Abstract
Tuberous sclerosis complex (TSC) is an autosomal dominant disease characterized by hamartomatous tumours of the brain, heart, skin, lung and kidney. Patients with TSC show a diverse range of neurological features (including seizures, cognitive disability and autism) and renal manifestations (including angiomyolipomas, epithelial cysts and renal cell carcinoma (RCC)). TSC is caused by inactivating mutations in TSC1 and TSC2, which encode hamartin and tuberin, respectively. These two proteins form a complex that negatively regulates mechanistic target of rapamycin complex 1 (mTORC1), a master regulator of cellular growth and metabolism. In clinical trials, allosteric inhibitors of mTORC1 decrease angiomyolipoma size, but the tumours regrow after treatment cessation. Therefore, the development of strategies to eliminate rather than suppress angiomyolipomas remains a high priority. This Review describes important advances in the TSC field and highlights several remaining critical knowledge gaps: the factors that promote aggressive behaviour by a subset of TSC-associated RCCs; the molecular mechanisms underlying early-onset cystogenesis in TSC2–PKD1 contiguous gene deletion syndrome; the effect of early, long-term mTORC1 inhibition on the development of TSC renal disease; and the identification of the cell or cells of origin of angiomyolipomas.
Key points
-
Tuberous sclerosis complex (TSC) is an autosomal dominant syndrome caused by germline inactivating mutations in either allele of the tumour suppressor genes TSC1 or TSC2.
-
Annual surveillance of renal disease is recommended for most patients with TSC.
-
Nephron-sparing treatments for TSC-related renal disease (rapalogues, embolization and partial nephrectomy) should always be prioritized.
-
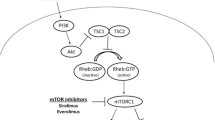
Activation of mechanistic target of rapamycin complex 1 (mTORC1) promotes TSC by accelerating cell growth and inhibiting autophagy.
-
mTORC1 hyperactivation increases oxidative stress, rendering TSC-related tumour cells vulnerable to agents that increase oxidative stress or inhibit antioxidant biosynthesis.
-
The interactions of TSC-related tumour cells with their microenvironment remain a key area of research; for example, tumour cells might be responsive to immune checkpoint inhibition.
-
Critical knowledge gaps include the angiomyolipoma cell of origin, aggressive behaviour of renal cell carcinomas and molecular mechanisms underlying early-onset cystic disease in PKD1–TSC2 contiguous gene deletion syndrome.





Similar content being viewed by others
References
Henske, E. P., Jozwiak, S., Kingswood, J. C., Sampson, J. R. & Thiele, E. A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2, 16035 (2016).
Crino, P. B., Nathanson, K. L. & Henske, E. P. The tuberous sclerosis complex. N. Engl. J. Med. 355, 1345–1356 (2006).
Curatolo, P., Bombardieri, R. & Jozwiak, S. Tuberous sclerosis. Lancet 372, 657–668 (2008).
Krueger, D. A. & Northrup, H. International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr. Neurol. 49, 255–265 (2013).
Robertson, F. M., Cendron, M., Klauber, G. T. & Harris, B. H. Renal cell carcinoma in association with tuberous sclerosis in children. J. Pediatr. Surg. 31, 729–730 (1996).
Kulkarni, S., Uddar, M., Deshpande, S. G., Vaid, S. & Wadia, R. S. Renal cell carcinoma as significant manifestation of tuberous sclerosis complex. J. Assoc. Physicians India 48, 351–353 (2000).
Yang, P. et al. Renal cell carcinoma in tuberous sclerosis complex. Am. J. Surg. Pathol. 38, 895–909 (2014).
Holt, J. F. & Dickerson, W. W. The osseous lesions of tuberous sclerosis. Radiology 58, 1–8 (1952).
Hizawa, K. et al. Gastrointestinal involvement in tuberous sclerosis. Two case reports. J. Clin. Gastroenterol. 19, 46–49 (1994).
Gould, S. R. Hamartomatous rectal polyps are common in tuberous sclerosis. Ann. NY Acad. Sci. 615, 71–80 (1991).
Jozwiak, S., Michalowicz, R., Pedich, M. & Rajszys, P. Hepatic hamartoma in tuberous sclerosis. Lancet 339, 180 (1992).
Larson, A. M. et al. Pancreatic neuroendocrine tumors in patients with tuberous sclerosis complex. Clin. Genet. 82, 558–563 (2012).
Wang, J. H. et al. Multi-modality imaging findings of splenic hamartoma: a report of nine cases and review of the literature. Abdom. Imag. 38, 154–162 (2013).
O’Callaghan, F. J., Noakes, M. J., Martyn, C. N. & Osborne, J. P. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int. 94, 853–857 (2004).
Osborne, J. P., Fryer, A. & Webb, D. Epidemiology of tuberous sclerosis. Ann. NY Acad. Sci. 615, 125–127 (1991).
Henske, E. P. & McCormack, F. X. Lymphangioleiomyomatosis — a wolf in sheep’s clothing. J. Clin. Invest. 122, 3807–3816 (2012).
Rakowski, S. K. et al. Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int. 70, 1777–1782 (2006).
Henske, E. P. et al. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer 13, 295–298 (1995).
van Slegtenhorst, M. et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277, 805–808 (1997).
Plank, T. L., Yeung, R. S. & Henske, E. P. Hamartin, the product of the tuberous sclerosis 1 (TSC1) gene, interacts with tuberin and appears to be localized to cytoplasmic vesicles. Cancer Res. 58, 4766–4770 (1998).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Dibble, C. C. & Cantley, L. C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 25, 545–555 (2015).
Kwiatkowski, D. J. & Manning, B. D. Molecular basis of giant cells in tuberous sclerosis complex. N. Engl. J. Med. 371, 778–780 (2014).
Im, K. et al. Altered structural brain networks in tuberous sclerosis complex. Cereb. Cortex 26, 2046–2058 (2016).
Tsai, P. T. et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647–651 (2012).
Tyburczy, M. E. et al. A shower of second hit events as the cause of multifocal renal cell carcinoma in tuberous sclerosis complex. Hum. Mol. Genet. 24, 1836–1842 (2015).
Jones, A. C. et al. Molecular genetic and phenotypic analysis reveals differences between TSC1 and TSC2 associated familial and sporadic tuberous sclerosis. Hum. Mol. Genet. 6, 2155–2161 (1997).
Dabora, S. L. et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 68, 64–80 (2001).
Tyburczy, M. E. et al. Mosaic and intronic mutations in TSC1/TSC2 explain the majority of TSC patients with no mutation identified by conventional testing.PLOS Genet. 11, e1005637 (2015).
Jones, A. C. et al. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am. J. Hum. Genet. 64, 1305–1315 (1999).
van Eeghen, A. M., Black, M. E., Pulsifer, M. B., Kwiatkowski, D. J. & Thiele, E. A. Genotype and cognitive phenotype of patients with tuberous sclerosis complex. Eur. J. Hum. Genet. 20, 510–515 (2012).
de Vries, P. J. et al. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND checklist. Pediatr. Neurol. 52, 25–35 (2015).
Yu, J. J. et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc. Natl Acad. Sci. USA 106, 2635–2640 (2009).
McCormack, F. X. et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 364, 1595–1606 (2011).
Muir, T. E. et al. Micronodular pneumocyte hyperplasia. Am. J. Surg. Pathol. 22, 465–472 (1998).
Hayashi, T. et al. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod. Pathol. 23, 1251–1260 (2010).
Shepherd, C. W., Gomez, M. R., Lie, J. T. & Crowson, C. S. Causes of death in patients with tuberous sclerosis. Mayo Clin. Proc. 66, 792–796 (1991).
Eijkemans, M. J. et al. Long-term follow-up assessing renal angiomyolipoma treatment patterns, morbidity, and mortality: an observational study in tuberous sclerosis complex patients in the Netherlands. Am. J. Kidney. Dis. 66, 638–645 (2015).
European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75, 1305–1315 (1993).
Bissler, J. J. et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381, 817–824 (2013).
Bissler, J. J. et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 358, 140–151 (2008).
Dabora, S. L. et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLOS ONE 6, e23379 (2011).
Li, J., Kim, S. G. & Blenis, J. Rapamycin: one drug, many effects. Cell Metab. 19, 373–379 (2014).
Krueger, D. A. et al. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology 80, 574–580 (2013).
Krueger, D. A. et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 363, 1801–1811 (2010).
Krueger, D. A. et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex.Ann. Neurol. 74, 679–687 (2013).
Krueger, D. A. et al. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology 87, 2408–2415 (2016).
Siroky, B. J. et al. Improvement in renal cystic disease of tuberous sclerosis complex after treatment with mammalian target of rapamycin inhibitor. J. Pediatr. 187, 318–322.e2 (2017).
Franz, D. N. et al. Long-term use of everolimus in patients with tuberous sclerosis complex: final results from the EXIST-1 study. PLOS ONE 11, e0158476 (2016).
French, J. A. et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet 388, 2153–2163 (2016).
Amin, S. et al. Causes of mortality in individuals with tuberous sclerosis complex. Dev. Med. Child Neurol. 59, 612–617 (2017).
Ewalt, D. H., Sheffield, E., Sparagana, S. P., Delgado, M. R. & Roach, E. S. Renal lesion growth in children with tuberous sclerosis complex. J. Urol. 160, 141–145 (1998).
Guo, J. et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am. J. Surg. Pathol. 38, 1457–1467 (2014).
Henske, E. P. et al. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am. J. Hum. Genet. 59, 400–406 (1996).
Henske, E. P. et al. Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am. J. Pathol. 151, 1639–1647 (1997).
Karbowniczek, M., Yu, J. & Henske, E. P. Renal angiomyolipomas from patients with sporadic lymphangiomyomatosis contain both neoplastic and non-neoplastic vascular structures. Am. J. Pathol. 162, 491–500 (2003).
Delaney, S. P., Julian, L. M. & Stanford, W. L. The neural crest lineage as a driver of disease heterogeneity in tuberous sclerosis complex and lymphangioleiomyomatosis. Front. Cell Dev. Biol. 2, 69 (2014).
Siroky, B. J. et al. Evidence for pericyte origin of TSC-associated renal angiomyolipomas and implications for angiotensin receptor inhibition therapy. Am. J. Physiol. Renal Physiol. 307, F560–F570 (2014).
Goncalves, A. F. et al. Evidence of renal angiomyolipoma neoplastic stem cells arising from renal epithelial cells. Nat. Commun. 8, 1466 (2017).
Webb, D. W., Kabala, J. & Osborne, J. P. A population study of renal disease in patients with tuberous sclerosis. Br. J. Urol. 74, 151–154 (1994).
Robert, A. et al. Renal involvement in tuberous sclerosis complex with emphasis on cystic lesions. Radiol. Med. 121, 402–408 (2016).
Cook, J. A., Oliver, K., Mueller, R. F. & Sampson, J. A cross sectional study of renal involvement in tuberous sclerosis. J. Med. Genet. 33, 480–484 (1996).
Ren, S. et al. Inactivation of Tsc2 in mesoderm-derived cells causes polycystic kidney lesions and impairs lung alveolarization. Am. J. Pathol. 186, 3261–3272 (2016).
Brook-Carter, P. T. et al. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease.—.a contiguous gene syndrome. Nat. Genet. 8, 328–332 (1994).
Torra, R. et al. Facilitated diagnosis of the contiguous gene syndrome: tuberous sclerosis and polycystic kidneys by means of haplotype studies. Am. J. Kidney Dis. 31, 1038–1043 (1998).
Sampson, J. R. et al. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am. J. Hum. Genet. 61, 843–851 (1997).
Pema, M. et al. mTORC1-mediated inhibition of polycystin-1 expression drives renal cyst formation in tuberous sclerosis complex. Nat. Commun. 7, 10786 (2016).
Hartman, T. R. et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum. Mol. Genet. 18, 151–163 (2009).
Al-Saleem, T. et al. Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer 83, 2208–2216 (1998).
Bjornsson, J., Short, M. P., Kwiatkowski, D. J. & Henske, E. P. Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features. Am. J. Pathol. 149, 1201–1208 (1996).
Kubo, M., Iwashita, K., Oyachi, N., Oyama, T. & Yamamoto, T. Two different types of infantile renal cell carcinomas associated with tuberous sclerosis. J. Pediatr. Surg. 46, E37–E41 (2011).
Giannikou, K. et al. Whole exome sequencing identifies TSC1/TSC2 biallelic loss as the primary and sufficient driver event for renal angiomyolipoma development. PLOS Genet. 12, e1006242 (2016).
Kang, S. G. et al. Two different renal cell carcinomas and multiple angiomyolipomas in a patient with tuberous sclerosis. Kor. J. Urol. 51, 729–732 (2010).
Jimenez, R. E. et al. Concurrent angiomyolipoma and renal cell neoplasia: a study of 36 cases. Mod. Pathol. 14, 157–163 (2001).
Paul, E., Thiele, E. A., Shailam, R., Rosales, A. M. & Sadow, P. M. Case records of the Massachusetts general hospital. Case 26–2011. A 7-year-old boy with a complex cyst in the kidney. N. Engl. J. Med. 365, (743–751 (2011).
Buj Pradilla, M. J., Marti Balleste, T., Torra, R. & Villacampa Auba, F. Recommendations for imaging-based diagnosis and management of renal angiomyolipoma associated with tuberous sclerosis complex. Clin. Kidney J. 10, (728–737 (2017).
Jinzaki, M., Silverman, S. G., Akita, H., Mikami, S. & Oya, M. Diagnosis of renal angiomyolipomas: classic, fat-poor, and epithelioid types. Semin. Ultrasound CT MR 38, 37–46 (2017).
Potretzke, A. M. et al. Computed tomography and magnetic resonance findings of fat-poor angiomyolipomas. J. Endourol. 31, 119–128 (2017).
Kingswood, J. C. et al. Review of the tuberous sclerosis renal guidelines from the 2012 consensus conference: current data and future study. Nephron 134, 51–58 (2016).
Bissler, J. J. & Kingswood, J. C. Optimal treatment of tuberous sclerosis complex associated renal angiomyolipomata: a systematic review. Ther. Adv. Urol. 8, 279–290 (2016).
Yamakado, K. et al. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology 225, 78–82 (2002).
Williams, J. M., Racadio, J. M., Johnson, N. D., Donnelly, L. F. & Bissler, J. J. Embolization of renal angiomyolipomata in patients with tuberous sclerosis complex. Am. J. Kidney Dis. 47, 95–102 (2006).
Bissler, J. J. et al. The effect of everolimus on renal angiomyolipoma in pediatric patients with tuberous sclerosis being treated for subependymal giant cell astrocytoma. Pediatr. Nephrol. 33, 101–109 (2018).
Bissler, J. J. et al. Everolimus long-term use in patients with tuberous sclerosis complex: four-year update of the EXIST-2 study. PLOS ONE 12, e0180939 (2017).
Malinowska, I. A. et al. Similar trends in serum VEGF-D levels and kidney angiomyolipoma responses with longer duration sirolimus treatment in adults with tuberous sclerosis. PLOS ONE 8, e56199 (2013).
Franz, D. N. et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 15, 1513–1520 (2014).
Cardamone, M. et al. Mammalian target of rapamycin inhibitors for intractable epilepsy and subependymal giant cell astrocytomas in tuberous sclerosis complex. J. Pediatr. 164, 1195–1200 (2014).
Cheng, S., Hawkins, C., Taylor, M. D. & Bartels, U. Pathological findings of a subependymal giant cell astrocytoma following treatment with rapamycin. Pediatr. Neurol. 53, 238–242 (2015).
Davies, D. M. et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N. Engl. J. Med. 358, 200–203 (2008).
Franz, D. N. et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381, 125–132 (2013).
Bissler, J. J. et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol. Dial. Transplant. 31, 111–119 (2016).
Brakemeier, S. et al. Treatment effect of mTOR-inhibition on tissue composition of renal angiomyolipomas in tuberous sclerosis complex (TSC). PLOS ONE 12, e0189132 (2017).
Samuels, J. A. Treatment of renal angiomyolipoma and other hamartomas in patients with tuberous sclerosis complex. Clin. J. Am. Soc. Nephrol. 12, 1196–1202 (2017).
Sheth, R. A., Feldman, A. S., Paul, E., Thiele, E. A. & Walker, T. G. Sporadic versus tuberous sclerosis complex-associated angiomyolipomas: predictors for long-term outcomes following transcatheter embolization. J. Vasc. Interv. Radiol. 27, 1542–1549 (2016).
Bissler, J. J., Racadio, J., Donnelly, L. F. & Johnson, N. D. Reduction of postembolization syndrome after ablation of renal angiomyolipoma. Am. J. Kidney Dis. 39, 966–971 (2002).
Shillingford, J. M. et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl Acad. Sci. USA 103, 5466–5471 (2006).
Bonnet, C. S. et al. Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum. Mol. Genet. 18, 2166–2176 (2009).
Braun, W. E., Schold, J. D., Stephany, B. R., Spirko, R. A. & Herts, B. R. Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study. Clin. J. Am. Soc. Nephrol. 9, 881–888 (2014).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Perico, N. et al. Sirolimus therapy to halt the progression of ADPKD. J. Am. Soc. Nephrol. 21, 1031–1040 (2010).
Kim, H. S. et al. The use of everolimus to target carcinogenic pathways in a patient with renal cell carcinoma and tuberous sclerosis complex: a case report. J. Med. Case Rep. 8, 95 (2014).
Alsidawi, S. & Kasi, P. M. Exceptional response to everolimus in a novel tuberous sclerosis complex-2 mutation-associated metastatic renal-cell carcinoma. Cold Spring Harb. Mol. Case Stud. 4, a002220 (2018).
Yu, J., Astrinidis, A., Howard, S. & Henske, E. P. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L694–L700 (2004).
Ogorek, B. et al. TSC2 regulates microRNA biogenesis via mTORC1 and GSK3β. Hum. Mol. Genet. 27, 1654–1663 (2018).
Parkhitko, A. A. et al. Autophagy-dependent metabolic reprogramming sensitizes TSC2-deficient cells to the antimetabolite 6-aminonicotinamide. Mol. Cancer Res. 12, 48–57 (2014).
Filippakis, H. et al. Lysosomal regulation of cholesterol homeostasis in tuberous sclerosis complex is mediated via NPC1 and LDL-R. Oncotarget 8, 38099–38112 (2017).
Liu, H. J. et al. TSC2-deficient tumors have evidence of T cell exhaustion and respond to anti-PD-1/anti-CTLA-4 immunotherapy. JCI Insight 3, 98674 (2018).
Csibi, A. et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 153, 840–854 (2013).
Lam, H. C. et al. p62/SQSTM1 cooperates with hyperactive mTORC1 to regulate glutathione production, maintain mitochondrial integrity, and promote tumorigenesis. Cancer Res. 77, 3255–3267 (2017).
Onda, H., Lueck, A., Marks, P. W., Warren, H. B. & Kwiatkowski, D. J. Tsc2 +/− mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Invest. 104, 687–695 (1999).
Lee, L. et al. Efficacy of a rapamycin analog (CCI-779) and IFN-γ in tuberous sclerosis mouse models. Genes Chromosomes Cancer 42, 213–227 (2005).
Liang, N. et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J. Exp. Med. 211, 2249–2263 (2014).
Traykova-Brauch, M. et al. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat. Med. 14, 979–984 (2008).
Zhou, J., Brugarolas, J. & Parada, L. F. Loss of Tsc1, but not Pten, in renal tubular cells causes polycystic kidney disease by activating mTORC1. Hum. Mol. Genet. 18, 4428–4441 (2009).
Buller, C. L. et al. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am. J. Physiol. Cell Physiol. 295, C836–C843 (2008).
Csibi, A. & Blenis, J. Appetite for destruction: the inhibition of glycolysis as a therapy for tuberous sclerosis complex-related tumors. BMC Biol. 9, 69 (2011).
Choo, A. Y. et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol. Cell 38, 487–499 (2010).
Ben-Sahra, I., Howell, J. J., Asara, J. M. & Manning, B. D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328 (2013).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Priolo, C. et al. Tuberous sclerosis complex 2 loss increases lysophosphatidylcholine synthesis in lymphangioleiomyomatosis. Am. J. Respir. Cell. Mol. Biol. 53, 33–41 (2015).
Lee, G. et al. Post-transcriptional regulation of de novo lipogenesis by mTORC1-S6K1-SRPK2 signaling. Cell 171, 1545–1558 (2017).
Goncharova, E. A. et al. mTORC2 is required for proliferation and survival of TSC2-null cells. Mol. Cell. Biol. 31, 2484–2498 (2011).
Taveira-DaSilva, A. M., Jones, A. M., Julien-Williams, P. A., Stylianou, M. & Moss, J. Retrospective review of combined sirolimus and simvastatin therapy in lymphangioleiomyomatosis. Chest 147, 180–187 (2015).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02061397 (2017).
Li, C. et al. Estradiol and mTORC2 cooperate to enhance prostaglandin biosynthesis and tumorigenesis in TSC2-deficient LAM cells. J. Exp. Med. 211, 15–28 (2014).
US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02484664 (2018).
Siroky, B. J. et al. Human TSC-associated renal angiomyolipoma cells are hypersensitive to ER stress. Am. J. Physiol. Renal Physiol. 303, F831–F844 (2012).
Di Nardo, A. et al. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J. Neurosci. 29, 5926–5937 (2009).
Zhang, Y. et al. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 513, 440–443 (2014).
Morita, M. et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 18, 698–711 (2013).
Morita, M. et al. mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol. Cell. 67, 922–935 (2017).
Ebrahimi-Fakhari, D. et al. Impaired mitochondrial dynamics and mitophagy in neuronal models of tuberous sclerosis complex. Cell Rep. 17, 1053–1070 (2016).
Cunningham, J. T. et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450, 736–740 (2007).
Filipczak, P. T. et al. TSC2 deficiency unmasks a novel necrosis pathway that is suppressed by the RIP1/RIP3/MLKL signaling cascade. Cancer Res. 76, 7130–7139 (2016).
Medvetz, D. et al. High-throughput drug screen identifies chelerythrine as a selective inducer of death in a TSC2-null setting. Mol. Cancer Res. 13, 50–62 (2015).
Li, J. et al. Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells. Proc. Natl Acad. Sci. USA 112, E21–E29 (2015).
Dunlop, E. A., Johnson, C. E., Wiltshire, M., Errington, R. J. & Tee, A. R. Targeting protein homeostasis with nelfinavir/salinomycin dual therapy effectively induces death of mTORC1 hyperactive cells. Oncotarget 8, 48711–48724 (2017).
Parkhitko, A. et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc. Natl Acad. Sci. USA 108, 12455–12460 (2011).
El-Chemaly, S. et al. Sirolimus and autophagy inhibition in lymphangioleiomyomatosis: results of a phase I clinical trial. Chest 151, 1302–1310 (2017).
Alayev, A. et al. Effects of combining rapamycin and resveratrol on apoptosis and growth of TSC2-deficient xenograft tumors. Am. J. Respir. Cell. Mol. Biol. 53, 637–646 (2015).
Cui, Y. et al. Aberrant SYK kinase signaling is essential for tumorigenesis induced by TSC2 inactivation. Cancer Res. 77, 1492–1502 (2017).
Kingswood, J. C. et al. Renal angiomyolipoma in patients with tuberous sclerosis complex: findings from the tuberous sclerosis registry to increase disease awareness. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfy063 (2018).
Kwiatkowski, D. J. et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 11, 525–534 (2002).
Hernandez, O., Way, S., McKenna, J. 3rd & Gambello, M. J. Generation of a conditional disruption of the Tsc2 gene. Genesis 45, 101–106 (2007).
Finlay, G. A., Malhowski, A. J., Polizzi, K., Malinowska-Kolodziej, I. & Kwiatkowski, D. J. Renal and liver tumors in Tsc2 +/− mice, a model of tuberous sclerosis complex, do not respond to treatment with atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Mol. Cancer Ther. 8, 1799–1807 (2009).
Lee, N. et al. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 9, 8 (2009).
Zhang, D. et al. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 202, 289.e1–289.e9 (2010).
Li, J. et al. mTORC1-Driven tumor cells are highly sensitive to therapeutic targeting by antagonists of oxidative stress. Cancer Res. 76, 4816–4827 (2016).
Valvezan, A. J. et al. mTORC1 couples nucleotide synthesis to nucleotide demand resulting in a targetable metabolic vulnerability. Cancer Cell 32, 624–638 (2017).
Acknowledgements
The authors thank J. Nijmeh, N. Alesi and C. Filippakis (Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA) for critical help in table generation, image acquisition and proofreading.
Reviewer information
Nature Reviews Nephrology thanks I. Frew, J. Kingswood and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors participated in researching data for the article, discussions of its content, writing the draft and review or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Tuberous sclerosis complex
-
(TSC). A rare tumour suppressor genetic disorder that causes benign tumours in various organs, primarily the brain, eyes, heart, kidneys, skin and lungs; this disorder is also the leading genetic cause of both epilepsy and autism.
- Renal cell carcinoma
-
(RCC). The most common kidney cancer, originating in the lining of the proximal convoluted tubes in the kidney. Occurs in both adults and children with tuberous sclerosis complex.
- Lymphangioleiomyomatosis
-
A rare progressive lung disease that is the pulmonary manifestation of tuberous sclerois complex, primarily affecting women of childbearing age, that typically results in cystic lung destruction.
- Mechanistic target of rapamycin (mTOR) complex 1
-
(mTORC1). A protein complex that functions as a master regulator. This complex senses and integrates nutrient, energy and redox signals and either potentiates growth and anabolic processes or limits catabolic processes, such as autophagy.
- GTP-binding protein Rheb
-
Also known as Ras homologue enriched in brain, this protein is ubiquitously expressed in humans and other mammals and is principally involved in the mechanistic target of rapamycin pathway and in regulation of the cell cycle.
- Autophagy
-
A conserved regulated intracellular process for degradation and recycling of the cellular components in enzyme-filled compartments, known as autophagosomes.
- Knudson two-hit hypothesis
-
Most loss-of-function mutations in tumour suppressor genes are recessive, meaning that both alleles of the gene must be mutated for function to be lost and for the cell to become cancerous. The germline mutation is the first hit, but disease onset requires a second-hit event affecting the other allele.
- Angiomyolipomas
-
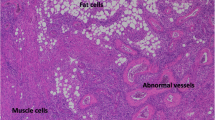
A form of perivascular epithelioid cell tumour characterized by varying contributions of adipocytes, smooth muscle cells and abnormal vasculature.
- Mosaicism
-
The presence, within the same individual, of different cell populations with distinct genetic make-up.
- Pneumothorax
-
A condition in which air is trapped between the lungs and chest wall, leading a portion or all of the lung to collapse.
- Multifocal micronodular pneumocyte hyperplasia
-
A subtype of pneumocytic hyperplasia that presents as a diffuse, benign nodular proliferation of epithelial cells and occurs in many patients with tuberous sclerosis.
- Sirolimus
-
An FDA-approved drug with immunosuppressive and antiproliferative properties due to inhibition of mechanistic target of rapamycin.
- Everolimus
-
An FDA-approved mechanistic target of rapamycin inhibitor that is a derivative of sirolimus and has a similar mechanism of action.
- Autosomal dominant polycystic kidney disease
-
(ADPKD). One of the most common, life-threatening genetic diseases. It causes small, fluid-filled sacs called cysts to develop and enlarge in the kidneys, which eventually leads to kidney failure.
- Mizoribine
-
An FDA-approved selective inhibitor of inosine monophosphate synthetase and GMP synthetase, resulting in the inhibition of de novo nucleotide synthesis, which inhibits DNA synthesis. Also has immunosuppressive properties.
- Simvastatin
-
An FDA-approved statin that works by slowing the production of cholesterol in the body to prevent atherosclerosis-related complications.
- Celecoxib
-
An FDA-approved nonsteroidal anti-inflammatory drug (NSAID), specifically a cyclooxygenase 2 inhibitor, that relieves pain and swelling by reducing prostaglandins.
- Endoplasmic reticulum stress
-
An accumulation of unfolded proteins in the endoplasmic reticulum that overloads its peptide processing and recycling capacity.
- Unfolded protein response
-
An adaptive mechanism, initiated in response to the accumulation of unfolded proteins, that involves transcriptional activation of genes responsible for enhancing the protein-folding capacity of the endoplasmic reticulum and promotes endoplasmic reticulum-associated misfolded protein degradation.
- l-Buthionine sulfoximine
-
(BSO). A compound that inhibits γ-glutamyl cysteine synthetase, the enzyme required in the first step of glutathione synthesis, thus reducing glutathione levels.
- Chelerythrine chloride
-
A potent and selective inhibitor of protein kinase C.
- Nelfinavir
-
An FDA-approved antiretroviral drug that belongs to the protease inhibitor family.
- Salinomycin
-
An antibacterial drug that has been shown in mice to kill cancer stem cells.
- Chloroquine
-
An FDA-approved drug that neutralizes lysosomal acidification, thus blocking autophagosomal degradation, and mildly suppresses the immune system.
- Pentose phosphate pathway
-
An alternative to the glucose-oxidizing metabolic pathway. It is the major source to generate NADPH but also generates pentoses (five-carbon sugars) as well as ribose 5-phosphate, a precursor for the synthesis of nucleotides.
- 6-Aminonicotinamide
-
An inhibitor of 6-phosphogluconate dehydrogenase, thus used to block the pentose phosphate pathway.
- Resveratrol
-
A type of natural phenol found in plants that acts as an antioxidant.
- T cell exhaustion
-
The loss of T cells’ ability to perform their effector functions efficiently, which leads to low proliferative capacity and poor survival following antigen stimulation. Exhausted T cells co-express multiple inhibitory receptors that negatively regulate their function, such as programmed cell death protein 1, a major target of clinical immunotherapy.
Rights and permissions
About this article
Cite this article
Lam, H.C., Siroky, B.J. & Henske, E.P. Renal disease in tuberous sclerosis complex: pathogenesis and therapy. Nat Rev Nephrol 14, 704–716 (2018). https://doi.org/10.1038/s41581-018-0059-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0059-6
- Springer Nature Limited
This article is cited by
-
Giant cell angiofibroma of gingiva in tuberous sclerosis complex: a case report and literature review
Diagnostic Pathology (2024)
-
Childhood tuberous sclerosis complex in southern Sweden: a paradigm shift in diagnosis and treatment
BMC Pediatrics (2023)
-
Cystic kidney disease in tuberous sclerosis complex: current knowledge and unresolved questions
Pediatric Nephrology (2023)
-
Prevalence of thoracoabdominal imaging findings in tuberous sclerosis complex
Orphanet Journal of Rare Diseases (2022)
-
Midkine expression by stem-like tumor cells drives persistence to mTOR inhibition and an immune-suppressive microenvironment
Nature Communications (2022)