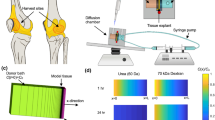
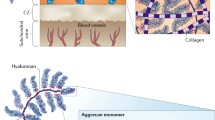
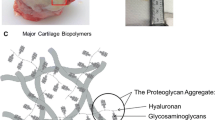
Abstract
Developing therapeutic molecules that target chondrocytes and locally produced inflammatory factors within arthritic cartilage is an active area of investigation. The extensive studies that have been conducted over the past 50 years have enabled the accurate prediction and reliable optimization of the transport of a wide variety of molecules into cartilage. In this Review, the factors that can be used to tune the transport kinetics of therapeutics are summarized. Overall, the most crucial factor when designing new therapeutic molecules is solute size. The diffusivity and partition coefficient of a solute both decrease with increasing solute size as indicated by molecular mass or by hydrodynamic radius. Surprisingly, despite having an effective pore size of ~6 nm, molecules of ~16 nm radius can diffuse through the cartilage matrix. Alteration of the shape or charge of a solute and the application of physiological loading to cartilage can be used to predictably improve solute transport kinetics, and this knowledge can be used to improve the development of therapeutic agents for osteoarthritis that target the cartilage.
Key points
-
Therapeutic agents for arthritis treatment vary widely in their capacity to diffuse through cartilage.
-
Solute size and molecular mass strongly influence the diffusivity of a molecule in cartilage.
-
Linear, flexible solutes (such as dextrans) exhibit fundamentally different transport kinetics compared with those of spherical solutes (such as antibodies).
-
Altering the solute shape or charge and applying physiological loading to cartilage can be used to predictably increase the transport of therapeutics into cartilage.
-
Even molecules that are larger than the effective pore size of cartilage (~6 nm) can diffuse through the entire depth of healthy cartilage.



Similar content being viewed by others
References
Evans, C. H., Kraus, V. B. & Setton, L. A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 10, 11–22 (2013).
Evans, C. H. Drug delivery to chondrocytes. Osteoarthritis Cartilage 24, 1–3 (2016).
Bajpayee, A. G. & Grodzinsky, A. J. Cartilage-targeting drug delivery: can electrostatic interactions help? Nat. Rev. Rheumatol. 13, 183–193 (2017).
Moos, V., Fickert, S., Müller, B., Weber, U. & Sieper, J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J. Rheumatol. 26, 870–879 (1999).
Fernandes, J. C., Martel-Pelletier, J. & Pelletier, J.-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 39, 237–246 (2002).
Hampel, U. et al. Chemokine and cytokine levels in osteoarthritis and rheumatoid arthritis synovial fluid. J. Immunol. Methods 396, 134–139 (2013).
Feldmann, M., Brennan, F. M. & Maini, R. N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14, 397–440 (1996).
Steiner, G. et al. Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology 38, 202–213 (1999).
Feldmann, M. & Maini, R. N. Anti -TNFα therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol. 19, 163–196 (2001).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Gerwin, N., Hops, C. & Lucke, A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliv. Rev. 58, 226–242 (2006).
McAlindon, T. E. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22, 363–388 (2014).
da Costa, B. R. et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 390, e21–e33 (2017).
McCabe, P. S., Maricar, N., Parkes, M. J., Felson, D. T. & O’Neill, T. W. The efficacy of intra-articular steroids in hip osteoarthritis: a systematic review. Osteoarthritis Cartilage 24, 1509–1517 (2016).
Raynauld, J.-P. et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 48, 370–377 (2003).
Garg, N., Perry, L. & Deodhar, A. Intra-articular and soft tissue injections, a systematic review of relative efficacy of various corticosteroids. Clin. Rheumatol. 33, 1695–1706 (2014).
Carubbi, F. et al. Safety and efficacy of intra-articular anti-tumor necrosis factor α agents compared to corticosteroids in a treat-to-target strategy in patients with inflammatory arthritis and monoarthritis flare. Int. J. Immunopathol. Pharmacol. 29, 252–266 (2016).
Urech, D. M. et al. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-TNFα single-chain Fv antibody (ESBA105) designed for local therapeutic use. Ann. Rheum. Dis. 69, 443–449 (2010).
Hunter, D. J. Are there promising biologic therapies for osteoarthritis? Curr. Rheumatol. Rep. 10, 19–25 (2008).
Mow, V. C., Holmes, M. H. & Michael Lai, W. Fluid transport and mechanical properties of articular cartilage: a review. J. Biomech. 17, 377–394 (1984).
Poole, A. R. et al. Composition and structure of articular cartilage: a template for tissue repair. Clin. Orthop. Relat. Res. 1, S26–S33 (2001).
Hwang, W. S. et al. Collagen fibril structure of normal, aging, and osteoarthritic cartilage. J. Pathol. 167, 425–433 (1992).
Maroudas, A. Physicochemical properties of cartilage in the light of ion exchange theory. Biophys. J. 8, 575–595 (1968).
Maroudas, A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology 12, 233–248 (1975).
Kim, Y. J., Bonassar, L. J. & Grodzinsky, A. J. The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J. Biomech. 28, 1055–1066 (1995).
Zhu, W., Mow, V. C., Koob, T. J. & Eyre, D. R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J. Orthop. Res. 11, 771–781 (1993).
Fortin, M., Soulhat, J., Shirazi-Adl, A., Hunziker, E. B. & Buschmann, M. D. Unconfined compression of articular cartilage: nonlinear behavior and comparison with a fibril-reinforced biphasic model. J. Biomech. Eng. 122, 189–195 (2000).
Albro, M. B. et al. Dynamic loading of immature epiphyseal cartilage pumps nutrients out of vascular canals. J. Biomech. 44, 1654–1659 (2011).
Sophia Fox, A. J., Bedi, A. & Rodeo, S. A. The basic science of articular cartilage: structure, composition and function. Orthopaedics 1, 461–468 (2009).
Garcia, A. M., Frank, E. H., Grimshaw, P. E. & Grodzinsky, A. J. Contributions of fluid convection and electrical migration to transport in cartilage: relevance to loading. Arch. Biochem. Biophys. 333, 317–325 (1996).
Maroudas, A. Distribution and diffusion of solutes in articular cartilage. Biophys. J. 10, 365–379 (1970).
Leddy, H. A. & Guilak, F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann. Biomed. Eng. 31, 753–760 (2003).
Leddy, H. A., Haider, M. A. & Guilak, F. Diffusional anisotropy in collagenous tissues: fluorescence imaging of continuous point photobleaching. Biophys. J. 91, 311–316 (2006).
Travascio, F. & Gu, W. Y. Simultaneous measurement of anisotropic solute diffusivity and binding reaction rates in biological tissues by FRAP. Ann. Biomed. Eng. 39, 53–65 (2011).
Fetter, N. L., Leddy, H. A., Guilak, F. & Nunley, J. A. Composition and transport properties of human ankle and knee cartilage. J. Orthop. Res. 24, 211–219 (2006).
Garcia, A. M. et al. Transport and binding of insulin-like growth factor I through articular cartilage. Arch. Biochem. Biophys. 415, 69–79 (2003).
Byun, S. et al. Transport and equilibrium uptake of a peptide inhibitor of PACE4 into articular cartilage is dominated by electrostatic interactions. Arch. Biochem. Biophys. 499, 32–39 (2010).
Maroudas, A. Transport of solutes through cartilage: permeability to large molecules. J. Anat. 122, 335–347 (1976).
Maroudas, A. & Bullough, P. Permeability of articular cartilage. Nature 219, 1260–1261 (1968).
Bajpayee, A. G., Wong, C. R., Bawendi, M. G., Frank, E. H. & Grodzinsky, A. J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials 35, 538–549 (2014).
Nimer, E., Schneiderman, R. & Maroudas, A. Diffusion and partition of solutes in cartilage under static load. Biophys. Chem. 106, 125–146 (2003).
Bonassar, L. J., Grodzinsky, A. J., Srinivasan, A., Davila, S. G. & Trippel, S. B. Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch. Biochem. Biophys. 379, 57–63 (2000).
Bonassar, L. J. et al. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J. Orthop. Res. 19, 11–17 (2001).
Buschmann, M. D. et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch. Biochem. Biophys. 366, 1–7 (1999).
Allen, K. D., Adams, S. B. & Setton, L. A. Evaluating intra-articular drug delivery for the treatment of osteoarthritis in a rat model. Tissue Eng. Part B. Rev. 16, 81–92 (2010).
Torzilli, P. A., Arduino, J. M., Gregory, J. D. & Bansal, M. Effect of proteoglycan removal on solute mobility in articular cartilage. J. Biomech. 30, 895–902 (1997).
Quinn, T. M., Kocian, P. & Meister, J. J. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch. Biochem. Biophys. 384, 327–334 (2000).
Albro, M. B., Li, R., Banerjee, R. E., Hung, C. T. & Ateshian, G. A. Validation of theoretical framework explaining active solute uptake in dynamically loaded porous media. J. Biomech. 43, 2267–2273 (2010).
Quinn, T. M., Morel, V. & Meister, J. J. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J. Biomech. 34, 1463–1469 (2001).
Chin, H. C., Moeini, M. & Quinn, T. M. Solute transport across the articular surface of injured cartilage. Arch. Biochem. Biophys. 535, 241–247 (2013).
Torzilli, P. A., Grande, D. A. & Arduino, J. M. Diffusive properties of immature articular cartilage. J. Biomed. Mater. Res. 40, 132–138 (1998).
Torzilli, P. A., Adams, T. C. & Mis, R. J. Transient solute diffusion in articular cartilage. J. Biomech. 20, 203–214 (1987).
Allhands, R. V., Torzilli, P. A. & Kallfelz, F. A. Measurement of diffusion of uncharged molecules in articular cartilage. Cornell Vet. 74, 111–123 (1984).
Evans, R. C. & Quinn, T. M. Dynamic compression augments interstitial transport of a glucose-like solute in articular cartilage. Biophys. J. 91, 1541–1547 (2006).
O’Hara, B. P., Urban, J. P. & Maroudas, A. Influence of cyclic loading on the nutrition of articular cartilage. Ann. Rheum. Dis. 49, 536–539 (1990).
Kokkonen, H. T., Chin, H. C., Töyräs, J., Jurvelin, J. S. & Quinn, T. M. Solute transport of negatively charged contrast agents across articular surface of injured cartilage. Ann. Biomed. Eng. 45, 973–981 (2017).
Shafieyan, Y., Khosravi, N., Moeini, M. & Quinn, T. M. Diffusion of MRI and CT contrast agents in articular cartilage under static compression. Biophys. J. 107, 485–492 (2014).
Decker, S. G. A., Moeini, M., Chin, H. C., Rosenzweig, D. H. & Quinn, T. M. Adsorption and distribution of fluorescent solutes near the articular surface of mechanically injured cartilage. Biophys. J. 105, 2427–2436 (2013).
Burstein, D., Gray, M. L., Hartman, A. L., Gipe, R. & Foy, B. D. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J. Orthop. Res. 11, 465–478 (1993).
Yin, Y., Zhao, C., Kuroki, S. & Ando, I. Diffusion of rodlike polypeptides with different main-chain lengths in the thermotropic liquid crystalline state as studied by the field-gradient 1H NMR method. Macromolecules 35, 2335–2338 (2002).
Foy, B. D. & Blake, J. Diffusion of paramagnetically labeled proteins in cartilage: enhancement of the 1D NMR imaging technique. J. Magn. Reson. 148, 126–134 (2001).
Honkanen, J. T. J. et al. Cationic contrast agent diffusion differs between cartilage and meniscus. Ann. Biomed. Eng. 44, 2913–2921 (2016).
Kulmala, K. A. M. et al. Diffusion coefficients of articular cartilage for different CT and MRI contrast agents. Med. Eng. Phys. 32, 878–882 (2010).
Silvast, T. S., Jurvelin, J. S., Tiitu, V., Quinn, T. M. & Töyräs, J. Bath concentration of anionic contrast agents does not affect their diffusion and distribution in articular cartilage in vitro. Cartilage 4, 42–51 (2013).
Arbabi, V., Pouran, B., Weinans, H. & Zadpoor, A. A. Transport of neutral solute across articular cartilage: the role of zonal diffusivities. J. Biomech. Eng. 137, 71001 (2015).
DiDomenico, C. D., Xiang Wang, Z. & Bonassar, L. J. Cyclic mechanical loading enhances transport of antibodies into articular cartilage. J. Biomech. Eng. 139, 11012 (2016).
DiDomenico, C. D. et al. The effect of antibody size and mechanical loading on solute diffusion through the articular surface of cartilage. J. Biomech. Eng. 139, 91005 (2017).
Ogston, A. G. The spaces in a uniform random suspension of fibres. Trans. Faraday Soc. 54, 1754 (1958).
Clague, D. S. & Phillips, R. J. Hindered diffusion of spherical macromolecules through dilute fibrous media. Phys. Fluids 8, 1720–1731 (1996).
Amsden, B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules 31, 8382–8395 (1998).
Renkin, E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J. Gen. Physiol. 38, 225–243 (1954).
Schneiderman, R. et al. Insulin-like growth factor-I and its complexes in normal human articular cartilage: studies of partition and diffusion. Arch. Biochem. Biophys. 324, 159–172 (1995).
Garcia, A. M., Lark, M. W., Trippel, S. B. & Grodzinsky, A. J. Transport of tissue inhibitor of metalloproteinases-1 through cartilage: contributions of fluid flow and electrical migration. J. Orthop. Res. 16, 734–742 (1998).
Ogston, A. G., Preston, B. N. & Wells, J. D. On the transport of compact particles through solutions of chain-polymers. Proc. R. Soc. A Math. Phys. Eng. Sci. 333, 297–316 (1973).
Ng, L. et al. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J. Struct. Biol. 143, 242–257 (2003).
Levick, J. R. Flow through interstitium and other fibrous matrices. Q. J. Exp. Physiol. 72, 409–437 (1987).
Stell, G. & Joslin, C. G. The donnan equilibrium: a theoretical study of the effects of interionic forces. Biophys. J. 50, 855–859 (1986).
Fredrickson, G. H. The theory of polymer dynamics. Curr. Opin. Solid State Mater. Sci. 1, 812–816 (1996).
de Gennes, P. G. Reptation of a polymer chain in the presence of fixed obstacles. J. Chem. Phys. 55, 572–579 (1971).
Deen, W. M. Hindered transport of large molecules in liquid-filled pores. AIChE J. 33, 1409–1425 (1987).
Pluen, A., Netti, P. A., Jain, R. K. & Berk, D. A. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys. J. 77, 542–552 (1999).
Deen, W. M., Bohrer, M. P. & Epstein, N. B. Effects of molecular size and configuration on diffusion in microporous membranes. AIChE J. 27, 952–959 (1981).
Baumgärtner, A. & Muthukumar, M. A trapped polymer chain in random porous media. J. Chem. Phys. 87, 3082 (1987).
Shen, H., Hu, Y. & Saltzman, W. M. DNA diffusion in mucus: effect of size, topology of DNAs, and transfection reagents. Biophys. J. 91, 639–644 (2006).
Yamakov, V. & Milchev, A. Diffusion of a polymer chain in porous media. Phys. Rev. E 55, 1704–1712 (1997).
Pajevic, S., Bansil, R. & Konák, C. Diffusion of linear polymer chains in methyl methacrylate gels. Macromolecules 26, 305–312 (1993).
Doi, M. & Edwards, S. F. in The theory of polymer dynamics (eds Ericksen, J. L., Kinderlehrer, D.) 218–379 (Oxford Univ. Press, 1988).
Tong, J. & Anderson, J. L. Partitioning and diffusion of proteins and linear polymers in polyacrylamide gels. Biophys. J. 70, 1505–1513 (1996).
Davidson, M. G., Suter, U. W. & Deen, W. M. Equilibrium partitioning of flexible macromolecules between bulk solution and cylindrical pores. Macromolecules 20, 1141–1146 (1987).
Truskey, G. A., Yuan, F. & Katz, D. F. Transport phenomena in biological systems (2nd Edition) (Pearson Education, 2009).
Evans, R. C. & Quinn, T. M. Solute diffusivity correlates with mechanical properties and matrix density of compressed articular cartilage. Arch. Biochem. Biophys. 442, 1–10 (2005).
Moeini, M. et al. Decreased solute adsorption onto cracked surfaces of mechanically injured articular cartilage: towards the design of cartilage-specific functional contrast agents. Biochim. Biophys. Acta 1840, 605–614 (2014).
Graham, B. T., Moore, A. C., Burris, D. L. & Price, C. Sliding enhances fluid and solute transport into buried articular cartilage contacts. Osteoarthritis Cartilage 25, 2100–2107 (2017).
Ateshian, G. A. & Weiss, J. A. in Computer Models in Biomechanics (eds Holzapfel, G. A. & Kuhl, E.) 231–249 (Springer, 2013).
Yao, H. & Gu, W. Y. Convection and diffusion in charged hydrated soft tissues: a mixture theory approach. Biomech. Model. Mechanobiol. 6, 63–72 (2007).
Evans, R. C. & Quinn, T. M. Solute convection in dynamically compressed cartilage. J. Biomech. 39, 1048–1055 (2006).
Gardiner, B. et al. Solute transport in cartilage undergoing cyclic deformation. Comput. Methods Biomech. Biomed. Engin. 10, 265–278 (2007).
Ferguson, S. J., Ito, K. & Nolte, L. P. Fluid flow and convective transport of solutes within the intervertebral disc. J. Biomech. 37, 213–221 (2004).
Quinn, T. M., Studer, C., Grodzinsky, A. J. & Meister, J. J. Preservation and analysis of nonequilibrium solute concentration distributions within mechanically compressed cartilage explants. J. Biochem. Biophys. Methods 52, 83–95 (2002).
Zhang, L., Gardiner, B. S., Smith, D. W., Pivonka, P. & Grodzinsky, A. The effect of cyclic deformation and solute binding on solute transport in cartilage. Arch. Biochem. Biophys. 457, 47–56 (2007).
Hung, C. T. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J. Biomech. Eng. 125, 602 (2003).
Entezari, V. et al. Effect of mechanical convection on the partitioning of an anionic iodinated contrast agent in intact patellar cartilage. J. Orthop. Res. 32, 1333–1340 (2014).
Nia, H. T. et al. High-bandwidth AFM-based rheology reveals that cartilage is most sensitive to high loading rates at early stages of impairment. Biophys. J. 104, 1529–1537 (2013).
Eckstein, F., Hudelmaier, M. & Putz, R. The effects of exercise on human articular cartilage. J. Anat. 208, 491–512 (2006).
Chevalier, X. et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J. Rheumatol. 32, 1317–1323 (2005).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res. 61, 344–352 (2009).
Bajpayee, A. G., Scheu, M., Grodzinsky, A. J. & Porter, R. M. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. J. Orthop. Res. 33, 660–667 (2015).
Ghosh, P. & Guidolin, D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin. Arthritis Rheum. 32, 10–37 (2002).
Winalski, C. S. et al. Enhancement of joint fluid with intravenously administered gadopentetate dimeglumine: technique, rationale, and implications. Radiology 187, 179–185 (1993).
Bajpayee, A. G., Quadir, M. A., Hammond, P. T. & Grodzinsky, A. J. Charge based intra-cartilage delivery of single dose dexamethasone using avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthritis Cartilage 24, 71–81 (2016).
Burstein, D. et al. Protocol issues for delayed Gd(DTPA)2—enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn. Reson. Med. 45, 36–41 (2001).
Hawezi, Z. K., Lammentausta, E., Svensson, J., Dahlberg, L. E. & Tiderius, C. J. In vivo transport of Gd-DTPA2- in human knee cartilage assessed by depth-wise dGEMRIC analysis. J. Magn. Reson. Imaging 34, 1352–1358 (2011).
Edwards, S. H. R. Intra-articular drug delivery: the challenge to extend drug residence time within the joint. Vet. J. 190, 15–21 (2011).
Chevalier, X., Eymard, F. & Richette, P. Biologic agents in osteoarthritis: hopes and disappointments. Nat. Rev. Rheumatol. 9, 400–410 (2013).
Owen, S., Francis, H. & Roberts, M. Disappearance kinetics of solutes from synovial fluid after intra- articular injection. Br. J. Clin. Pharmacol. 38, 349–355 (1994).
Arkill, K. P. & Winlove, C. P. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis Cartilage 16, 708–714 (2008).
Pan, J. et al. In situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 27, 1347–1352 (2009).
Hughes, C. et al. Human single-chain variable fragment that specifically targets arthritic cartilage. Arthritis Rheum. 62, 1007–1016 (2010).
Hughes, C. et al. Targeting of viral interleukin-10 with an antibody fragment specific to damaged arthritic cartilage improves its therapeutic potency. Arthritis Res. Ther. 16, R151 (2014).
Champion, J. A., Walker, A. & Mitragotri, S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 25, 1815–1821 (2008).
Horisawa, E. et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm. Res. 19, 132–139 (2002).
Palmieri, G. et al. Hyaluronic acid nanoporous microparticles with long in vivo joint residence time and sustained release. Part. Part. Syst. Charact. 34, 1600411 (2017).
Bottini, M. et al. Nanodrugs to target articular cartilage: an emerging platform for osteoarthritis therapy. Nanomedicine 12, 255–268 (2016).
Joshi, N. et al. Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 9, 1275 (2018).
Lynch, I. & Dawson, K. A. Protein-nanoparticle interactions. Nano Today 3, 40–47 (2008).
Poole, C. A. Articular cartilage chondrons: form, function and failure. J. Anat. 191, 1–13 (1997).
Alexopoulos, L. G., Haider, M. A., Vail, T. P. & Guilak, F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J. Biomech. Eng. 125, 323–333 (2003).
Leddy, H. A., Christensen, S. E. & Guilak, F. Microscale diffusion properties of the cartilage pericellular matrix measured using 3D scanning microphotolysis. J. Biomech. Eng. 130, 61002 (2008).
Maroudas, A., Bayliss, M. T. & Venn, M. F. Further studies on the composition of human femoral head cartilage. Ann. Rheum. Dis. 39, 514–523 (1980).
Venn, M. & Maroudas, A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann. Rheum. Dis. 36, 121–129 (1977).
Saarakkala, S. et al. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage 18, 73–81 (2010).
Manning, H. B. et al. Detection of cartilage matrix degradation by autofluorescence lifetime. Matrix Biol. 32, 32–38 (2013).
Kim, S. E. et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 11, 977–985 (2016).
Rothenfluh, D. A., Bermudez, H., O’Neil, C. P. & Hubbell, J. A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat. Mater. 7, 248–254 (2008).
Pi, Y. et al. Intra-articular delivery of anti-Hif-2α siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 22, 439–448 (2015).
Sacchetti, C. et al. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano 8, 12280–12291 (2014).
Pi, Y. et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials 32, 6324–6332 (2011).
DiDomenico, C. & Bonassar, L. Local solute transport kinetics are strongly correlated to local cartilage composition [abstract]. Trans. Annu. Meet. - Orthop. Res. Soc. 43, a0411 (2018).
Acknowledgements
The work of the authors was supported by the National Science Foundation (grant NSF-1536463; awarded to L.J.B. and I. Cohen). The authors also thank I. Cohen, L. Bartell, L. Fortier and M. Delco for their help in refining the message of the manuscript.
Referee accreditation statement
Nature Reviews Rheumatology thanks M. Bottini, N. E. Lane and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Review criteria
PubMed served as the primary database to identify all relevant articles using the search terms “articular cartilage” and “solute transport”. All articles that reported solute transport metrics in articular cartilage were included unless the data were collected using diseased or degraded tissue. For all other aspects of this manuscript, the most representative papers were chosen, with a bias towards articles that are recent and clinical.
Author information
Authors and Affiliations
Contributions
C.D.D. and L.J.B. wrote the article. All authors researched the data for the article, provided substantial contributions to discussions of its content, and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hydrodynamic radius
-
A molecular characteristic that quantifies the size of the solute; this characteristic assumes that the solute can be approximated as a sphere, so it might not be appropriate to use for all solutes.
- Anisotropic
-
The state of having properties that depend on direction (for example, parallel versus perpendicular to the articular surface).
- Fixed charge density
-
The concentration of charge that results from the constituent parts of the cartilage matrix, primarily determined by the presence of sulfated glycosaminoglycans.
- Poroelastic mechanical response
-
The time-dependent behaviour of cartilage, which arises from fluid movement through the porous matrix.
- Diffusion coefficient
-
A solute transport metric that quantifies how quickly diffusive transport occurs in a medium; this metric decreases rapidly with increasing solute size and also depends on other factors.
- Partition coefficient
-
A solute transport metric that quantifies the equilibrium concentration of a solute in cartilage compared with the concentration of the solute in synovial fluid; this metric often decreases with increasing solute size.
- Cyclical mechanical loading
-
The mechanical loading of cartilage tissue within the joint, which occurs over a wide range of frequencies and amplitudes, depending on the type of physical activity involved.
- Convective transport
-
Transport of solutes caused by induced fluid flow within cartilage; this type of transport can be caused by mechanical loading of the joint during walking or jumping.
- Donnan equilibrium
-
The unequal distribution of solutes across the cartilage–synovial fluid interface as a result of the high fixed charge density of the tissue, which produces high concentrations of cationic solutes and low concentrations of anionic solutes in cartilage.
- Isoelectric point
-
A molecular characteristic that quantifies the charge of a solute by calculating the pH at which the solute is neutrally charged. Values above 7 denote a positive charge, whereas values below 7 denote a negative charge.
- Linear solutes
-
Solutes with a flexible, chain-like molecular structure that can change shape from an extended linear geometry to a more compact geometry (random coil).
- Spherical solutes
-
Solutes with a generally spherical molecular structure that cannot substantially change their shape.
- Peclet number
-
A solute transport metric that quantifies the relative contributions of convective transport and diffusive transport. Values above 1 indicate that convection is more important than diffusion, whereas values below 1 indicate that diffusion is more important than convection.
Rights and permissions
About this article
Cite this article
DiDomenico, C.D., Lintz, M. & Bonassar, L.J. Molecular transport in articular cartilage — what have we learned from the past 50 years?. Nat Rev Rheumatol 14, 393–403 (2018). https://doi.org/10.1038/s41584-018-0033-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-018-0033-5
- Springer Nature Limited
This article is cited by
-
Extracellular vesicle–matrix interactions
Nature Reviews Materials (2023)
-
Mesenchymal stromal cells donate mitochondria to articular chondrocytes exposed to mitochondrial, environmental, and mechanical stress
Scientific Reports (2022)
-
A plant-derived natural photosynthetic system for improving cell anabolism
Nature (2022)
-
A noninvasive fluorescence imaging-based platform measures 3D anisotropic extracellular diffusion
Nature Communications (2021)
-
Size-Dependent Effective Diffusivity in Healthy Human and Porcine Joint Synovium
Annals of Biomedical Engineering (2021)