Abstract
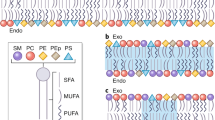
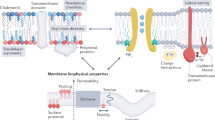
A fundamental feature of cellular plasma membranes (PMs) is an asymmetric lipid distribution between the bilayer leaflets. However, neither the detailed, comprehensive compositions of individual PM leaflets nor how these contribute to structural membrane asymmetries have been defined. We report the distinct lipidomes and biophysical properties of both monolayers in living mammalian PMs. Phospholipid unsaturation is dramatically asymmetric, with the cytoplasmic leaflet being approximately twofold more unsaturated than the exoplasmic leaflet. Atomistic simulations and spectroscopy of leaflet-selective fluorescent probes reveal that the outer PM leaflet is more packed and less diffusive than the inner leaflet, with this biophysical asymmetry maintained in the endocytic system. The structural asymmetry of the PM is reflected in the asymmetric structures of protein transmembrane domains. These structural asymmetries are conserved throughout Eukaryota, suggesting fundamental cellular design principles.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files) or are available from the corresponding author on reasonable request.
Change history
15 May 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41589-020-0564-3
References
Devaux, P. F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30, 1163–1173 (1991).
Op den Kamp, J. A. Lipid asymmetry in membranes. Annu. Rev. Biochem. 48, 47–71 (1979).
Verkleij, A. J. et al. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta 323, 178–193 (1973).
Schick, P. K., Kurica, K. B. & Chacko, G. K. Location of phosphatidylethanolamine and phosphatidylserine in the human platelet plasma membrane. J. Clin. Investig. 57, 1221–1226 (1976).
Sandra, A. & Pagano, R. E. Phospholipid asymmetry in LM cell plasma membrane derivatives: polar head group and acyl chain distributions. Biochemistry 17, 332–338 (1978).
Bollen, I. C. & Higgins, J. A. Phospholipid asymmetry in rough- and smooth-endoplasmic-reticulum membranes of untreated and phenobarbital-treated rat liver. Biochem. J. 189, 475–480 (1980).
Lin, Q. & London, E. The influence of natural lipid asymmetry upon the conformation of a membrane-inserted protein (perfringolysin O). J. Biol. Chem. 289, 5467–5478 (2014).
Doktorova, M. et al. Preparation of asymmetric phospholipid vesicles for use as cell membrane models. Nat. Protoc. 13, 2086–2101 (2018).
Enoki, T. A. & Feigenson, G. W. Asymmetric bilayers by hemifusion: method and leaflet behaviors. Biophys. J. 117, 1037–1050 (2019).
Chiantia, S. & London, E. Acyl chain length and saturation modulate interleaflet coupling in asymmetric bilayers: effects on dynamics and structural order. Biophys. J. 103, 2311–2319 (2012).
Heberle, F. A. et al. Subnanometer structure of an asymmetric model membrane: interleaflet coupling influences domain properties. Langmuir 32, 5195–5200 (2016).
Cheng, H. T. & Megha, London,E. Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation. J. Biol. Chem. 284, 6079–6092 (2009).
Doktorova, M. et al. Gramicidin increases lipid flip-flop in symmetric and asymmetric lipid vesicles. Biophys. J. 116, 860–873 (2019).
Sharpe, H. J., Stevens, T. J. & Munro, S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158–169 (2010).
Morrot, G. et al. Asymmetric lateral mobility of phospholipids in the human erythrocyte membrane. Proc. Natl Acad. Sci. USA 83, 6863–6867 (1986).
el Hage Chahine, J. M., Cribier, S. & Devaux, P. F. Phospholipid transmembrane domains and lateral diffusion in fibroblasts. Proc. Natl Acad. Sci. USA 90, 447–451 (1993).
Tanaka, K. I. & Ohnishi, S. Heterogeneity in the fluidity of intact erythrocyte membrane and its homogenization upon hemolysis. Biochim. Biophys. Acta 426, 218–231 (1976).
Gupta, A., Korte, T., Herrmann, A. & Wohland, T. Plasma membrane asymmetry of lipid organization: fluorescence lifetime microscopy and correlation spectroscopy analysis. J. Lipid Res. 61, 252–266 (2020).
Schroeder, F. Differences in fluidity between bilayer halves of tumour cell plasma membranes. Nature 276, 528–530 (1978).
Schachter, D., Abbott, R. E., Cogan, U. & Flamm, M. Lipid fluidity of the individual hemileaflets of human erythrocyte membranes. Ann. N. Y. Acad. Sci. 414, 19–28 (1983).
Rimon, G., Meyerstein, N. & Henis, Y. I. Lateral mobility of phospholipids in the external and internal leaflets of normal and hereditary spherocytic human erythrocytes. Biochim. Biophys. Acta 775, 283–290 (1984).
Harayama, T. et al. Lysophospholipid acyltransferases mediate phosphatidylcholine diversification to achieve the physical properties required in vivo. Cell Metab. 20, 295–305 (2014).
Yeung, T. et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 (2008).
Levental, I., Janmey, P. A. & Cebers, A. Electrostatic contribution to the surface pressure of charged monolayers containing polyphosphoinositides. Biophys. J. 95, 1199–1205 (2008).
Sodt, A. J., Venable, R. M., Lyman, E. & Pastor, R. W. Nonadditive compositional curvature energetics of lipid bilayers. Phys. Rev. Lett. 117, 138104 (2016).
Ingolfsson, H. I. et al. Lipid organization of the plasma membrane. J. Am. Chem. Soc. 136, 14554–14559 (2014).
Cui, H., Lyman, E. & Voth, G. A. Mechanism of membrane curvature sensing by amphipathic helix containing proteins. Biophys. J. 100, 1271–1279 (2011).
Jin, L. et al. Characterization and application of a new optical probe for membrane lipid domains. Biophys. J. 90, 2563–2575 (2006).
Owen, D. M. et al. Fluorescence lifetime imaging provides enhanced contrast when imaging the phase-sensitive dye di-4-ANEPPDHQ in model membranes and live cells. Biophys. J. 90, L80–L82 (2006).
Sezgin, E., Sadowski, T. & Simons, K. Measuring lipid packing of model and cellular membranes with environment sensitive probes. Langmuir 30, 8160–8166 (2014).
Wesseling, M. C. et al. Novel insights in the regulation of phosphatidylserine exposure in human red blood cells. Cell Physiol. Biochem. 39, 1941–1954 (2016).
Diaz-Rohrer, B. B., Levental, K. R., Simons, K. & Levental, I. Membrane raft association is a determinant of plasma membrane localization. Proc. Natl Acad. Sci. USA 111, 8500–8505 (2014).
Lorent, J. H. et al. Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 8, 1219 (2017).
Kobayashi, T. & Menon, A. K. Transbilayer lipid asymmetry. Curr. Biol. 28, R386–R391 (2018).
Yamakawa, T. & Nagai, Y. Glycolipids at the cell surface and their biological functions. Trends Biochem. Sci. 3, 128–131 (1978).
Levental, I., Cebers, A. & Janmey, P. A. Combined electrostatics and hydrogen bonding determine intermolecular interactions between polyphosphoinositides. J. Am. Chem. Soc. 130, 9025–9030 (2008).
Steck, T. L. & Lange, Y. Transverse distribution of plasma membrane bilayer cholesterol: picking sides. Traffic 19, 750–760 (2018).
Courtney, K. C. et al. C24 sphingolipids govern the transbilayer asymmetry of cholesterol and lateral organization of model and live-cell plasma membranes. Cell Rep. 24, 1037–1049 (2018).
Liu, S. L. et al. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nat. Chem. Biol. 13, 268–274 (2017).
Steck, T. L. & Lange, Y. How slow is the transbilayer diffusion (flip-flop) of cholesterol? Biophys. J. 102, 945–946 (2012); author reply 102, 947–949.
Iaea, D. B. & Maxfield, F. R. Membrane order in the plasma membrane and endocytic recycling compartment. PLoS ONE 12, e0188041 (2017).
Levental, I., Levental, K. R. & Heberle, F. A. Lipid rafts: controversies resolved, mysteries remain. Trends Cell Biol. 30, 341–353 (2020).
Lin, Q. & London, E. Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles. Biophys. J. 108, 2212–2222 (2015).
Kiessling, V., Crane, J. M. & Tamm, L. K. Transbilayer effects of raft-like lipid domains in asymmetric planar bilayers measured by single molecule tracking. Biophys. J. 91, 3313–3326 (2006).
Veatch, S. L. & Keller, S. L. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 94, 148101 (2005).
Kusumi, A. et al. Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu. Rev. Cell Dev. Biol. 28, 215–250 (2012).
Honigmann, A. et al. A lipid bound actin meshwork organizes liquid phase separation in model membranes. eLife 3, e01671 (2014).
Levental, K. R. et al. Homeostatic remodeling of mammalian membranes in response to dietary lipids is essential for cellular fitness. Nat. Commun. 11, 1339 (2020).
Hussain, N. F., Siegel, A. P., Ge, Y., Jordan, R. & Naumann, C. A. Bilayer asymmetry influences integrin sequestering in raft-mimicking lipid mixtures. Biophys. J. 104, 2212–2221 (2013).
Frewein, M., Kollmitzer, B., Heftberger, P. & Pabst, G. Lateral pressure-mediated protein partitioning into liquid-ordered/liquid-disordered domains. Soft Matter 12, 3189–3195 (2016).
McIntyre, J. C. & Sleight, R. G. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry 30, 11819–11827 (1991).
Levental, K. R. et al. Omega-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Sci. Adv. 3, eaao1193 (2017).
Surma, M. A. et al. An automated shotgun lipidomics platform for high throughput, comprehensive and quantitative analysis of blood plasma intact lipids. Eur. J. Lipid Sci. Technol. 117, 1540–1549 (2015).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Venable, RichardM. et al. CHARMM all-atom additive force field for sphingomyelin: elucidation of hydrogen bonding and of positive curvature. Biophys. J. 107, 134–145 (2014).
Shan, Y., Klepeis, J. L., Eastwood, M. P., Dror, R. O. & Shaw, D. E. Gaussian split Ewald: a fast Ewald mesh method for molecular simulation. J. Chem. Phys. 122, 054101 (2005).
Camley, B. A., Lerner, M. G., Pastor, R. W. & Brown, F. L. H. Strong influence of periodic boundary conditions on lateral diffusion in lipid bilayer membranes. J. Chem. Phys. 143, 243113 (2015).
Li, Q., Wang, X., Ma, S., Zhang, Y. & Han, X. Electroformation of giant unilamellar vesicles in saline solution. Colloids Surf. B Biointerfaces 147, 368–375 (2016).
Steinkuhler, J., De Tillieux, P., Knorr, R. L., Lipowsky, R. & Dimova, R. Charged giant unilamellar vesicles prepared by electroformation exhibit nanotubes and transbilayer lipid asymmetry. Sci. Rep. 8, 11838 (2018).
Acknowledgements
All fluorescence microscopy was performed at the Center for Advanced Microscopy, Department of Integrative Biology & Pharmacology at McGovern Medical School, UTHealth. We thank N. Waxham for his generous sharing of the microinjection system. We acknowledge K. Simons, T. Steck, Y. Lange and G. Feigenson for their critical feedback on this manuscript. Funding for this work was provided by the NIH/National Institute of General Medical Sciences (GM114282, GM124072, GM120351 and GM134949), the Volkswagen Foundation (grant no. 93091) and the Human Frontiers Science Program (RGP0059/2019). E.S. is funded by Newton-Katip Ҫelebi Institutional Links grant no. 352333122. Anton2 computer time was provided by the National Resource for Biomedical Supercomputing (NRBSC), the Pittsburgh Supercomputing Center (PSC) and the Biomedical Technology Research Center for Multiscale Modeling of Biological Systems through grant no. P41GM103712-S1 from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.H.L., I.L., E.L. and K.R.L. designed the study. J.H.L., K.R.L., L.G., G.R.-L., M.D. and E.S. performed experiments. E.L. performed and analyzed the molecular dynamics simulations. J.H.L. carried out the bioinformatics analysis. J.H.L., K.R.L. and I.L. analyzed the experimental results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Supplementary Figs. 1–15.
Supplementary Dataset
Asymmetric lipidomes.
Rights and permissions
About this article
Cite this article
Lorent, J.H., Levental, K.R., Ganesan, L. et al. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol 16, 644–652 (2020). https://doi.org/10.1038/s41589-020-0529-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0529-6
- Springer Nature America, Inc.
This article is cited by
-
Is cholesterol both the lock and key to abnormal transmembrane signals in Autism Spectrum Disorder?
Lipids in Health and Disease (2024)
-
Hydrophobic mismatch drives self-organization of designer proteins into synthetic membranes
Nature Communications (2024)
-
Giant organelle vesicles to uncover intracellular membrane mechanics and plasticity
Nature Communications (2024)
-
MemPrep, a new technology for isolating organellar membranes provides fingerprints of lipid bilayer stress
The EMBO Journal (2024)
-
Molecular mechanism of GPCR spatial organization at the plasma membrane
Nature Chemical Biology (2024)