratio of 90:10). Cells were collected by anaerobic centrifugation for 30 min at 6,000g at 4 °C and a final yield of 51 g of cells (wet weight) was obtained. The cell pellets were transferred in a sealed bottle, gassed with 0.3 × 105 Pa N2, flash frozen in liquid N2 and stored at −80 °C.
Genome sequencing of M. thermolithotrophicus
M. thermolithotrophicus was anaerobically grown in the above-described medium and 2 mM Na2S was used as a sulfur source. A total culture volume of 20 ml was used. Cells were aerobically collected by centrifugation (30 min, 6,000g at 4 °C). DNA was extracted and purified based on ref. 43. Quality control, library preparation and sequencing (PacBio Sequel II) were performed in the Max Planck-Genome-Centre (Cologne).
Purification of Fsr
All steps were performed under the strict exclusion of oxygen and daylight. Protein purifications were carried out in a Coy tent with an N2 and H2 atmosphere (97:3) at 20 °C under yellow light. For both Fsr, three to five chromatography steps were used with some variations. Fsr purification was further followed via activity assays and on the basis of absorbance peaks at wavelengths of 280, 420 and 595 nm. Each elution profile was systematically controlled by SDS–PAGE to select the purest fractions.
Purification of MjFsr
M. jannaschii cells (13.5 g wet weight) were thawed under warm water and transferred in an anaerobic tent (N2/CO2 atmosphere at a ratio of 90:10). Cells were diluted by three volumes of lysis buffer (50 mM Tricine/NaOH pH 8.0, 2 mM dithiothreitol (DTT)) and disrupted by sonication: 7 cycles at 62% intensity with 30 pulses followed by 1 min break (probe MS76, SONOPULS Bandelin). Cell debris was removed anaerobically via centrifugation (21,000g, one hour, room temperature). The protein concentration (measured by Bradford) of the supernatant was estimated to 4.68 mg ml−1. The supernatant was transferred to a Coy tent (N2/H2 atmosphere of 97:3) under yellow light at 20 °C. The sample was diluted with two volumes of lysis buffer and passed through a 0.2-µm filter (Sartorius). The filtered sample was loaded on a 10-ml Q Sepharose high-performance column (GE Healthcare), which was previously equilibrated with 5 column volumes (CV) of lysis buffer. The column was then washed with 2 CV of lysis buffer. MjFsr was eluted by a gradient of NaCl (from 0.1 to 0.6 M) in 27 CV at a flow rate of 1.5 ml min−1 in fraction sizes of 3.5 ml. MjFsr eluted between 0.37 and 0.41 M NaCl. The fractions of interest were pooled and 1:1 diluted with HIC buffer (25 mM Tris–HCl pH 7.6, 2 M (NH4)2SO4 and 2 mM DTT). The sample was filtered and applied to a Source15Phe 4.6/100 PE column (GE Healthcare) previously equilibrated with the HIC buffer. The column was then washed with 2 CV of 25 mM Tris–HCl pH 7.6, 1.4 M (NH4)2SO4 and 2 mM DTT buffer. The elution was performed at a flow rate of 0.8 ml min−1 by a decreasing gradient of (NH4)2SO4 (1.4 to 0 M) over 90 min, with a fractionation size of 2 ml. Fsr eluted in the fractions at 0.9 to 0.78 M (NH4)2SO4. Those fractions were merged and concentrated using a 30-kDa-cutoff filter (Merck Millipore). The concentrated sample was passed through a 0.2-µm filter and injected on a Superdex 200 Increase 10/300 GL (GE Healthcare) equilibrated in storage buffer (25 mM Tris–HCl pH 7.6, containing 10% v/v glycerol and 2 mM DTT). The elution was performed at a flow rate of 0.4 ml min−1 in the storage buffer. MjFsr eluted as a sharp Gaussian peak at 10.4 ml. The pooled samples were concentrated by passing them through a 30-kDa-cutoff filter, and the final concentration was measured by the Bradford method (BioRad). The sample was immediately crystallized at a concentration of 6.1 mg ml−1.
Purification of MtFsr for crystallization
Cells (19.25 g wet weight) derived from a fermenter were thawed under warm water and transferred to an anaerobic tent containing an atmosphere of N2/CO2 (90:10). Cells were lysed by osmotic shock through the addition of 60 ml lysis buffer (50 mM Tricine/NaOH pH 8.0, 2 mM DTT). Cell lysate was homogenized by sonication: 3 cycles at 70% intensity with 30 pulses followed by 1 min break (probe MS76, SONOPULS Bandelin) and cell debris was removed anaerobically via centrifugation (21,000g, one hour at 4 °C). The supernatant was transferred in a Coy tent (N2/H2 atmosphere of 97:3), with yellow light at 20 °C. The sample was filtered through a 0.2-µm filter (Sartorius) and was passed onto a DEAE fast-flow column (30 ml), equilibrated with lysis buffer. The column was then washed with 2 CV of lysis buffer. MtFsr was eluted with a gradient of 0.1 to 0.6 M NaCl in 120 min at a flow rate of 2.5 ml min−1 and in fractionation sizes of 4 ml. MtFsr eluted between 0.3 and 0.39 M NaCl. The fractions of interest were merged, diluted by 3 volumes of lysis buffer and filtered through a 0.2-µm filter. The filtered sample was loaded on a 15-ml Q Sepharose high-performance column, equilibrated with lysis buffer. The column was washed with 2 CV of lysis buffer. A gradient of 0.15 to 0.55 M NaCl in 120 min with a flow rate of 1 ml min−1 was performed and fractions of 1.5 ml were collected. MtFsr eluted between 0.49 and 0.53 M NaCl. Fractions of interest were pooled and diluted with 2 volumes of HAP buffer (20 mM K2HPO4/HCl pH 7.0 and 2 mM DTT) and subsequently filtered through a 0.2-µm filter. The filtered sample was applied to a 10-ml hydroxyapatite column type 1 (Bio-Scale Mini CHT cartridges, BioRad) equilibrated with HAP buffer. The column was washed with 2 CV of HAP buffer and a gradient of 0.02 to 0.5 M K2HPO4 for 60 min at a flow rate of 2 ml min−1 was performed and 3-ml fractions were collected. MtFsr eluted between 0.28 and 0.39 M K2HPO4 and the respective fractions were pooled. The pool was diluted 1:3 with 25 mM Tris–HCl pH 7.6, 2 M (NH4)2SO4 and 2 mM DTT (HIC buffer). The filtered sample was applied to a Source15Phe 4.6/100 PE column (GE Healthcare) previously equilibrated with the HIC buffer. The column was then washed with 2 CV of HIC buffer. A gradient of (NH4)2SO4 ranging from 2 to 1 M was performed for 30 min at a flow rate of 0.8 ml min−1 with a fractionation size of 1 ml. MtFsr eluted between 1.38 and 1.23 M (NH4)2SO4 and the respective fractions were pooled. The buffer was exchanged for the storage buffer (25 mM Tris–HCl pH 7.6, containing 10% v/v glycerol and 2 mM DTT) by using a 30-kDa-cutoff filter (6 ml, Merck Millipore) and MtFsr was concentrated to 11.06 mg ml−1 in a volume of 120 µl. The protein concentration was estimated by the Bradford method. The sample was immediately crystallized.
Purification of MtFsr for enzyme activity assays
SO32−-grown cells (8 g wet weight) were thawed under warm water and transferred to an anaerobic tent containing an atmosphere of N2/CO2 (90:10). Cells were lysed by osmotic shock through the addition of 60 ml lysis buffer (50 mM Tricine/NaOH pH 8.0, 2 mM DTT). Cell lysate was homogenized by sonication: 9 cycles at 75% intensity with 30 pulses followed by 1 min break (probe KE76, SONOPULS Bandelin) and cell debris was removed anaerobically via centrifugation (21,000g, one hour at 4 °C). The supernatant was transferred to a Coy tent (N2/H2 atmosphere of 97:3) under yellow light at 20 °C and was diluted with 90 ml lysis buffer, filtered through a 0.2-µm filter. The filtered sample was applied to a 10-ml DEAE fast-flow column (GE Healthcare), which was previously equilibrated with lysis buffer. The column was then washed with 2 CV of lysis buffer. A gradient of 0.1 to 0.6 M NaCl was applied for 120 min at a flow rate of 2.5 ml min−1 and fractions of 4 ml were collected. MtFsr eluted between 0.34 and 0.4 M NaCl. The fractions of interest were merged and diluted by 3 volumes of lysis buffer. The filtered sample was loaded on a 10-ml Q Sepharose high-performance column (GE Healthcare) and a gradient of 0.15 to 0.55 M NaCl was applied for 120 min with a flow rate of 1 ml min−1. Fractions of 1.5 ml were collected. MtFsr eluted between 0.49 and 0.53 M NaCl. The MtFsr fractions were pooled, and three times diluted with HAP buffer (20 mM K2HPO4/HCl pH 7.0 and 2 mM DTT). The filtered sample was applied to a 10-ml hydroxyapatite type 1 (Bio-Scale Mini CHT cartridges, BioRad) equilibrated with HAP buffer. The column was washed with 2 CV of HAP buffer and a gradient of 0.02 to 0.5 M K2HPO4 in 60 min at a flow rate of 2 ml min−1 was performed. Fraction sizes of 1.5-ml were collected. MtFsr eluted between 0.25 and 0.42 M K2HPO4 and the respective fractions were pooled. The pool was diluted with 3 volumes of HIC buffer (25 mM Tris–HCl pH 7.6, 2 M (NH4)2SO4 and 2 mM DTT). The filtered sample was applied to a Source15Phe 4.6/100 PE column (GE Healthcare) previously equilibrated with the HIC buffer. The column was then washed with 2 CV of 25 mM Tris–HCl pH 7.6, 1.6 M (NH4)2SO4 and 2 mM DTT buffer. MtFsr was eluted in a gradient of 1.6 to 0.8 M of (NH4)2SO4 in 25 min at a flow rate of 0.8 ml min−1 and a fractionation size of 1 ml. MtFsr eluted between 1.43 and 1.28 M (NH4)2SO4 and the respective fractions were pooled. The buffer was exchanged for the storage buffer (25 mM Tris–HCl pH 7.6, containing 10% v/v glycerol and 2 mM DTT) by using a 30-kDa-cutoff filter (6 ml, Merck Millipore) and MtFsr was concentrated to 900 µl. The concentrated sample was passed onto a Superdex 200 Increase 10/300 GL (GE Healthcare), equilibrated in storage buffer. MtFsr eluted at a flow rate 0.4 ml min−1 in a sharp Gaussian peak at an elution volume of 10.01 ml (Extended Data Fig. 2g). To determine the apparent molecular weight of MtFsr, standard proteins (conalbumin, aldolase and ferritin, purchased from GE Healthcare) were passed at the same flow rate and in the same buffer. The fractions of interest containing MtFsr were concentrated with a 30-kDa-cutoff centrifugal concentrator to 1 ml and the protein was directly used for enzymatic activity assays. The concentration of purified MtFsr, estimated by the Bradford method, was 3.41 mg ml−1.
Purification of MtFsr for titrations and EPR spectroscopy
For the titrations and EPR spectroscopic measurements two separate purifications were carried out starting either with 34 g cells (wet weight) derived from a SO32−-grown fermenter, or with 49.5 g cells (wet weight) derived from a SO42−-grown fermenter. Cells were thawed under warm water and transferred to an anaerobic tent containing an atmosphere of N2/CO2 (90:10). Cells were lysed by osmotic shock through the addition of 180 ml and 240 ml lysis buffer (50 mM Tricine/NaOH pH 8.0, 2 mM DTT), respectively. The cell lysates were homogenized by sonication: 4 cycles at 72% intensity with 60 pulses followed by 1.30 minute break (probe MS76, SONOPULS Bandelin) and the cell debris was removed anaerobically via centrifugation (21,000g, 1 h at 10 °C). The supernatant was transferred in a Coy tent (N2/H2 atmosphere of 97:3), with yellow light at 20 °C.
The purification steps were carried out as described in ‘Purification of MtFsr for crystallization’. In the final purification step the buffer was exchanged by dilution and concentration in storage buffer (25 mM Tris–HCl pH 7.6, containing 10% v/v glycerol and 2 mM DTT) by using 30-kDa-cutoff filter (6 ml, Merck Millipore). MtFsr derived from the SO32−-grown fermenter was concentrated to 18 mg ml−1 in a volume of 4.54 ml, and for the SO42−-grown fermenter MtFsr was concentrated to 20 mg ml−1 in a volume of 1.24 ml. The protein concentrations were estimated by the Bradford method.
Mass spectrometry identification
Purified MtFsr (1 μg) was digested with trypsin and analyzed by mass spectrometry (ThermoFisher Q Exactive HF coupled to an Easy-nLC 1200) as described in ref. 44.
Protein crystallization
The purified enzymes were kept in 25 mM Tris–HCl pH 7.6, 10% v/v glycerol and 2 mM DTT. Fresh, unfrozen samples were immediately used for crystallization. Crystals were obtained anaerobically (N2/H2, 97:3) by initial screening at 20 °C using the sitting-drop method on 96-well MRC two-drop crystallization plates in polystyrene (SWISSCI) containing 90 µl of crystallization solution in the reservoir.
Crystallization of MjFsr
MjFsr (0.5 µl) at a concentration of 6.1 mg ml−1 was mixed with 0.5 µl reservoir solution. Black, long, plate-shaped crystals appeared after a few days in the following crystallization conditions: 45% v/v 2-methyl-2,4-pentanediol, 100 mM Bis–Tris pH 5.5 and 200 mM calcium chloride.
Crystallization of MtFsr
MtFsr at a concentration of 11 mg ml−1 was cocrystallized with FAD (0.5 mM final concentration) and F420H2 (15.5 µM final concentration). The protein sample (0.6 µl) was mixed with 0.6 µl reservoir solution. Thick, square-shaped, brown crystals appeared after a few days. The reservoir solution contained 200 mM lithium sulfate, 100 mM Bis–Tris, pH 5.5 and 25% w/v polyethylene glycol 3350.
X-ray crystallography and structural analysis
Crystal handling was done inside the Coy tent under anaerobic atmosphere (N2/H2, 97:3). MjFsr crystals were directly plunged in liquid nitrogen, whereas MtFsr crystals were soaked in their crystallization solution supplemented with 20% v/v ethylene glycol as a cryo-protectant before being frozen in liquid nitrogen. Crystals were tested and collected at 100 K at the Synchrotron Source Optimisée de Lumière d’Énergie Intermédiaire du LURE (SOLEIL), PROXIMA-1 beamline; the Swiss Light Source, X06DA–PXIII; and at PETRA III, P11.
MjFsr
After an X-ray fluorescence spectrum on the Fe K-edge, datasets were collected at 1.74013 Å to perform the single-wavelength anomalous dispersion experiment. Native datasets were collected at a wavelength of 0.97857 Å on the same crystal. Data were processed and scaled with autoPROC45. The resolution limits in each cell direction were as follows: a = 2.43 Å, b = 2.62 Å and c = 2.19 Å. Phasing (obtained maximum CFOM for the substructure determination was 69), density modification and automatic building were performed with CRANK-2 (ref. 46). The asymmetric unit of MjFsr contains two half homotetramers. The model was then manually built with Coot and further refined with PHENIX47,48. X-ray crystallographic data were twinned, and the refinement was performed by applying the following twin law -k, -h, -l. During the refinement translational-liberation screw was applied.
MtFsr
Data were processed and scaled with autoPROC. The resolution limits in each cell direction were as follows: a = 1.69 Å, b = 1.55 Å and c = 1.81 Å. The structure was solved by molecular replacement with phaser from PHENIX, using MjFsr as a template48. The asymmetric unit of MtFsr contains four homotetramers. This crystalline form presents a notable translational noncrystallographic symmetry (14%). The model was then manually rebuilt with Coot and further refined with PHENIX. During the refinement, noncrystallographic symmetry and translational-liberation screw were applied. In the last refinement cycles, hydrogens were added in riding positions. Hydrogens were omitted from the final deposited model. In one of the chains (chain N), the lid region 204–253 has two different conformations, and both were tentatively modeled.
All models were validated through the MolProbity server (http://molprobity.biochem.duke.edu)49. B-factors, MolProbity scores and rotamer outliers in Extended Data Table 1 were calculated based on the available PDB structures with PHENIX. The other values in Extended Data Table 1 were derived from the original first PDB reports. Data collection and refinement statistics, as well as PDB identification codes for the deposited models and structure factors, are listed in Extended Data Table 1. Figures were generated with PyMOL (Schrödinger, LLC). Structural comparison was performed with the dissimilatory sulfite reductases from D. vulgaris (2V4J), A. fulgidus (3MM5) and with the assimilatory sulfite reductase from E. coli (1AOP) and Z. mays (5H92).
Purification of the F420-reducing hydrogenase from M. thermolithotrophicus
MtFrh was required to reduce F420 and was purified from the same batch of cells as MtFsr used for crystallization. The activity of MtFrh after each purification step was followed by the reduction of methyl viologen in the N2/H2 tent (97:3). The assay was performed in 120 µl of 0.5 M KH2PO4/NaOH pH 7.6 containing 1.7 mM of oxidized methyl viologen. The addition of 2 µl from the fractions containing Frh led to a blue coloration.
MtFrh was in the same pool as MtFsr used for crystallization, for the DEAE and the Q Sepharose columns. The Q Sepharose column performed the separation of the two target proteins. MtFrh eluted between 0.48 and 0.49 M NaCl from the Q Sepharose column. The filtered sample was applied to a 10-ml hydroxyapatite type 1 (Bio-Scale Mini CHT cartridges, BioRad) equilibrated with HAP buffer (20 mM K2HPO4/HCl pH 7.0 and 2 mM DTT). The column was then washed with 2 CV of HAP buffer. The elution was performed with a gradient of 0.02 to 0.5 M K2HPO4 in 60 min at a flow rate of 2 ml min−1 with 3-ml fractions. MtFrh eluted between 0.22 and 0.37 M K2HPO4 and the respective fractions were pooled. The pool was diluted 1:1 with the HIC buffer (25 mM Tris–HCl pH 7.6, 2 M (NH4)2SO4 and 2 mM DTT). The filtered sample was applied onto a Source15Phe 4.6/100 PE column (GE Healthcare) previously equilibrated with the HIC buffer. The column was then washed with 2 CV of 25 mM Tris–HCl pH 7.6, 1.0 M (NH4)2SO4 and 2 mM DTT buffer. MtFrh was eluted in a gradient of 1 to 0 M (NH4)2SO4 in 30 min at a flow rate of 0.8 ml min−1 and a fractionation size of 1 ml. MtFrh eluted between 0.4 and 0.15 M (NH4)2SO4 and the respective fractions were pooled. The buffer was exchanged for the storage buffer (25 mM Tris–HCl pH 7.6, containing 10% v/v glycerol and 2 mM DTT) by using a 30-kDa-cutoff filter (6 ml, Merck Millipore) and MtFrh was concentrated to 4.97 mg ml−1 in 100 µl. The purified sample was aliquoted and anaerobically flash frozen in liquid N2 and stored at −80 °C. MtFrh lost its activity after more than one cycle of thawing-freezing.
Purification of oxidized F420
Since F420 is highly sensitive to light, all steps were carried out under yellow light or by covering the sample with aluminum foil. About 10 g (wet weight) of M. thermolithotrophicus cells from a 1.5-l fermenter were anaerobically lysed by osmotic shock and sonication (see above). The sample was centrifuged at 45,000g for 60 min at 4 °C. The supernatant was transferred in a Coy tent containing an atmosphere of N2/H2 (97:3). The sample was filtered and passed onto a 30-ml DEAE Sepharose column equilibrated with 50 mM Tricine/NaOH pH 8.0 and 2 mM DTT. F420 was eluted by a gradient of 0 to 0.6 M NaCl. The samples containing F420 were determined on the basis of the absorbance profile at 420 nm and eluted between 0.48 M and 0.58 M NaCl. Pooled fractions were moved outside the tent and diluted with one volume of HIC-F420 buffer (25 mM Tris HCl pH 7.6, 2 M (NH4)2SO4). (NH4)2SO4 powder was directly added to the diluted sample to reach a final concentration of 3 M (NH4)2SO4 and was stirred for one hour at room temperature. The sample was centrifuged at 4,000g for 20 minutes at room temperature. The supernatant was filtered through a 0.2-µm filter and loaded on a 30-ml Phenyl-Sepharose high-performance column, equilibrated with HIC-F420 buffer. F420 was eluted by washing the column with the HIC-F420 buffer, at a flow rate of 2 ml min−1 and 1-ml fractions were collected. The fractions containing F420 were pooled and filtered through a 0.2-µm filter. The sample was diluted by 50 volumes of 5 mM Tris–HCl pH 8.0 and loaded overnight on a 5-ml Q Sepharose high-performance column, equilibrated in 5 mM Tris–HCl pH 8.0. The following steps were performed at 4 °C. The column containing the bound F420 was washed with 5 CV of 20 mM (NH4)HCO3 precooled at 4 °C. F420 elution was performed by adding 1 M (NH4)HCO3 and collected in a brown serum flask. (NH4)HCO3 was removed by evacuation at 37 °C for 2 hours under constant stirring. (NH4)HCO3-free F420 powder was obtained by freeze drying. The purity of the preparation was checked by measuring the ratio of Abs247/Abs420 in 25 mM Tris buffer pH 8.8. A pure sample would have a ratio value of 0.85 (ref. 50). F420 concentration was estimated by measuring the absorbance at 420 nm in 25 mM Tris buffer pH 7.5 (ε420 = 41.4 mM−1 cm−1). The final concentration of oxidized F420 used for this study was 3.15 mM and 7.53 mM.
Reduction of F420 for enzyme assays
For enzyme activity assays and cocrystallization of MtFsr with F420H2, the oxidized F420 needed to be reduced. Dithionite was not used since it contains 10–20% (m/m) sodium sulfite and generates further SO32− as product. All steps were performed under the strict exclusion of oxygen and under yellow light. First, the aerobic gas phase of the F420 stock was exchanged several times for N2. The sample was then transferred in a Coy tent with an atmosphere containing a N2/H2 mixture (97:3). The reduction took place in 1.4 ml 200 mM KH2PO4, pH 7.0, 0.5 mM F420, and 5 µl of 5 mg ml−1 purified MtFrh was added. Outside the tent, in a brown serum flask, the gas phase was exchanged three times for H2 and CO2 by evacuation and gassing with 1 × 105 Pa H2 and CO2 (80:20) at room temperature. The reduction of F420 was observed by the color shift from yellow to transparent. Frh was removed by passing the sample through a 10-kDa-cutoff filter. Since reduced F420 is not stable and oxidizes with time, aliquoted F420H2 without Frh was immediately flash frozen in liquid N2 and stored at −80 °C.
Reduction of F420 for redox titrations
F420 is the physiological electron donor for Fsr and was therefore used as the reductant for the redox titrations. Oxidized F420 was purified as described before. Since both the reduction of F420 with Frh is not complete and F420H2 is not stable over time, we reduced F420 with sodium borohydride, as previously described51. The reduction of F420 was performed in an anaerobic chamber with an N2/H2 atmosphere of 97:3 at 25 °C. F420H2 was generated by reducing 100 µl F420 at 7.53 mM with a few sodium borohydride crystals in a 10 mM Tris-HCl solution at pH 7.6, followed by destruction of excess borohydride by acidification with 50 µl 1 M hydrochloric acid. After the hydrogen evolution ceased, the pH was readjusted by the addition of 50 µl 1 M Tris–HCl pH 8.0. The generated F420H2 was prepared freshly for each experiment and used immediately.
Enzymatic assays
Enzymatic Fsr measurements were performed in 200 mM KH2PO4 buffer pH 7.0 under strict exclusion of hydrogen and oxygen. F420 was reduced by Frh as previously described. The oxidation of the reduced electron donor F420 was followed spectrophotometrically at 420 nm. For F420H2, a molecular extinction coefficient of 33.82 mM−1 cm−1 at 420 nm was experimentally determined for the above-mentioned conditions.
The assays for the specific enzyme activity were performed at 65 °C in a 1-ml quartz cuvette closed with a butyl rubber stopper. The gas phase of the cuvette was exchanged several times with N2. To monitor the reduction of SO32−, 1.4 mM Na2SO3 and 47.3 µM F420H2 were added to the KH2PO4 buffer. Once the spectrophotometer (Agilent Cary 60 UV–Vis) displayed a stable signal, the reaction was started by the addition of 0.19 µg MtFsr. To investigate whether MtFsr can use substrates other than SO32−, we provided 1.4 mM of disodium thiosulfate (S2O32−), 1.4 mM sodium nitrite (NO2−) or 1.4 mM disodium selenite (SeO32−). We further tested whether MtFsr can function in the reverse way by providing 1.4 mM Na2S as an electron donor and 47.3 µM of oxidized F420. All experiments were performed in triplicate.
The appKm and appVmax of MtFsr for SO32− and NO2− were determined at 50 °C under an anaerobic atmosphere (100% N2). The assays were performed in 96-deep-well plates and monitored spectrophotometrically (FLUOstar Omega Multi-Mode Microplate Reader). To determine the appKm and appVmax of MtFsr, 0–500 µM Na2SO3 or NaNO2 and 50 µM F420H2 were added to the 200 mM KH2PO4 buffer pH 7.0 and the reaction was started by the addition of 3.8 ng MtFsr. All experiments were performed in triplicate with a standard deviation represented by the ± sign. Kinetic parameters were calculated based on the ic50.tk server by applying a Hill coefficient of 1 (http://www.ic50.tk/kmvmax.html).
EPR spectroscopy
The midpoint potentials of the [4Fe‒4S] centers and the siroheme of MtFsr were determined from EPR signal intensities and EPR integrals of the various redox states. All titrations were performed in a Coy tent (N2/H2, 97:3), at 25 °C in the dark. A volume of 3.32 or 3 ml for the reductive or oxidative titrations with F420H2 or potassium ferricyanide at an initial MtFsr concentration of 4.07 or 2.7 mg ml−1 (in 100 mM Tris–HCl, pH 7.6), respectively, was stirred under anaerobic conditions. The solution potential was measured with an InLab ARGENTHAL (Mettler) microelectrode (Ag/AgCl, +207 mV versus H2/H+ with in-built platinum counter electrode) in the presence of the respective mediator mix. MtFsr was preincubated for 30 minutes before each titration with the mediator mix and assay buffer. The amount of MtFsr available and the necessary protein concentration to obtain a satisfying signal-to-noise ratio for the EPR spectra precluded multiple titrations. Thus, values reported were from a single redox titration for the siroheme and from two redox titrations for the Fe/S signals.
The mediator mix for the reductive titration contained methylene blue, resorufin, indigo carmine, 2-hydroxy-1,4-naphthoquinone (50 µM), sodium anthraquinone-2-sulfonate, phenosafranin, safranin T, neutral red, benzyl and methyl viologen (all at a final concentration of 25 µM, except 2-hydroxy-1,4-naphthoquinone). For the oxidative titration the mediator mix contained methylene blue, resorufin, indigo carmine, 2-hydroxy-1,4-naphthoquinone (all at a final concentration of 20 µM). After adjustment of the potential by microliter additions of F420H2 or potassium ferricyanide and 3 minutes equilibration, EPR samples were taken. For this, 300 µl of the mix were withdrawn, removed from the anaerobic glovebox in EPR tubes after attachment of a 5-cm piece of 3 mm × 7 mm (internal diameter × outer diameter) natural rubber tubing sealed with a 5-mm outer diameter acrylic glass stick at the other end. The samples were stored in liquid nitrogen until EPR spectra were recorded.
MtFsr as isolated was already in a partially reduced state. To obtain the completely oxidized form, 675 µl Fsr at 20 mg ml−1 was incubated for 30 minutes with 2 mM methylene blue. The sample was then passed through a Sephadex G-25M column (previously equilibrated with 100 mM Tris–HCl pH 7.6) to remove the methylene blue. This methylene blue-treated Fsr (1.28 ml) was collected at a concentration of 5.65 mg ml−1 and 300 µl was directly taken frozen for EPR spectroscopy of Fsr in its oxidized form.
Samples from the same methylene blue-treated Fsr (passed through a Sephadex G-25M column) at 5.09 mg ml−1 final concentration were incubated for 5 minutes with 10 mM Na2SO3, and then stored in liquid nitrogen.
All EPR spectra were recorded on a Bruker Elexsys E580 X band spectrometer (digitally upgraded) with a 4122HQE cavity linked to an ESR 900 Oxford Instruments helium flow cryostat. Cryocooling was performed by a Stinger (Cold Edge Technologies) closed-cycle cryostat driven by an F-70 Sumitomo helium compressor. Our local glassblower produced EPR tubes from Ilmasil PN tubing (outer diameter 4.7 mm and 0.5 mm wall thickness, Qsil). Before use, the tubes were extensively cleaned with pipe cleaners to remove inadvertent contaminants. EPR spectra were simulated with Easyspin52. The concentration of Fsr for the spin integration (using a 1 mM Cu2+–EDTA solution as standard) was obtained by dividing the Fe concentration, as determined with the ferene method29, by 24, since siroheme does not release Fe. Fitting to the Nernst equation was performed in Excel.
High-resolution clear-native PAGE
To visualize the expression levels of Fsr in HS−- versus SO32−-grown cultures, and to estimate the oligomerization of Fsr, high-resolution clear-native–PAGE (hrCN–PAGE) was performed. 10 ml of M. thermolithotrophicus and M. jannaschii cultures, with either 2 mM Na2S or 2 mM Na2SO3 as sulfur source, were grown for one night at 65 °C, with standing. Cells were collected by anaerobic centrifugation at 6,000g for 20 min at room temperature and the cell pellets were resuspended in 2 ml of 50 mM Tricine/NaOH pH 8.0 and 2 mM DTT. The cells were anaerobically sonicated four times at 70% intensity for 10 seconds, followed by a 30-second break (MS 73 probe, SONOPULS Bandelin). The hrCN–PAGE was run anaerobically and the protocol was adapted from ref. 53. Linear polyacrylamide gradient gels (8–15%) were prepared under aerobic conditions but then transferred into an anoxic chamber (atmosphere of N2/CO2, 90:10), where the gels were equilibrated in anaerobic cathode buffer (50 mM Tricine; 15 mM Bis–Tris, pH 7.0; 0.05% w/v sodium deoxycholate; 0.01% w/v dodecyl maltoside and 2 mM DTT) overnight. Fresh and anaerobic samples were diluted with the lysis buffer to a final concentration of 1 mg ml−1 and a volume of 12 µl per sample was loaded onto the gel, as well as 2 µl of the NativeMark Unstained Protein Standard ladder (ThermoFisher). Glycerol (20% v/v final) was added to each sample and 0.001% w/v Ponceau S served as a marker for protein migration. The electrophoresis anode buffer contained 50 mM Bis–Tris buffer pH 7.0 and 2 mM DTT. The hrCN gels were run with a constant 40-mA current (PowerPac Basic Power Supply, BioRad). After electrophoresis, the protein bands were aerobically stained with Instant Blue (Expedeon).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The crystal structures have been deposited in the Protein Data Bank under accession codes: 7NP8 for MjFsr and 7NPA for MtFsr. Raw crystallographic data have been deposited on Zenodo: https://doi.org/10.5281/zenodo.4751125. The data for this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Jannasch, H. W. & Mottl, M. J. Geomicrobiology of deep-sea hydrothermal vents. Science 229, 717–725 (1985).
Balderston, W. L. & Payne, W. J. Inhibition of methanogenesis in salt marsh sediments and whole-cell suspensions of methanogenic bacteria by nitrogen oxides. Appl. Environ. Microbiol. 32, 264–269 (1976).
Jones, W. J., Leigh, J. A., Mayer, F., Woese, C. R. & Wolfe, R. S. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136, 254–261 (1983).
Daniels, L., Belay, N. & Rajagopal, B. S. Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl. Environ. Microbiol. 51, 703–709 (1986).
Johnson, E. F. & Mukhopadhyay, B. A new type of sulfite reductase, a novel coenzyme F420-dependent enzyme, from the methanarchaeon Methanocaldococcus jannaschii. J. Biol. Chem. 280, 38776–38786 (2005).
Yu, H. et al. Comparative genomics and proteomic analysis of assimilatory sulfate reduction pathways in anaerobic methanotrophic archaea. Front. Microbiol. 9, 2917 (2018).
Johnson, E. F. & Mukhopadhyay, B. Coenzyme F420-dependent sulfite reductase-enabled sulfite detoxification and use of sulfite as a sole sulfur source by Methanococcus maripaludis. Appl. Environ. Microbiol. 74, 3591–3595 (2008).
Susanti, D., Frazier, M. C. & Mukhopadhyay, B. A genetic system for Methanocaldococcus jannaschii: an evolutionary deeply rooted hyperthermophilic Methanarchaeon. Front. Microbiol. 10, 1256 (2019).
Crane, B. R., Siegel, L. M. & Getzoff, E. D. Sulfite reductase structure at 1.6 Å: evolution and catalysis for reduction of inorganic anions. Science 270, 59–67 (1995).
Brânzanic, A. M. V., Ryde, U. & Silaghi-Dumitrescu, R. Why does sulfite reductase employ siroheme. Chem. Commun. 55, 14047–14049 (2019).
Crane, B. R. & Getzoff, E. D. The relationship between structure and function for the sulfite reductases. Curr. Opin. Struct. Biol. 6, 744–756 (1996).
Dhillon, A., Goswami, S., Riley, M., Teske, A. & Sogin, M. Domain evolution and functional diversification of sulfite reductases. Astrobiology 5, 18–29 (2005).
Oliveira, T. F. et al. The crystal structure of Desulfovibrio vulgaris dissimilatory sulfite reductase bound to DsrC provides novel insights into the mechanism of sulfate respiration. J. Biol. Chem. 283, 34141–34149 (2008).
Schiffer, A. et al. Structure of the dissimilatory sulfite reductase from the hyperthermophilic archaeon Archaeoglobus fulgidus. J. Mol. Biol. 379, 1063–1074 (2008).
Santos, A. A. et al. A protein trisulfide couples dissimilatory sulfate reduction to energy conservation. Science 350, 1541–1545 (2015).
Parey, K., Warkentin, E., Kroneck, P. M. H. & Ermler, U. Reaction cycle of the dissimilatory sulfite reductase from Archaeoglobus fulgidus. Biochemistry 49, 8912–8921 (2010).
Heryakusuma, C. et al. A reduced F420-dependent nitrite reductase in an anaerobic methanotrophic archaeon. J Bacteriol. 204, e00078-22 (2022).
Susanti, D. & Mukhopadhyay, B. An intertwined evolutionary history of methanogenic archaea and sulfate reduction. PLoS ONE 7, e45313 (2012).
Johnson, E. F. & Mukhopadhyay, B. in Microbial Sulfur Metabolism (eds. Dahl, C. & Friedrich, C. G.) Ch. 16 (Springer, 2008).
Shah, M. V. et al. Cofactor F420-dependent enzymes: an under-explored resource for asymmetric redox biocatalysis. Catalysts 9, 868 (2019).
DiMarco, A. A., Bobik, T. A. & Wolfe, R. S. Unusual coenzymes of methanogenesis. Annu. Rev. Biochem. 59, 355–394 (1990).
Greening, C. et al. Physiology, biochemistry, and applications of F420- and F0-dependent redox reactions. Microbiol. Mol. Biol. Rev. 80, 451–493 (2016).
Wagner, T., Koch, J., Ermler, U. & Shima, S. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe-4S] clusters for reduction. Science 357, 699–703 (2017).
Moura, I. et al. Low-spin sulfite reductases: a new homologous group of non-heme iron-siroheme proteins in anaerobic bacteria. Biochem. Biophys. Res. Commun. 141, 1032–1041 (1986).
Vitt, S. et al. The F420-reducing [NiFe]-hydrogenase complex from Methanothermobacter marburgensis, the first X-ray structure of a group 3 family member. J. Mol. Biol. 426, 2813–2826 (2014).
Ilina, Y. et al. X-ray crystallography and vibrational spectroscopy reveal the key determinants of biocatalytic dihydrogen cycling by [NiFe] hydrogenases. Angew. Chem. Int. Ed. Engl. 58, 18710–18714 (2019).
Janick, P. A. & Siegel, L. M. Electron paramagnetic resonance and optical spectroscopic evidence for interaction between siroheme and Fe4S4 prosthetic groups in Escherichia coli sulfite reductase hemoprotein subunit. Biochemistry 21, 3538–3547 (1982).
Huynh, B. H., Kang, L., DerVartanian, D. V., Peck, H. D. & LeGall, J. Characterization of a sulfite reductase from Desulfovibrio vulgaris. Evidence for the presence of a low-spin siroheme and an exchange-coupled siroheme-[4Fe–4S] unit. J. Biol. Chem. 259, 15373–15376 (1984).
Pierik, A. J. & Hagen, W. R. S = 9/2 EPR signals are evidence against coupling between the siroheme and the Fe/S cluster prosthetic groups in Desulfovibrio vulgaris (Hildenborough) dissimilatory sulfite reductase. Eur. J. Biochem. 195, 505–516 (1991).
Day, E. P. et al. Magnetization of the sulfite and nitrite complexes of oxidized sulfite and nitrite reductases: EPR silent spin S = 1/2 states. Biochemistry 27, 2126–2132 (1988).
Prince, R. C. & Adams, M. W. W. Oxidation-reduction properties of the two Fe4S4 clusters in Clostridium pasteurianum ferredoxin. J. Biol. Chem. 262, 5125–5128 (1987).
Christner, J. A. et al. Exchange coupling between siroheme and [4Fe-4S] cluster in E. coli sulfite reductase. Mössbauer studies and coupling models for a 2-electron reduced enzyme state and complexes with sulfide. J. Am. Chem. Soc. 106, 6786–6794 (1984).
Guo, M., Sulc, F., Ribbe, M. W., Farmer, P. J. & Burgess, B. K. Direct assessment of the reduction potential of the [4Fe–4S](1+/0) couple of the Fe protein from Azotobacter vinelandii. J. Am. Chem. Soc. 124, 12100–12101 (2002).
Askenasy, I. et al. The N-terminal domain of Escherichia coli assimilatory NADPH-sulfite reductase hemoprotein is an oligomerization domain that mediates holoenzyme assembly. J. Biol. Chem. 290, 19319–19333 (2015).
Kim, J. Y., Nakayama, M., Toyota, H., Kurisu, G. & Hase, T. Structural and mutational studies of an electron transfer complex of maize sulfite reductase and ferredoxin. J. Biochem. 160, 101–109 (2016).
Belay, N., Jung, K. Y., Rajagopal, B. S., Kremer, J. D. & Daniels, L. Nitrate as a sole nitrogen source for Methanococcus thermolithotrophicus and its effect on growth of several methanogenic bacteria. Curr. Microbiol. 21, 193–198 (1990).
Codorniu-Hernández, E. & Kusalik, P. G. Probing the mechanisms of proton transfer in liquid water. Proc. Natl Acad. Sci. USA 110, 13697–13698 (2013).
Loy, A. et al. Reverse dissimilatory sulfite reductase as phylogenetic marker for a subgroup of sulfur-oxidizing prokaryotes. Environ. Microbiol. 11, 289–299 (2009).
Canfield, D. E., Habicht, K. S. & Thamdrup, B. The Archean sulfur cycle and the early history of atmospheric oxygen. Science 288, 658–661 (2000).
Ueno, Y., Yamada, K., Yoshida, N., Maruyama, S. & Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 440, 516–519 (2006).
Liu, Y. F. et al. Genomic and transcriptomic evidence supports methane metabolism in Archaeoglobi. mSystems 5, e00651-19 (2020).
Mukhopadhyay, B., Johnson, E. F. & Wolfe, R. S. Reactor-scale cultivation of the hyperthermophilic methanarchaeon Methanococcus jannaschii to high cell densities. Appl. Environ. Microbiol. 65, 5059–5065 (1999).
Martín-Platero, A. M., Valdivia, E., Maqueda, M. & Martínez-Bueno, M. Fast, convenient, and economical method for isolating genomic DNA from lactic acid bacteria using a modification of the protein ‘salting-out’ procedure. Anal. Biochem. 366, 102–104 (2007).
Kulak, N. A., Pichler, G., Paron, I., Nagaraj, N. & Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 11, 319–324 (2014).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302 (2011).
Pannu, N. S. et al. Recent advances in the CRANK software suite for experimental phasing. Acta Crystallogr. D Biol. Crystallogr. 67, 331–337 (2011).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Biol. Crystallogr. 75, 861–877 (2019).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Schönheit, P., Keweloh, H. & Thauer, R. K. Factor F420 degradation in Methanobacterium thermoautotrophicum during exposure to oxygen. FEMS Microbiol. Lett. 12, 347–349 (1981).
Deppenmeier, U., Blaut, M., Mahlmann, A. & Gottschalk, G. Membrane bound F420H2-dependent heterodisulfide reductase in methanogenic bacterium strain Göl and Methanolobus tindarius. FEBS Lett. 261, 199–203 (1990).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Lemaire, O. N. et al. Small membranous proteins of the TorE/NapE family, crutches for cognate respiratory systems in Proteobacteria. Sci. Rep. 8, 13576 (2018).
Wagner, T., Wegner, C. E., Kahnt, J., Ermler, U. & Shima, S. Phylogenetic and structural comparisons of the three types of methyl coenzyme M reductase from Methanococcales and Methanobacteriales. J. Bacteriol. 199, e00197-17 (2017).
Murphy, M. J., Siegel, L. M., Tove, S. R. & Kamin, H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc. Natl Acad. Sci. USA 71, 612–616 (1974).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Krissinel, E. & Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 (2004).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Ebrahim, A. et al. Dose-resolved serial synchrotron and XFEL structures of radiation-sensitive metalloproteins. IUCrJ 6, 543–551 (2019).
Acknowledgements
We thank the Max Planck Institute for Marine Microbiology and the Max Planck Society for continuous support. We are grateful for the genome sequencing performed in the Max Planck-Genome-Centre (Cologne) and specifically B. Huettel. We thank J. Soares for the EPR data acquisition and measurement of the Fe content. We acknowledge the SOLEIL synchrotron for beam time allocation and the beamline staff of Proxima-1 for assistance with data collection. Furthermore, we thank the staff of beamline PXIII from SLS and P11 at PETRA III. We thank M. Räschle (Center for Mass Analytics, University of Kaiserslautern-Landau, Campus Kaiserslautern) and L. Hewener for analysis of the peptide fingerprints and sample preparation, respectively. We also thank C. Probian and R. Appel for their continuous support in the Microbial Metabolism laboratory and O. Lemaire for his support. We thank B. L. Boehman for her deep reviewing of the manuscript. This research was funded by the Max-Planck-Gesellschaft and supported by the Deutsche Forschungsgemeinschaft (DFG) Schwerpunktprogram 1927 ‘Iron-sulfur for Life’ (WA 4053/1-1, to T.W., and Pl 610/2-2, to A.J.P.). The upgrade of the EPR spectrometer (A.J.P.) was funded by the DFG (248/320-1, project number 444947649) and the government of Rhineland-Palatinate.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
M.J. cultivated both methanogens, and purified and crystallized both Fsr. M.J. performed all biochemical characterizations. M.J. and T.W. collected X-ray data and solved the structures. M.J. refined both Fsr models and M.J. with T.W. validated the models. M.J. and A.J.P. performed the redox titration experiments, and A.J.P. the spectroscopic analyses. T.W. and M.J. designed the research. All co-authors contributed to the writing of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks José Brito, Guenter Schwarz and Elizabeth Stroupe for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Structural and functional organization of assimilatory (aSir) and dissimilatory (dSir refer here as DsrAB) sulfite reductases.
Distinct and conserved domains in aSir as well as dSir are shown in the top panel. The [4Fe-4S]-cluster binding motifs in the proximity of the siroheme or sirohydrochlorin are highlighted. Bottom panel: aSirs (left) are functional monomers that probably evolved through a gene duplication event, where one gene lost its cluster binding motif. The N-terminal half abbreviated as aSir-a (light pink) has a structural function and the C-terminal half abbreviated as aSir-b (red) harbours the active [4Fe-4S]-siroheme. aSirs indirectly use electrons from NADPH (bacteria) or directly via a [2Fe-2S]-cluster containing ferredoxin (plants) to reduce SO32− to HS− in a six-electron reduction reaction11,35. The produced sulfide will be used for sulfur assimilation. dSirs (right) are composed of two DsrA (light pink) and two DsrB (red) subunits and receive electrons from reduced ferredoxins (Fdred2−) or so far unknown donors15. In absence of DsrC (cyan), DsrAB turns SO32− to thionates (that is S2O32−, S3O62−) and HS−. In presence of DsrC, the intermediate sulfur species bound on the siroheme is transferred to DsrC. In the case of Desulfovibrio species, the membrane DsrMKJOP complex (green) fully reduces the DsrC-trisulfide (4 electrons transfer) probably by using the menaquinol pool and generates DsrC and HS− via the trisulfide pathway, a key process for energy conservation15.
Extended Data Fig. 2 Physiological and biochemical profiles of Fsr from Methanococcales.
a, Final OD600 nm of M. thermolithotrophicus grown on sulfide (S2-) and different sulfite (SO32−) concentrations as a sole sulfur source after 22 hours (mean ± s.d., n = 3 biologically independent replicates). b, c, hrCN-PAGE of cell extracts (12 µg loaded) from M. jannaschii (b, n = 1 independent experiment) and M. thermolithotrophicus (c, n = 3 independent experiments), grown on 2 mM Na2S or 2 mM Na2SO3 as a sole sulfur source. Purified MCR from M. thermolithotrophicus (1.7 µg loaded) was used as a control for the hrCN-PAGE54. d, e, SDS-PAGE profile of purified MjFsr (d, n = 1 independent experiment) and MtFsr (e, n = 3 independent experiments). f,. UV-visible spectrum of 0.33 mg MtFsr measured anaerobically (100 % N2) in 25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 10 % v/v glycerol and 2 mM DTT. MtFsr displays the typical spectra of [Fe-S]-cluster and siroheme containing enzymes55, similar to the UV spectrum of MjFsr previously determined exhibiting three peaks at 280 nm, 395 nm and 593 nm5. g, Molecular weight estimation of MtFsr via size exclusion chromatography (Superdex 200 Increase 10/300 GL from GE Healthcare). Apparent molecular weight of purified MtFsr (monomeric molecular weight = 69.145 kDa) was estimated to 282 kDa. MtFsr is therefore apparently organized as a homotetramer (theoretical molecular weight of the protein in the homotetramer: 276.58 kDa).
Extended Data Fig. 3 Homotetrameric arrangement of Fsr.
a, Superposition of MtFsr (black) with MjFsr (orange, rmsd of 0.456 Å for 544-Cα aligned). Ligands are shown in balls and sticks and coloured in black and orange for MtFsr and MjFsr, respectively. b, Surface area involved in the oligomerization in Fsr. Monomers of MtFsr are shown in surface representation, with one monomer being displayed in cartoon and coloured by its domain composition: the N-terminal ferredoxin domain in dark blue, F420H2-oxidase in green, the sulfite reductase domain in red and its inserted ferredoxin domain in orange. The C-terminal segment involved in the oligomerization is coloured in light pink with the C-terminus highlighted as a ball. The monomer-monomer contacts are shown as a green surface and contacts to the adjacent dimer are visualized by a cyan surface. The basic monomer-monomer interface of 2,902-Å2 for MtFsr and 2,971-Å2 for MjFsr is established by the sulfite reductase domain and the two additional ferredoxin domains. The C-terminal part of the sulfite reductase domain (562–618 in MtFsr, 562–620 in MjFsr), the second ferredoxin domain and the loop 171–189 of the F420H2-oxidase domain generate the dimer-dimer interface, totalling an area of 3,055-Å2 for MtFsr and 3,037-Å2 for MjFsr. Most of these contacts involve salt bridges. In MjFsr, the tetrameric structure is supported by two divalent cations, modelled as calcium ions that are each coordinated by a conserved aspartate from the opposite monomers (Asp511 and water molecules)56.
Extended Data Fig. 4 Asymmetric unit content and B-factor profile of MtFsr.
a, The four homotetramers contained in the asymmetric unit of MtFsr are shown in cartoon and the 96 [4Fe-4S]-clusters, the 16 FADs (in yellow) and 16 sirohemes (in pink) are shown in balls and sticks. To our knowledge, MtFsr contains the highest number of clusters seen in an asymmetric unit so far. b, Superposition of all sixteen chains from the asymmetric unit in MtFsr, with an average rmsd of 0.14 Å for 514-Cα aligned. The N- and C-terminus of each chain are shown by a blue and red sphere, respectively. The models are coloured according to their B-factor values; blue to red indicate low to high B-factors, respectively. c, Averaged B-factor values (in black) for each residue from the 16 chains composing the asymmetric unit of MtFsr. The averaged root mean square deviations (rmsd, in red) of the corresponding Cα is overlaid on the same graph. Averaged rmsd were calculated by the software superpose57.
Extended Data Fig. 5 Cluster coordination in Fsr.
The top panel shows the monomeric arrangement of MtFsr (in cartoon) coloured by domains. The siroheme (purple), FAD (yellow) and the [4Fe-4S]-clusters are represented in balls and sticks. Nitrogen, oxygen, sulfur, and iron atoms are coloured respectively, in blue, red, yellow and orange. In the bottom panel, cysteines and the glutamate involved in direct [4Fe-4S]-cluster binding are highlighted, as well as the different domains of Fsr. Sequence alignment was done by Clustal Omega58, secondary structure prediction was performed with ESPript 3.059. Cluster 6 is electronically connected to the siroheme. The black stars (*) indicate residues near the siroheme, proposed to bind SO32−.
Extended Data Fig. 6 EPR spectra of the dye-mediated redox titrations of MtFsr and g-values as function of J/D and E/D as described by J. A. Christner et al. 1984.
a, b and d The redox potentials at which samples were frozen are indicated. EPR intensities were scaled to correct for differences in concentration. EPR conditions: temperature, 10 K; modulation frequency, 100 kHz; modulation amplitude, 1.0 mT; microwave frequency, 9.353 GHz; microwave power, 20 mW (panel d 0.2 mW). c, Contours of the two highest g-values of the coupled ferrous siroheme-[4Fe-4S]1+ system as function of J/D and E/D according to Fig. 4 from32. The blue points are from E. coli sulfite reductase: A and B, KCl (two species); C, KF or KBr; E, urea; F, sodium formate; G, (Gdm)2SO4; H, KBr; D, spinach nitrite reductase; MtFsR is shows as red point.
Extended Data Fig. 7 Overall structural comparison between Fsr, aSir and dSir.
Cut-through view shown in cartoon of one dimer for Fsr and DsrAB. Ligands are shown as balls and sticks. a, The sulfite reductase domain with the inserted ferredoxin domain of MtFsr. Fsr´ corresponds to the opposite monomer. b, aSir from Zea mays and its [2Fe-2S]-ferredoxin coloured in light green (PDB 5H92). c, DsrAB from A. fulgidus (PDB 3MM5) and d, DsrABC from D. vulgaris (PDB 2V4J). The inserted ferredoxin domains of Fsr, DsrA and DsrB are coloured in orange. The catalytic siroheme in DsrAB is coloured in purple and the structural siroheme is coloured in black. DsrAB from D. vulgaris contains sirohydrochlorin instead of siroheme.
Extended Data Fig. 8 Siroheme conformation within Fsr.
a, Electrostatic charge profile of MtFsr shown in surface is coloured in red and blue to represent acidic and basic patches, respectively. The siroheme is accessible via a positively charged solvent channel. Carbon, oxygen, nitrogen, sulfur and iron are coloured in green, red, blue, yellow and orange, respectively. b and c, Close up of the axial ligands bound on the siroheme of MjFsr (b) and MtFsr (c). The 2Fo-Fc map of the siroheme and SO32− are contoured to 1.5-σ in MjFsr, while the siroheme and HS− is contoured to 3-σ in MtFsr. In MjFsr the Fe-siroheme is equidistant (2.3 Å) to the sulfur from the modelled SO32− and the bridging-sulfur of the cysteine 472, suggesting a tight covalent binding. In MtFsr, the bridging-sulfur of the cysteine 472 is at a distance of 2.6 Å to the Fe-siroheme and the sulfur from the modelled HS− is 2.9 Å distant to the Fe-siroheme, indicating a loose binding of the HS−, which might result from a reduction event by X-ray radiation60. d, Siroheme superposition between aSirs (1AOP, 5H92), dSirs (3MM5, 2V4J) and Fsrs. Siroheme from aSirs and Fsr are coloured in green, structural siroheme/sirohydrochlorin from dSirs in black and dSirs functional sirohemes in blue. Superposition analysis shows that the functional sirohemes are arranged in a highly similar manner, whereas the conformation of the structural siroheme or sirohydrochlorin differ, which highlights the strong influence of the protein environment on the siroheme geometry.
Extended Data Fig. 9 Theoretical evolutionary scenario of sulfite reductases.
The proposed route is based on the assumption that aSir, dSir and Fsr could have evolved from a common ancestor. The primordial sulfite reductase model corresponds to the elementary sulfite reductase core of the MtFsr structure. The different steps that led to the evolution of this progenitor to modern Fsr can be hypothesized based on its modular organization. In a straightforward and simple model, a ferredoxin with 2 × [4Fe‒4 S]-cluster could have been inserted into the elementary sulfite reductase module. Then an F420H2-oxidase with a ferredoxin domain (Fqo/FpoF-like) would have been fused to the N-terminus of the sulfite reductase domain containing the inserted ferredoxin. Some members of the Sir superfamily might have arisen from one of these steps18. Such a hypothesis is exemplified by the similarities between the quaternary organization of Fsr and DsrAB and the active site of Fsr and aSir.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Table 1.
Source data
Source Data Table 1
Statistical source data for the nitrite/sulfite Km measurements for MtFsr.
Source Data Extended Data Fig. 2
Unprocessed gels for Extended Data Fig. 2.
Source Data Extended Data Fig. 2
Statistical source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 4
Statistical source data for Fig. 4c.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jespersen, M., Pierik, A.J. & Wagner, T. Structures of the sulfite detoxifying F420-dependent enzyme from Methanococcales. Nat Chem Biol 19, 695–702 (2023). https://doi.org/10.1038/s41589-022-01232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01232-y
- Springer Nature America, Inc.
This article is cited by
-
Differences in regulation mechanisms of glutamine synthetases from methanogenic archaea unveiled by structural investigations
Communications Biology (2024)
-
Assimilatory sulfate reduction in the marine methanogen Methanothermococcus thermolithotrophicus
Nature Microbiology (2023)