Abstract
The Cambrian radiation of euarthropods can be attributed to an adaptable body plan. Sophisticated brains and specialized feeding appendages, which are elaborations of serially repeated organ systems and jointed appendages, underpin the dominance of Euarthropoda in a broad suite of ecological settings. The origin of the euarthropod body plan from a grade of vermiform taxa with hydrostatic lobopodous appendages (‘lobopodian worms’)1,2 is founded on data from Burgess Shale-type fossils. However, the compaction associated with such preservation obscures internal anatomy3,4,5,6. Phosphatized microfossils provide a complementary three-dimensional perspective on early crown group euarthropods7, but few lobopodians8,9. Here we describe the internal and external anatomy of a three-dimensionally preserved euarthropod larva with lobopods, midgut glands and a sophisticated head. The architecture of the nervous system informs the early configuration of the euarthropod brain and its associated appendages and sensory organs, clarifying homologies across Panarthropoda. The deep evolutionary position of Youti yuanshi gen. et sp. nov. informs the sequence of character acquisition during arthropod evolution, demonstrating a deep origin of sophisticated haemolymph circulatory systems, and illuminating the internal anatomical changes that propelled the rise and diversification of this enduringly successful group.
Similar content being viewed by others
Systematic palaeontology
Superphylum Panarthropoda
Lower stem group to Phylum Euarthropoda10
Youti yuanshi gen. et sp. nov.
LSID. urn:lsid:zoobank.org:act:28BD6A01-5FDC-40EC-973A-63AEB05328A4.
Etymology. From Pinyin yòutĭ, meaning larva, and yuánshĭ, meaning primitive; reflecting the early developmental stage of the fossil and its bearing on the origin of the euarthropod body plan.
Holotype. YKLP 12387 (Figs. 1–3 and Extended Data Figs. 1–4), recovered by 5% acetic acid digestion of carbonate nodules from black shales of the Yu’anshan Formation (Eoredlichia–Wutingaspis Biozone, approximately late Atdabanian stage, Cambrian Period Series 2, Stage 3), Xiaotan section, Yongshan, Yunnan Province.
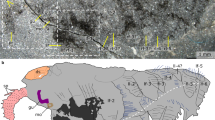
YKLP 12387. a, External scanning electron microscopy, right side. Damage to posterior epidermis exposes lining of perivisceral cavity, demonstrating blind gut. b, External scanning electron microscopy, left side. c,g–j, Median virtual dissection from X-ray computed tomography (XCT) data (c), showing location of transverse slices intersecting digestive glands (g,i) and transverse membrane (h,j). d, Semi-manual segmentation of internal chambers from XCT data, viewed from the left side. Dorsolateral aspects of the peripheral cavity are omitted for clarity. e,f, Virtual dissection parallel to coronal plane, looking ventrally (e) and dorsally (f), showing digestive glands, pericardial sinus, transverse membranes within perivisceral cavity, and oblique membranes within peripheral cavity. g–j, XCT sections at positions indicated in c at position of digestive glands (g,i) and at position of ventrolateral lacunae and transverse membrane (h,j). g,h, Sections close to the anterior trunk, reflecting segments at late developmental stage. i,j, Sections close to the posterior trunk, showing superior preservation of internal tissue. k, Segmentation of internal chambers from XCT data, viewed from the dorsal perspective at anterior, middle and posterior trunk. Aspects of peripheral cavity are omitted for clarity. a, appendage; cb, central body of brain; db, dorsolateral body of brain; dia, diagenetic grain; dg, digestive gland; dm, dorsal membrane; dp, dorsal projection; dv, dorsal vessel; fb, frontal body of brain; irr, irregular chamber; lig, ligament; om, oblique membrane; pc, pericardial sinus; pph, peripheral cavity; pn, perineural sinus; pv, perivisceral cavity; tm, transverse membrane; vl, ventrolateral sinus; vv, ventral vessel. Scale bars, 200 μm.
YKLP 12387. a,b, Virtual dissections of dorsolateral trunk, looking posteriad, showing internal structure of tenth (a) and eighth (b) digestive glands, oblique membrane within peripheral cavity and ligaments associated with gut. c, Virtual dissection through 14th digestive gland, looking anteriad, showing connection of digestive glands to dorsal gut. d, Virtual dissection showing blind termination of posterior gut; dashed line marks anterior limit of gut preservation. e–g, Disposition of chambers within trunk appendages, showing extensions of perivisceral and peripheral cavities into appendage. Virtual dissections oriented parallel (e), oblique (f) and subperpendicular (g) to appendages. A, anterior; P, posterior. Colour scheme as in Fig. 1. dgp, digestive gland process; lac, lacuna of the ventrolateral sinus. Scale bars, 100 μm.
YKLP 12387. a–d, XCT sections from ventral (a,b) to dorsal (c,d) surface of dorsal lobe, showing internal structure of head and trunk. e–j, Transverse XCT sections from anterior (e) to posterior (j) of head. D, dorsal; V, ventral. k–m, Ventral view of head, showing external XCT volume render (k) and segmentation of chambers without (l) and with (m) ventral structures. n, Sublateral view. Colour scheme as in Fig. 1. an, anterior compartment of first appendage; cc, circumpharyngeal connective; co, circumoral ring; e, eye; mm, transverse medial membrane of the central body; po, posterior compartment of first appendage; sp, subpharyngeal gland; µb, microboring. Scale bars, 200 μm.
Diagnosis. Euarthropod with paired glands dorsal to lobopods. Bulbous anterior appendages adjacent to ventral mouth. Perivisceral cavity incompletely partitioned by transverse membranes of connective tissue. Ventrolateral sinuses with serially repeated dorsal lacunae. Prominent dorsal head lobe with paired dorsal projections. Subdivided brain with discrete frontal body.
Preservation. Orsten-type preservation11 typically replicates chitinous cuticle in amorphous apatite; preservation of more labile tissue12 is rare. Concretions within the Yu’anshan Formation are exceptional in preserving non-chitinous material, including coprolites and muscle, at exquisite resolution9,13. This material is often penetrated by post-phosphatization microborings13 with diameters on the scale of 10 µm. Secondary encrustations of diagenetic phosphate, although evident in similar deposits14, are absent. Small grains of diagenetic minerals (Figs. 1f and 2b) are readily identified by their higher X-ray attenuation, which corresponds to a higher greyscale value.
Although the limited material available cannot support a detailed taphonomic model, the high fidelity indicates an early onset of phosphatization, with differential preservation of different tissue types9. In YKLP 12387, preservation is restricted to the integument and connective tissue, leaving behind voids that correspond to the outlines of non-phosphatized tissue.
Description
YKLP 12387 is curved, with 20 segments (Fig. 1a–d). Paired ventrolateral lobopods emerge from the midpoint of each segment (Figs. 1a–d,g–j and 2a,c–g). The specimen is 3,900 µm long and reaches 900 µm high by 360 µm wide. Appendage one is highly modified; appendage two is incompletely preserved (Figs. 1a,b and 3a). Appendage four is the longest, with each subsequent appendage being progressively shorter. The appendages transition from subcylindrical to subconical posteriad (Fig. 1a,b). The main cavity within the trunk, interpreted as perivisceral, surrounds the gut and extends into the appendages (Figs. 1c–k and 2 and Extended Data Figs. 1 and 2). It is flanked by dorsal, ventral and ventrolateral sinuses, and surrounded by a peripheral cavity (Figs. 1c–k and 2c,d and Extended Data Figs. 1 and 2).
The gut is an unornamented tube that lies centrally within the perivisceral cavity, opening directly through the mouth (with no buccal cavity), and terminating blindly in the final segment (Figs. 1a–d,i–k and 2c,d). There is no evidence of differentiation within the gut, although the gut is not preserved between segments 2–12 (Extended Data Figs. 1 and 2). We interpret the abrupt change in gut preservation at the posterior limit of segment 1 (Fig. 1c and Extended Data Figs. 3d–h and 4k–n) as denoting the end of the foregut and thus the position of the stomodeum15.
The gut attaches to the wall of the perivisceral cavity with lateral and ventral ligaments and a dorsal membrane (Figs. 1g–j and 2a,d). The dorsal membrane connects to a tubular dorsal sinus (Extended Data Figs. 1b and 2b) with a fenestrated wall (Fig. 1f and Extended Data Fig. 5b) and a fluctuating width (Fig. 1f–k and Extended Data Figs. 2b and 5b): it is sub-circular in transverse section where the legs emerge from each segment, but four times narrower between these points. On the basis of its position, size, shape and surface texture, we interpret this sinus as pericardial.
A pair of ventrolateral sinuses run along the base of the perivisceral cavity (Figs. 1d,g–k and 2c,d and Extended Data Figs. 1e and 2e). In the posterior region of each segment, a lacuna extends dorsolaterally from each sinus, following the floor of the perivisceral cavity (Figs. 1d,h,j and 2c,f,g). A further ventral sinus opens dorsally into the perivisceral cavity through fenestrae situated between these lacunae (Fig. 1e,g,j and Extended Data Figs. 1f, 2f and 4s,t). The anterior of this sinus connects to a ring that surrounds the opening of the mouth (Figs. 1d and 3e–j,m,n and Extended Data Figs. 1f, 2f, 3l–o and 4a–o). We interpret the sinus as a perineural sinus, and the ring around the mouth as housing a circumoral nerve ring.
The perineural sinus opens ventrally into a peripheral cavity that surrounds the perivisceral cavity (Extended Data Figs. 1g and 4o–t), and is interrupted at irregular intervals (approximately every three appendages) by oblique membranes (Figs. 1f,k and 2b and Extended Data Fig. 5). The peripheral cavity forms the main chamber within each appendage, which surrounds a sacculus of the perivisceral cavity (Figs. 1d,g,i,j and 2e–g and Extended Data Figs. 3k–o, 4t and 5).
A dorsolateral pair of lunate voids occur at the midpoint of each segment (Extended Data Figs. 1c and 2c). These extend dorsally to the margin of the pericardial sinus, and ventrally to the level of the appendages (Fig. 1d–k); they connect to the dorsal gut via a central process (Fig. 2c). Internally, each void contains branching or anastomosing tubes that extend from its medial inner surface to its outer margin (Fig. 2a,b). We interpret the voids as digestive glands, as their morphology, position and internal structure correspond to digestive glands in Cambrian euarthropods16.
The perivisceral cavity is incompletely partitioned by serially repeated transverse membranes of connective tissue that house the digestive glands and the lacunae of the ventrolateral sinuses, and to which the gut ligaments attach (Figs. 1c–j and 2a–d). A diminutive vessel runs along the ventral midline of the cavity (Figs. 1e,i and 2c and Extended Data Fig. 5).
Taking these organ systems together, we can thus define a typical body segment as containing: one pair of appendages; one pair of ventrolateral sinus lacunae; one pair of digestive glands; and ligaments that link the gut to a connective membrane (Fig. 4). The only notable differences between trunk segments are changes in the relative proportions of each lacunar system in line with the posteriad reduction in segment size. The exception is the highly modified first segment, which we term the ‘head’. Like other segments, it contains a pair of (modified) appendages, a single (reduced) pair of lacunae of the ventrolateral sinuses, a single pair of digestive glands, and a single (enlarged) transverse membrane within the (significantly reduced) perivisceral cavity (Fig. 3). It additionally bears a dorsal lobe, comprising a pair of dorsal projections, a complex of connected chambers that presumably housed the brain, and further structures that have no equivalents in subsequent segments (Figs. 1a–d,k and 3).
The head appendages form a pair of domed protrusions lateral to the mouth (Figs. 1a–d and 3k–n). Internally, each of these appendages houses a teardrop-shaped chamber that is divided into an anterior and posterior compartment (Fig. 3h–j,l–n and Extended Data Figs. 3i–k and 4e–j). The base of the anterior compartment is contiguous with the circumoral nerve ring (Fig. 3i,m and Extended Data Figs. 3k–m and 4h); by comparison with subsequent appendages, we interpret the posterior compartment as representing a detached sacculus of the perivisceral cavity (Figs. 1d,j, 2d–g and 3i,j,l and Extended Data Figs. 3h–k and 4g–l). The dorsal projections, by contrast, comprise a single undivided cavity that is continuous with the brain (Figs. 1a,k and 3b,c,j,n and Extended Data Figs. 2g, 3b–e and 4g–m), demonstrating that they are not appendicular in origin.
The brain itself—or strictly, the void in which it sat—comprises a frontal, central and dorsolateral body (Fig. 3a–i,l–n and Extended Data Fig. 2g,h). The frontal body is a largely undifferentiated wedge-shaped structure, separate from the rest of the brain except for a posterior connection with the central body (Fig. 3b,g and Extended Data Fig. 3e–j). A transverse median membrane divides the central body into anterior and posterior divisions, which fuse laterally (Fig. 3b,c,h and Extended Data Fig. 3a–k). The anterior division of the central body connects to the perineural sinus, via ventral circumpharyngeal connectives (Fig. 3a,g,h,l); and into the dorsolateral body (Fig. 3a–c,f–i), which caps the head and fills the dorsal projections (Fig. 3a–j,l–n and Extended Data Figs. 2h and 4e–g).
The head also contains subsidiary chambers. We interpret lateral sub-spherical voids (Fig. 3a,g–i,l–n and Extended Data Figs. 3e–i and 4d–i) as eyes. Immediately behind the mouth, within and slightly dorsal to the circumoral nerve ring, is a self-contained three-lobed structure, potentially opening ventro-anteriad into the digestive tract (Figs. 1d and 3i–j,m and Extended Data Figs. 3l–n and 4h–j). This subpharyngeal structure is tentatively interpreted as a digestive or masticatory gland, although comparison might also be made with the subpharyngeal ganglion of certain tardigrades17. Finally, an irregular chamber overlies the dorsolateral body of the brain and continues into the second segment on the right-hand side of the body, external to all other cavities (Figs. 1c,d,k and 3c,d,j and Extended Data Figs. 2i and 4i–t). Given its asymmetric location and its position dorsal to the brain and peripheral cavity, we interpret this as dorsal extra-embryonic ectoderm forming an originally yolk-filled trophic vesicle18. At points, the ventral wall of this chamber connects (possibly taphonomically) into a medial vessel of varying width that also runs dorsal to the peripheral cavity, extending posteriad from the dorsolateral body of the brain to the posterior limit of segment four (Figs. 1k and 3c,d and Extended Data Figs. 1h, 2g, 3a,b and 4l–t).
Affinity
The dorsal ectoderm, blind gut and curved body identify YKLP 12387 as a larva. The preserved morphology (Fig. 4) combines euarthropod synapomorphies such as serially repeated digestive glands16 with panarthropod plesiomorphies not found in the euarthropod crown group, such as fluid-filled lobopods19. In combination, these characters place Youti in the euarthropod stem group.
a, Organ system disposition in sagittal view. Dotted lines denote location of sections shown in e,f. b, Organ system disposition in transverse view. c,d, Head, from lateral perspective (c) and as medial transverse section (d). e,f, Transverse sections through trunk at location of digestive glands (e) and transverse membranes (f). g–j, Coronal sections through head, from ventral (g) to dorsal (j) planes. Colour scheme as in Fig. 1.
The first-order relationships of stem group euarthropods are reasonably established (Fig. 5), with many key nodes consistently recovered and defined by morphological synapomorphies10. As this phylogenetic framework emphasizes adult morphology, the interpretation of Youti requires a degree of caution: the absence of features such as annulations, claws, setal blades, or compound eyes, or the location of its first appendages, may reflect its ontogenetic stage rather than its phylogenetic position.
Phylogenetic analysis situates Youti yuanshi within the AOPK clade containing Anomalocaris, Opabinia, Pambdelurion and Kerygmachela. Under our preferred model, the circumoral brain ring of cycloneuralians corresponds to the panarthropod prosocerebrum, which innervates the first appendage pair (onychophoran antennae, tardigrade stylets or euarthropod labrum). We interpret the archicerebrum as a distinct development dorsal to the prosocerebrum, associated with sensory receptors: specifically the eyes, and the dorsal projections (Kerygmachela rostral spines, tardigrade cirri, crustacean frontal filaments or anterior paired projections of stem euarthropods; homology with the anteriormost onychophoran lip papillae is plausible, but may not be parsimonious). The taxa depicted in this figure are selected in order to depict the evolutionary context of Youti; the relationships shown are recovered under all analytical conditions.
This said, the presence of midgut glands places the fossil crownwards of Hadranax (Fig. 5). The anterodorsal head lobe supports a position within the ‘AOPK’ group containing Anomalocaris, Opabinia, Pambdelurion and Kerygmachela, whereas the ventral position of the mouth and the apparent absence of dorsal nodes point to a position crownward of Kerygmachela, and—unless sclerotization and segmented appendages arose at a later ontogenetic stage—outside the sub-clade containing radiodonts and crown euarthropods.
Quantitative phylogenetic analysis (summarized in Fig. 5 and Supplementary Information) confirms this position as the most parsimonious, despite the absence of any obvious precursor to dorsal flaps or setal blades: flaps may have evolved multiple times in derived groups20, or be secondarily or ontogenetically absent in Youti. To explore the possible influence of ontogeny, we repeated our phylogenetic analyses after re-coding as ambiguous any character in Youti whose state at adulthood is not unequivocal. This extremely conservative analysis also places Youti immediately stemwards of radiodonts, though with less resolution regarding its relationship to Pambdelurion and Kerygmachela. Whereas our interpretation is not contingent on the relationships between the panarthropod phyla, our analyses recover very strong support (Bayes factor = 11.6) for Tactopoda over Arthropoda (Supplementary Information).
Discussion
Digestive glands
Gut glands, which denote carnivory16, are offset posteriad within the segments of Youti, as in lobopodians16,21, dinocaridids22 and upper-stem euarthropods5, showing that this offset denotes the ancestral euarthropod condition.
Circulatory system
The onychophoran-like19 configuration of the peripheral haemolymph system in Youti appendages corroborates the functional equivalence of Cambrian and modern lobopods. The proximity of digestive glands to the pericardial sinus presages the integration of gut glands into the euarthropod (and specifically crustacean) lacunar system23,24, and the distribution of the pericardial and ventrolateral sinuses corroborates reconstructions of the ancestral euarthropod vascular system based on modern19,25 and fossil3,26 representatives, alleviating concerns that vascular systems have been misinterpreted in compression fossils27.
In turn, the broad peripheral lacuna that extends into appendages presents a biological interpretation for enigmatic structures in fossil taxa, such as the internal structure at the centre of many lobopods4,28, the ambiguous internal structure in Thanahita29, and the ‘tonguelets’30,31 and the interpreted alimentary canal32 of radiodonts and higher euarthropods. The perivisceral cavity offers a potential interpretation for the axial structure of Opabinia33, which circumscribes digestive glands (fig. 3c in ref. 33) and extends into lobopods20,21; and the ‘pharynx’ of Megadictyon (fig. 1a,b in ref. 16), which extends into the proximal lobopods. Structures interpreted as a nervous system in Cardiodictyon15 match the position and arrangement of the perineural sinus and peripheral cavity in Youti, including a ventral region that extends into the appendages, and a thin peripheral sheath that encloses the lateral flanks of the trunk.
Nervous system
The panarthropod protocerebrum comprises a developmentally anterior prosocerebrum34, associated with the expression of six335,36 (also known as optix), which innervates the first appendage pair and is associated with the mouth early in development37; and a posterior archicerebrum, associated with orthodenticle, which innervates the eyes and frontal sensory elements (Fig. 5). In Youti, we interpret the circumoral nerve ring as prosocerebral based on its integration with the mouth and first appendages, and the dorsal lobe as archicerebral due to its association with the eyes and non-appendicular dorsal projections. The Youti archicerebrum likely corresponds to the concentration of neural tissue within the Kerygmachela anterior lobe38; the ‘median eyes’ in Stanleycaris31, Kylinxia39 and Opabinia40; and the frontal organ of higher euarthropods41, whose archicerebral nature is corroborated by its position, sensory role, and association with stalked eyes41,42,43,44 (Fig. 5).
Given its deep position in the euarthropod stem group, we expect Youti to retain some characteristics inherited from the ancestral panarthropod, as well as displaying a subset of derived euarthropod characteristics. Features shared with onychophorans are presumably inherited from their common ancestor with euarthropods: (1) the connection of the perineural sinus(es) to the perivisceral sinus and to the main lobopod cavities19; and (2) the configuration of the protocerebrum from an undifferentiated frontal body and a central body that is subdivided by a transverse membrane into laterally fusing anterior and posterior divisions45.
Characters inferred to be inherited from the ancestral panarthropod include: (1) the protocerebral brain, confirming that the euarthropod deutocerebrum has a separate origin from that of onychophorans10,36; (2) the frontal filaments44,46; and (3) the circumoral component of the brain, whose vestiges may be represented by circumoesophageal neural tissue in radiodonts31 (Fig. 5).
If the prosocerebral nerve ring of Youti corresponds to the circumoral nerve ring of cycloneuralians47,48 (Fig. 5), then the archicerebrum can be considered as a posterodorsal elaboration of the brain within the panarthropod lineage, potentially accompanying the specialization of sensory structures within this group. The tardigrade brain may reflect an intermediate situation, in which a prosocerebrum, which innervates the appendages (stylets)17, and together with the outer connectives forms a ring around the digestive tract49, is not prominently differentiated37 from an outer, dorsolateral archicerebrum that innervates the eyes and frontal sensory elements (cirri)17,50 (Fig. 5).
Conclusion
The three-dimensionally preserved organ systems in Youti offer a revised template for the interpretation of carbonaceous compression fossils. The lacunar system provides a compelling interpretation for structures otherwise attributed to the digestive or nervous systems, or dismissed as taphonomic artefacts. The separate integrations of the deutocerebrum into euarthropod and onychophoran heads denote parallel increases in complexity, indicating that early euarthropod fossils are expected to exhibit protocerebral brains. Segmentally arranged haemolymph chambers and digestive glands denote the sophisticated internal anatomy attained by euarthropods before the arthropodization of the body wall. Together, these observations clarify the sequence of evolutionary events that established Euarthropoda as a diverse and dominant presence in Phanerozoic ecosystems.
Methods
X-ray computed tomography
XCT was conducted on the imaging branch of beamline I13, Diamond Light Source under a polychromatic (pink) beam using a 3.2 mm aluminium filter and a 120 ms exposure. One thousand projections were collected during a 180° rotation of the fossil at 4× magnification on a pco.edge camera with an effective pixel size of 1.625 µm. Data were reconstructed using I13 standard filter back projection protocols.
3D visualization and analysis
The XCT data were visualized and analysed in Avizo 3D 2022.2 (Thermo Fisher Scientific). Gross anatomy was visualized using ‘standard’ volume rendering of the greyscale reconstructed data, highlighting finer details with the Edge 2D and Edge 3D functions and ‘diffuse’ lighting. Digestive gland anatomy was rendered using ‘physical’ rendering, ‘phong’ lighting and a ‘glossy’ material style. Virtual dissections were achieved using an optimally rotated orthoslice.
The distinct organ structures were semi-manually segmented (Segmentation Workroom) using a combination of the ‘magic wand’ tool (to select a specific range of greyscale values within an additional contrast limit) and the ‘paint brush’ tool for further refinement and subjective delimitation of adjacent and continuous structures into discrete labelled objects. Small structures with complex geometry were manually segmented in each slice, while larger and simpler structures were segmented in every 5th–20th slice (at a resolution sufficient to capture their morphological variation), with the volumes defined by interpolation. Surfaces of the refined labelled objects were rendered using an unconstrained smoothing (extents varying between 3 and 9 depending on the size of the structure). Surface renders were compiled and adjusted as layers in the GNU Image Manipulation Program, version 2.10.32. Additional virtual greyscale dissections were visualized in Dragonfly (Object Research Systems); colour palettes were generated using iwanthue (M. Jacomy).
Electron microscopy
Scanning electron microscopy was conducted using a Phillips scanning electron microscope at 20 kV and a JCM-6000 bench-top scanning electron microscope at 10 kV.
Phylogenetic analysis
We conducted phylogenetic analysis on a matrix of 59 taxa and 154 morphological characters (MorphoBank51 project 3927); 149 characters were drawn from previous studies3,4,52,53,54,55,56,57,58,59,60,61,62, and 5 further characters added to capture details pertinent to this study. All character formulations were updated to reflect the homology framework of ref. 44. Taxa were scored according to a conservative survey of published literature, following principles of best practice63,64,65.
Parsimony analysis used implied66 and equal weights, using the R67 package TreeSearch68 to conduct heuristic search with the parsimony ratchet69 using an approximate correction for inapplicable characters64,70. Convergence of tree search onto optimal trees was verified by inspecting the progress of tree search on two-dimensional mappings of the clustering information distance between trees68,71,72.
Bayesian analysis employed the Mk model73 with gamma-distributed rate variation across characters73, and a Dirichlet prior distribution on branch lengths74,75. We ran four runs of eight chains in MrBayes 3.2.7a76, discarding the first 100,000 generations as burn-in before sampling every 500th generation for 900,000 generations. Convergence was indicated by minimum estimated sample sizes >500 and potential scale reduction factors77 of 1.000 for all parameters. The information content of summary trees was maximized by omitting rogue taxa78.
Schematics in Fig. 5 follow data presented in refs. 17,26,31,35,36,37,38,44,45,49,79,80,81,82,83.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Fossil material is accessioned at the Key Laboratory for Palaeobiology, Yunnan University, Kunming, China (YKLP). Scanning electron microscopy data, virtual dissections and raw XCT data are available at FigShare84. Phylogenetic data, results and scripts for analysis are available at MorphoBank51 (https://morphobank.org/permalink/?P3927).
Code availability
Templates for our phylogenetic workflows are available at https://github.com/smithlabdurham/phylo-workflow and are archived at Zenodo85.
References
Budd, G. E. The morphology and phylogenetic significance of Kerygmachela kierkegaardi Budd (Buen Formation, Lower Cambrian, N Greenland). Trans. R. S. Edinb. Earth Sci. 89, 249–290 (1998).
Smith, M. R. & Ortega-Hernández, J. Hallucigenia’s onychophoran-like claws and the case for Tactopoda. Nature 514, 363–366 (2014).
Ma, X.-Y., Cong, P.-Y., Hou, X.-G., Edgecombe, G. D. & Strausfeld, N. J. An exceptionally preserved arthropod cardiovascular system from the early Cambrian. Nat. Commun. 5, 3560 (2014).
Smith, M. R. & Caron, J.-B. Hallucigenia’s head and the pharyngeal armature of early ecdysozoans. Nature 523, 75–78 (2015).
Ortega-Hernández, J., Fu, D., Zhang, X. & Shu, D. Gut glands illuminate trunk segmentation in Cambrian fuxianhuiids. Curr. Biol. 28, R146–R147 (2018).
Ortega-Hernández, J., Lerosey-Aubril, R. & Pates, S. Proclivity of nervous system preservation in Cambrian Burgess Shale-type deposits. Proc. R. Soc. B 286, 20192370 (2019).
Hughes, N. C., Haug, J. T. & Waloszek, D. in Evolving Pathways: Key Themes in Evolutionary Developmental Biology (eds Minelli, A. & Fusco, G.) 281–298 (Cambridge Univ. Press, 2008).
Maas, A. & Waloszek, D. Cambrian derivatives of the early arthropod stem lineage, pentastomids, tardigrades and lobopodians — an ‘Orsten’ perspective. Zool. Anz. 240, 451–459 (2001).
Zhang, X.-G., Smith, M. R., Yang, J. & Hou, J.-B. Onychophoran-like musculature in a phosphatized Cambrian lobopodian. Biol. Lett. 12, 20160492 (2016).
Ortega-Hernández, J. Making sense of ‘lower’ and ‘upper’ stem-group Euarthropoda, with comments on the strict use of the name Arthropoda von Siebold, 1848. Biol. Rev. 91, 255–273 (2016).
Shen, C. et al. The search for Orsten-type fossils in southern China. Palaeoworld 22, 1–9 (2013).
Eriksson, M. E., Terfelt, F., Elofsson, R. & Marone, F. Internal soft-tissue anatomy of Cambrian ‘Orsten’ arthropods as revealed by synchrotron X-ray tomographic microscopy. PLoS ONE 7, e42582 (2012).
Zhang, X.-G. & Pratt, B. R. Microborings in Early Cambrian phosphatic and phosphatized fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 267, 185–195 (2008).
Peel, J. S. A phosphatised fossil Lagerstätte from the middle Cambrian (Wuliuan Stage) of North Greenland (Laurentia). Bull. Geol. Soc. Den. 72, 101–122 (2023).
Strausfeld, N. J., Hou, X.-G., Sayre, M. E. & Hirth, F. The lower Cambrian lobopodian Cardiodictyon resolves the origin of euarthropod brains. Science 378, 905–909 (2022).
Vannier, J., Liu, J.-N., Lerosey-Aubril, R., Vinther, J. & Daley, A. C. Sophisticated digestive systems in early arthropods. Nat. Commun. 5, 3641 (2014).
Persson, D. K., Halberg, K. A., Jørgensen, A., Møbjerg, N. & Kristensen, R. M. Brain anatomy of the marine tardigrade Actinarctus doryphorus (Arthrotardigrada). J. Morphol. 275, 173–190 (2014).
Walker, M. H. & Tait, N. N. Studies of embryonic development and the reproductive cycle in ovoviviparous Australian Onychophora (Peripatopsidae). J. Zool. 264, 333–354 (2004).
Jahn, H., Hammel, J. U., Göpel, T., Wirkner, C. S. & Mayer, G. A multiscale approach reveals elaborate circulatory system and intermittent heartbeat in velvet worms (Onychophora). Commun. Biol. 6, 468 (2023).
Lerosey-Aubril, R. & Ortega-Hernández, J. A new lobopodian from the middle Cambrian of Utah: did swimming body flaps convergently evolve in stem-group arthropods? Pap. Palaeontol. 8, e1450 (2022).
Budd, G. E. & Daley, A. C. The lobes and lobopods of Opabinia regalis from the middle Cambrian Burgess Shale. Lethaia 45, 83–95 (2012).
Briggs, D. E. G. & Robison, R. A. Exceptionally preserved nontrilobite arthropods and Anomalocaris from the Middle Cambrian of Utah. Univ. Kansas Paleontol. Contrib. 111, http://hdl.handle.net/1808/3656 (1984).
Göpel, T. & Wirkner, C. S. The circulatory system of Penaeus vannamei Boone, 1931—lacunar function and a reconsideration of the ‘open vs. closed system’ debate. J. Morphol. 281, 500–512 (2020).
Wirkner, C. S. & Richter, S. in Functional Morphology and Diversity Vol. 1 (eds Watling, L. & Thiel, M.) 376–412 (Oxford Univ. Press, 2013).
Wirkner, C. S., Tögel, M. & Pass, G. in Arthropod Biology and Evolution (eds Minelli, A., Boxshall, G. & Fusco, G.) 343–391 (Springer, 2013).
Cong, P.-Y., Ma, X.-Y., Hou, X.-G., Edgecombe, G. D. & Strausfeld, N. J. Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513, 538–542 (2014).
Liu, J.-N., Steiner, M., Dunlop, J. A. & Shu, D. Microbial decay analysis challenges interpretation of putative organ systems in Cambrian fuxianhuiids. Proc. R. Soc. B 285, 20180051 (2018).
Vannier, J. & Martin, E. L. O. Worm-lobopodian assemblages from the Early Cambrian Chengjiang biota: Insight into the ‘pre-arthropodan ecology’? Palaeogeogr. Palaeoclimatol. Palaeoecol. 468, 373–387 (2017).
Siveter, D. J., Briggs, D. E. G., Siveter, D. J., Sutton, M. D. & Legg, D. A three-dimensionally preserved lobopodian from the Herefordshire (Silurian) Lagerstätte, UK. R. Soc. Op. Sci. 5, 172101 (2018).
Aria, C., Caron, J.-B. & Gaines, R. A large new leanchoiliid from the Burgess Shale and the influence of inapplicable states on stem arthropod phylogeny. Palaeontology 58, 629–660 (2015).
Moysiuk, J. & Caron, J.-B. A three-eyed radiodont with fossilized neuroanatomy informs the origin of the arthropod head and segmentation. Curr. Biol. 32, 3302–3316.e2 (2022).
Berks, H. O. et al. A possibly deep branching artiopodan arthropod from the lower Cambrian Sirius Passet Lagerstätte (North Greenland). Pap. Palaeontol. 9, e1495 (2023).
Zhang, X.-L. & Briggs, D. E. G. The nature and significance of the appendages of Opabinia from the Middle Cambrian Burgess Shale. Lethaia 40, 161–173 (2007).
Urbach, R. & Technau, G. M. Segment polarity and DV patterning gene expression reveals segmental organization of the Drosophila brain. Development 130, 3607–3620 (2003).
Eriksson, B. J., Samadi, L. & Schmid, A. The expression pattern of the genes Engrailed, Pax6, Otd and Six3 with special respect to head and eye development in Euperipatoides kanangrensis Reid 1996 (Onychophora: Peripatopsidae). Dev. Genes Evol. 223, 237–246 (2013).
Smith, F. W., Cumming, M. & Goldstein, B. Analyses of nervous system patterning genes in the tardigrade Hypsibius exemplaris illuminate the evolution of panarthropod brains. EvoDevo 9, 19 (2018).
Smith, F. W., Bartels, P. J. & Goldstein, B. A hypothesis for the composition of the tardigrade brain and its implications for panarthropod brain evolution. Integr. Comp. Biol. 57, 546–559 (2017).
Park, T. Y. S. et al. Brain and eyes of Kerygmachela reveal protocerebral ancestry of the panarthropod head. Nat. Commun. 9, 1019 (2018).
O’Flynn, R. J. et al. The early Cambrian Kylinxia zhangi and evolution of the arthropod head. Curr. Biol. 33, 4006–4013.e2 (2023).
Dhungana, A. The origin and early evolution of Panarthropoda. PhD Thesis, Univ. Durham (2024).
Ortega-Hernández, J. Homology of head sclerites in Burgess Shale euarthropods. Curr. Biol. 25, 1625–1631 (2015).
Budd, G. E. Head structure in upper stem-group euarthropods. Palaeontology 51, 561–573 (2008).
Daley, A. C. & Edgecombe, G. D. Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88, 68–91 (2014).
Budd, G. E. The origin and evolution of the euarthropod labrum. Arthropod Struct. Dev. 62, 101048 (2021).
Martin, C. et al. The velvet worm brain unveils homologies and evolutionary novelties across panarthropods. BMC Biol. 20, 26 (2022).
Ortega-Hernández, J. & Budd, G. E. The nature of non-appendicular anterior paired projections in Palaeozoic total-group Euarthropoda. Arthropod Struct. Dev. 45, 185–199 (2016).
Schmidt-Rhaesa, A. & Henne, S. in Structure and Evolution of Invertebrate Nervous Systems (eds Schmidt-Rhaesa, A., Harzsch, S. & Purschke, G.) 368–382 (Oxford Univ. Press, 2015).
Henne, S., Friedrich, F., Hammel, J. U., Sombke, A. & Schmidt-Rhaesa, A. Reconstructing the anterior part of the nervous system of Gordius aquaticus (Nematomorpha, Cycloneuralia) by a multimethodological approach. J. Morphol. 278, 106–118 (2017).
Gross, V. et al. X-ray imaging of a water bear offers a new look at tardigrade internal anatomy. Zool. Lett. 5, 14 (2019).
Schulze, C. & Persson, D. in Structure and Evolution of Invertebrate Nervous Systems (eds Schmidt-Rhaesa, A., Harzsch, S. & Purschke, G.) 383–389 (Oxford Univ. Press, 2015).
O’Leary, M. A. & Kaufman, S. MorphoBank: phylophenomics in the ‘cloud’. Cladistics 27, 529–537 (2011).
Daley, A. C., Budd, G. E., Caron, J.-B., Edgecombe, G. D. & Collins, D. H. The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323, 1597–1600 (2009).
Tanaka, G., Hou, X.-G., Ma, X.-Y., Edgecombe, G. D. & Strausfeld, N. J. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364–367 (2013).
Vinther, J., Stein, M., Longrich, N. R. & Harper, D. A. T. A suspension-feeding anomalocarid from the Early Cambrian. Nature 507, 496–9 (2014).
Van Roy, P., Daley, A. C. & Briggs, D. E. G. Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522, 77–80 (2015).
Yang, J. et al. A superarmored lobopodian from the Cambrian of China and early disparity in the evolution of Onychophora. Proc. Natl Acad. Sci. USA 112, 8678–8683 (2015).
Yang, J. et al. Fuxianhuiid ventral nerve cord and early nervous system evolution in Panarthropoda. Proc. Natl Acad. Sci. USA 113, 2988–2993 (2016).
Aria, C. & Caron, J.-B. A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586–589 (2019).
Moysiuk, J. & Caron, J.-B. A new hurdiid radiodont from the Burgess Shale evinces the exploitation of Cambrian infaunal food sources. Proc. R. Soc. B 286, 20191079 (2019).
Zeng, H., Zhao, F., Niu, K., Zhu, M. & Huang, D. An early Cambrian euarthropod with radiodont-like raptorial appendages. Nature 588, 101–105 (2020).
Moysiuk, J. & Caron, J.-B. Exceptional multifunctionality in the feeding apparatus of a mid-Cambrian radiodont. Paleobiology 47, 704–724 (2021).
Pates, S., Wolfe, J. M., Lerosey-Aubril, R., Daley, A. C. & Ortega-Hernández, J. New opabiniid diversifies the weirdest wonders of the euarthropod stem group. Proc. R. Soc. B 289, 20212093 (2022).
Brazeau, M. D. Problematic character coding methods in morphology and their effects. Biol. J. Linn. Soc. 104, 489–498 (2011).
Brazeau, M. D., Guillerme, T. & Smith, M. R. An algorithm for morphological phylogenetic analysis with inapplicable data. Syst. Biol. 68, 619–631 (2019).
Simões, T. R., Caldwell, M. W., Palci, A. & Nydam, R. L. Giant taxon-character matrices: Quality of character constructions remains critical regardless of size. Cladistics 33, 198–219 (2017).
Goloboff, P. A. Estimating character weights during tree search. Cladistics 9, 83–91 (1993).
R Core Team. R: A Language and Environment for Statistical Computing. http://www.R-project.org/ (R Foundation for Statistical Computing, 2023).
Smith, M. R. TreeSearch: Morphological phylogenetic analysis in R. R J. 14, 305–315 (2023).
Nixon, K. C. The parsimony ratchet, a new method for rapid parsimony analysis. Cladistics 15, 407–414 (1999).
Brazeau, M. D., Smith, M. R. & Guillerme, T. MorphyLib: A library for phylogenetic analysis of categorical trait data with inapplicability. Zenodo https://doi.org/10.5281/zenodo.815372 (2017).
Smith, M. R. Information theoretic generalized Robinson–Foulds metrics for comparing phylogenetic trees. Bioinformatics 36, 5007–5013 (2020).
Smith, M. R. Robust analysis of phylogenetic tree space. Syst. Biol. 71, 1255–1270 (2022).
Lewis, P. O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001).
Rannala, B., Zhu, T. & Yang, Z. Tail paradox, partial identifiability, and influential priors in Bayesian branch length inference. Mol. Biol. Evol. 29, 325–335 (2012).
Zhang, C., Rannala, B. & Yang, Z. Robustness of compound Dirichlet priors for Bayesian inference of branch lengths. Syst. Biol. 61, 779–84 (2012).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Smith, M. R. Using information theory to detect rogue taxa and improve consensus trees. Syst. Biol. 71, 1088–1094 (2022).
Liu, Y., Hou, X.-G. & Bergström, J. Chengjiang arthropod Leanchoilia illecebrosa (Hou, 1987) reconsidered. GFF 129, 263–272 (2007).
Lan, T. et al. Leanchoiliidae reveals the ancestral organization of the stem euarthropod brain. Curr. Biol. 31, 4397–4404.e2 (2021).
Yang, J., Ortega-Hernández, J., Butterfield, N. J. & Zhang, X.-G. Specialized appendages in fuxianhuiids and the head organization of early euarthropods. Nature 494, 468–471 (2013).
Ma, X.-Y., Hou, X.-G., Edgecombe, G. D. & Strausfeld, N. J. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261 (2012).
Ma, X., Edgecombe, G. D., Hou, X., Goral, T. & Strausfeld, N. J. Preservational pathways of corresponding brains of a Cambrian euarthropod. Curr. Biol. 25, 2969–2975 (2015).
Smith, M. R. et al. Analysis of Youti yuanshi, YKLP 12387. Figshare https://doi.org/10.6084/m9.figshare.c.6490717.v2 (2024).
Smith, M. R. & Dhungana, A. Workflows for phylogenetic analysis of morphological data. Zenodo https://doi.org/10.5281/zenodo.7838056 (2023).
Acknowledgements
The authors thank H.-Q. Zhang for preparation of fossil material, and G. Edgecombe for comments on an early draft of the manuscript. M. Pankhurst and A. Bodey assisted with XCT analysis at Diamond Light Source (under proposal MT15461). We acknowledge funding from Dong Energy, the Leverhulme Trust (Research Project Grant 2019-223) and NERC (NE/M018687/2).
Author information
Authors and Affiliations
Contributions
M.R.S.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, software, validation, visualization and writing—original draft, review and editing. E.J.L.: data curation, formal analysis, funding acquisition, investigation, methodology, visualization, writing—original draft, review and editing. A.D.: data curation, methodology, validation and writing—review and editing. K.J.D.: funding acquisition, methodology, software, supervision and writing—review and editing. J.Y.: resources, investigation and writing—review and editing. X.Z.: resources, investigation and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Graham Budd and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Structure and relationship of major body cavities within Youti yuanshi.
Location of major organ systems within YKLP 12387, lateral view. a, perivisceral cavity; b, pericardial sinus; c, digestive glands, showing relationship to perivisceral cavity; d, gut and subpharyngeal gland; absence of medial gut represents non-preservation; e, ventrolateral sinus, showing relationship to perivisceral cavity; f, perineural sinus; g, peripheral cavity; h, dorsolateral body of brain with associated vessel. Abbreviations: co, circumoral ring; dv, dorsal vessel; pn, perineural sinus; sp, subpharyngeal gland.
Extended Data Fig. 2 Structure and relationship of major body cavities within Youti yuanshi.
Location of major organ systems in YKLP 12387, dorsal views. a, perivisceral cavity; b, pericardial sinus; c, digestive glands; d, preserved extent of gut; absence of medial gut represents non-preservation; e, ventrolateral sinus; f, perineural sinus; g, dorsolateral body of brain with associated vessel; h, central body of brain; i, irregular chamber, interpreted as dorsal extra-embryonic ectoderm. Abbreviations: cc, circumpharyngeal connective, co, circumoral ring; db, dorsolateral body of brain; dp, dorsal projection; dv, dorsal vessel; pn, perineural sinus.
Extended Data Fig. 3 Coronal sections through anterior of Youti yuanshi.
a–o, Virtual dissections of YKLP 12387 rendered from XCT data, from dorsal to ventral. Coloured regions identify organ systems. Raw images available at FigShare84. Abbreviations: a, appendage; an, anterior compartment of first appendage; cb, central body of brain; cc, circumpharyngeal connective; co, circumoral ring; db, dorsolateral body of brain; dg, digestive gland; dp, dorsal projection; dv, dorsal vessel; e, eye; fb, frontal body of brain; irr, irregular chamber; lac, lacuna; mm, transverse medial membrane of the central body; po, posterior compartment of first appendage; pph, peripheral cavity; sep, membrane separating compartments of the first appendage; sp, subpharyngeal gland; tm, transverse membrane; vl, ventrolateral sinus.
Extended Data Fig. 4 Transverse sections through anterior of Youti yuanshi.
a–t, Virtual dissections of YKLP 12387 rendered from XCT data, from anterior to posterior. Coloured regions identify organ systems; colours as for Extended Data Fig. 3. Raw images available at FigShare84. Abbreviations: an, anterior compartment of first appendage; cb, central body of brain; cc, circumpharyngeal connective; db, dorsolateral body of brain; dg, digestive gland; dv, dorsal vessel; e, eye; fen, fenestra; irr, irregular chamber; lac, lacuna; pc, pericardial sinus; pn, perineural sinus; po, posterior compartment of first appendage; pv, perivisceral sinus; sp, subpharyngeal gland; vl, ventrolateral sinus.
Extended Data Fig. 5 Virtual dissection of Youti yuanshi.
Enlargement of Fig. 1e–f, Rendered from XCT data parallel to coronal plane, looking a, ventrally; b, dorsally. Abbreviations: a, appendage; dg, digestive gland; dgp, digestive gland process; fen, fenestra; lig, ligament; om, oblique membrane; pc, pericardial sinus; pph, peripheral cavity; tm, transverse membrane; vv, ventral vessel.
Supplementary information
Supplementary Information
This file contains supplementary notes and Supplementary Figs. 1–8, detailing scoring of characters and summary of phylogenetic results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smith, M.R., Long, E.J., Dhungana, A. et al. Organ systems of a Cambrian euarthropod larva. Nature 633, 120–126 (2024). https://doi.org/10.1038/s41586-024-07756-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07756-8
- Springer Nature Limited